Development of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids: A Potential Breakthrough in Heterosis Fixation
Abstract
1. Introduction
2. Results
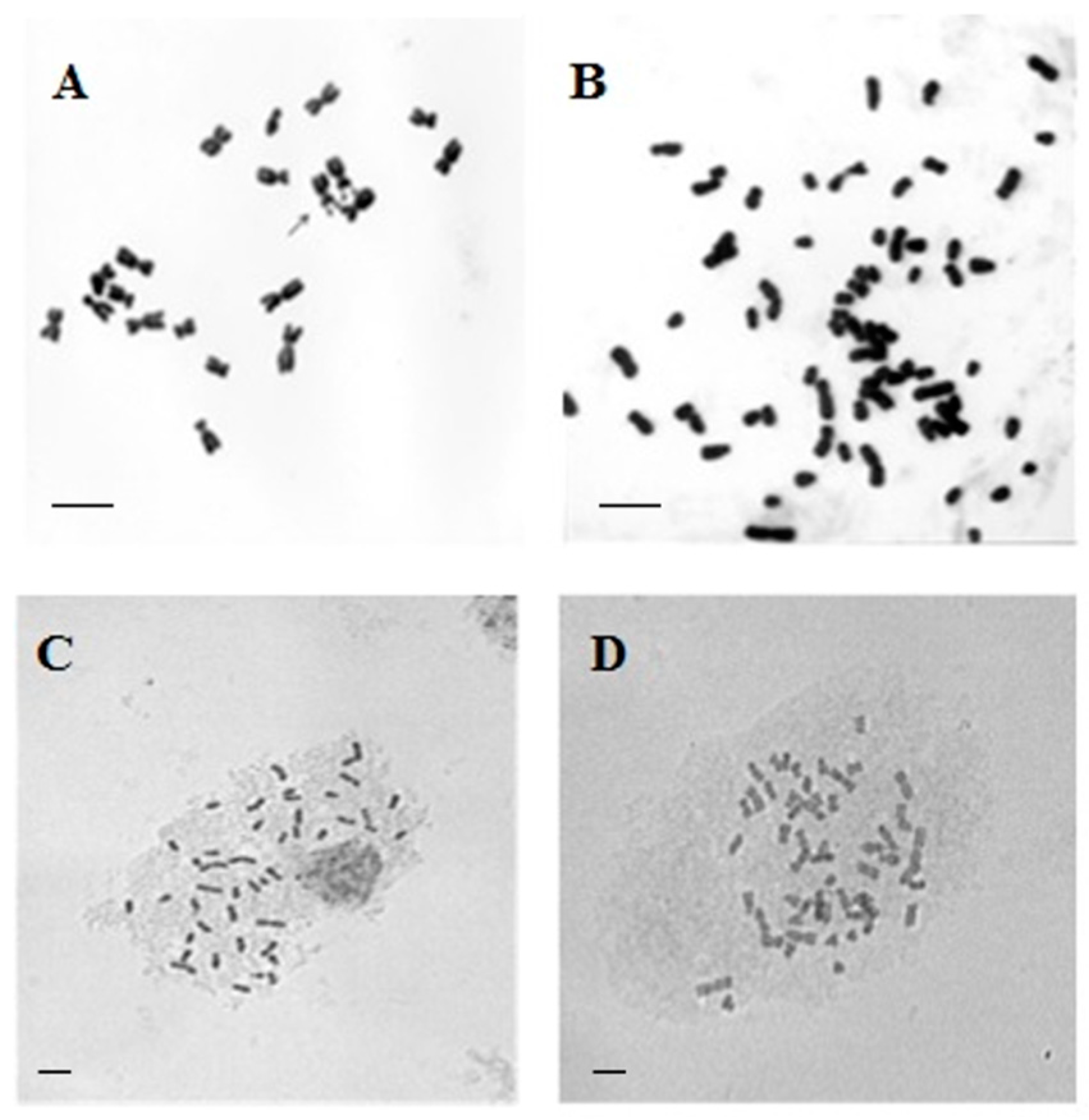
2.1. Obtaining 46-Chromosome Maize–Tripsacum Hybrids
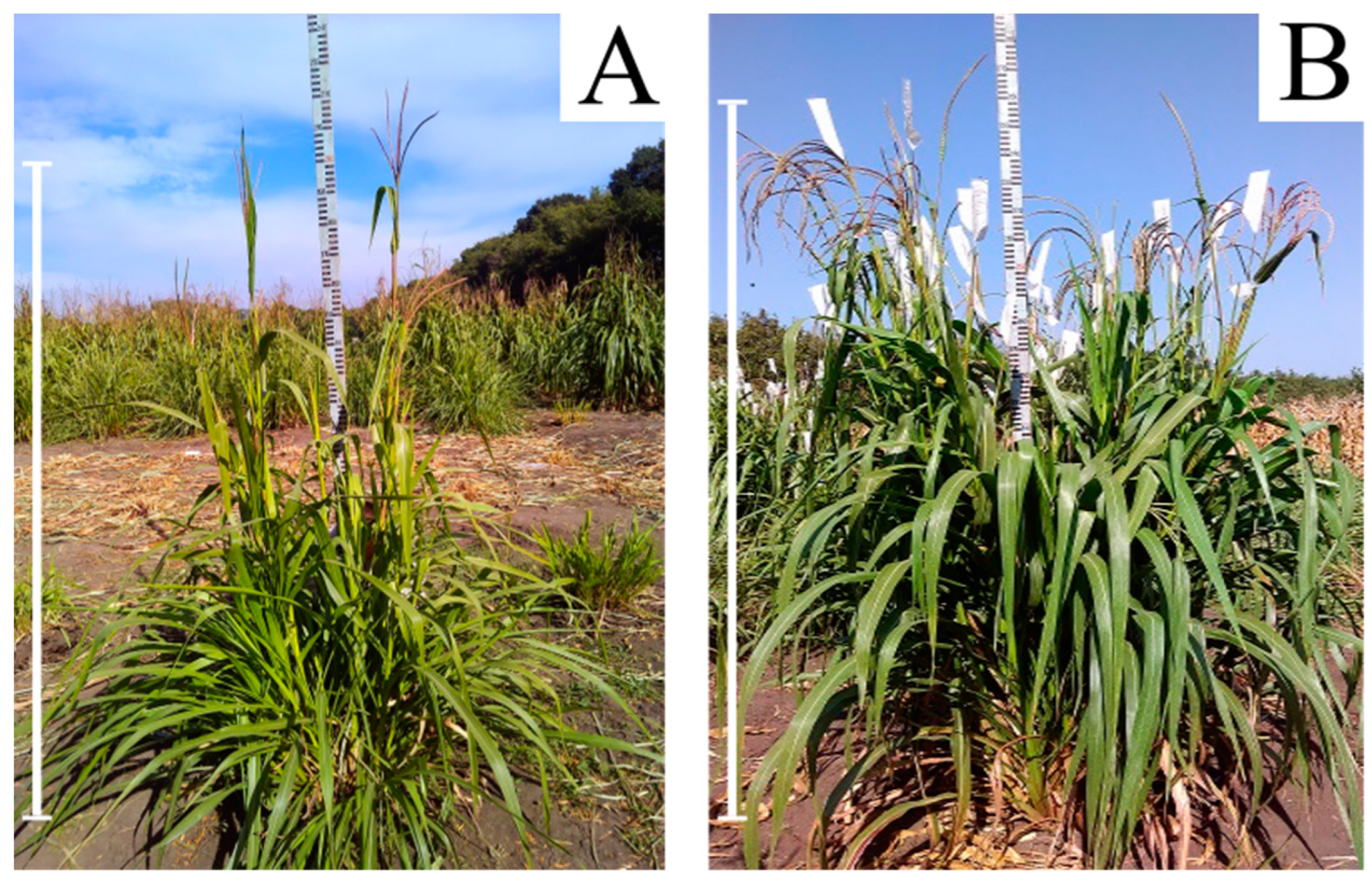
2.2. Production of 56-Chromosome Maize–Tripsacum Hybrids
- Plants produced by fertilization by a haploid maize sperm with an egg originating from a megaspore that has undergone meiosis and carries 18 Tripsacum chromosomes with maize chromosomes added is a BII hybridization;
- The 56-chromosome plants, which result from BIII hybridization when an unreduced egg carrying 46 chromosomes is fertilized by a haploid maize sperm;
- The 46-chromosome forms resulting from asexual reproduction.
2.3. Selection of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids
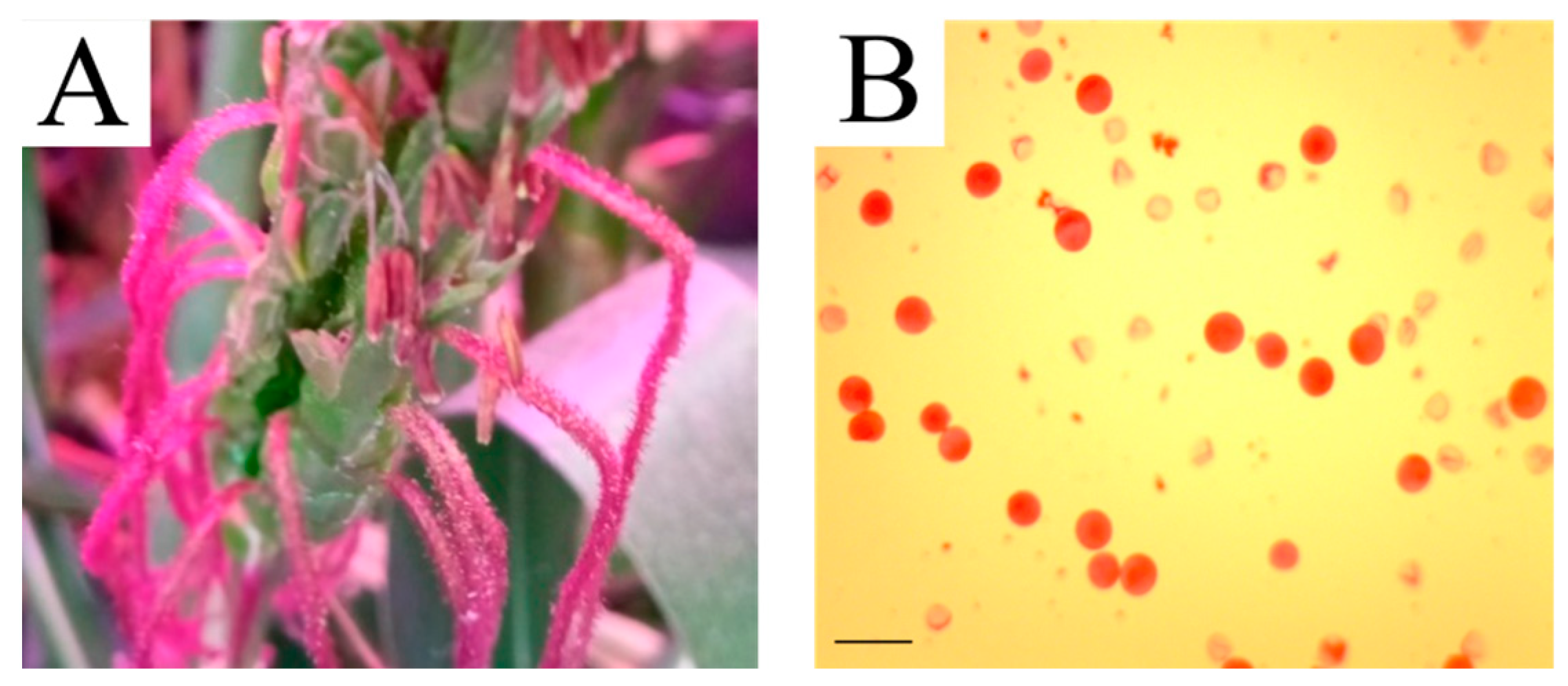
2.4. Selection of Apomictic 56-Chromosomal Plants with Fertile Pollen
2.5. Establishment and Analysis of Nucleotide Sequences of Pox3 and tRNA-Leu Genes in the Parental Lines and Hybrids
3. Discussion
4. Materials and Methods
4.1. Source Material and Hybridization
4.2. The Method of Determining Pollen Fertility
4.3. Determination of Modes of Reproduction
4.4. Molecular–Biological Testing of Hybridization Results: Total DNA Isolation, PCR Amplification and Nucleotide Sequence Determination
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Navashin, M.S. New views in selection. Semenovodstvo 1933, 2, 11–16. [Google Scholar]
- Nogler, G.A. Gametophytic Apomixis; Springer: Berlin/Heidelberg, Germany, 1984; pp. 475–518. [Google Scholar]
- Belousova, N.I.; Laikova, L.I.; Fokina, E.S. Apomixis in the second and third generations of maize × Trypsacum hybrids (Apomiksis vo vtorom i tretyem pokoleniyakh gibridov kukuruzy s tripsakum). Izvestiia Sibirskogo Otdeleniia AN SSSR 1972, 10, 43–45. [Google Scholar]
- Blakey, C.A.; Costich, D.; Sokolov, V.; Islam-Faridi, M.N. Tripsacum Genetics: From Observations along a River to Molecular Genomics. Maydica 2007, 52, 81. [Google Scholar]
- Petrov, D.F. Apomixis and distant hybridization (Apomiksis i otdalennaya gibridizatsiya). In Cytology and breeding of cultivated plants (Tsitologiya i selektsiya kulturnykh rasteniy); Petrov, D.F., Ed.; Ed. & Publ. Dept. of Sib. Div., USSR Acad. Sci.: Novosibirsk, Russia, 1964; pp. 6–17. [Google Scholar]
- Sokolov, V.A.; Kindiger, B.; Khatypova, I.V. 39-Chromosomal Maize-Tripsacum Apomictically Reproducing Hybrids. Genetika 1998, 34, 499–506. [Google Scholar]
- Yudin, B.F.; Sokolov, V.A. Genetic Man. Plants 1989, 5, 36–40. [Google Scholar]
- Mangelsdorf, P.C.; Reeves, R.G.; Mangelsdorf, P.C.; Reeves, R.G. The Origin of Indian Corn and Its Relatives. Tex. Agric. Exp. Stn. Bull. 1939, 574, 1–315. [Google Scholar]
- Harlan, J.R.; Wet, J.M.J.D. Pathways of Genetic Transfer from Tripsacum to Zea mays. Proc. Natl. Acad. Sci. USA 1977, 74, 3494–3497. [Google Scholar] [CrossRef]
- Kindiger, B.; Sokolov, V. Progress in the Development of Apomictic Maize. Trends Agron. 1997, 1, 75–94. [Google Scholar]
- Grimanelli, D.; Leblanc, O.; Espinosa, E.; Perotti, E.; González De León, D.; Savidan, Y. Non-Mendelian Transmission of Apomixis in Maize–Tripsacum Hybrids Caused by a Transmission Ratio Distortion. Heredity 1998, 80, 40–47. [Google Scholar] [CrossRef]
- Leblanc, O.; Grimanelli, D.; Islam-Faridi, N.; Berthaud, J.; Savidan, Y. Reproductive Behavior in Maize-Tripsacum Polyhaploid Plants: Implications for the Transfer of Apomixis into Maize. J. Hered. 1996, 87, 108–111. [Google Scholar] [CrossRef][Green Version]
- Schallau, A.; Arzenton, F.; Johnston, A.J.; Hähnel, U.; Koszegi, D.; Blattner, F.R.; Altschmied, L.; Haberer, G.; Barcaccia, G.; Bäumlein, H. Identification and Genetic Analysis of the APOSPORY Locus in Hypericum Perforatum L.: The APOSPORY Locus in Hypericum perforatum L. Plant J. 2010, 62, 773–784. [Google Scholar] [CrossRef]
- Corral, J.M.; Vogel, H.; Aliyu, O.M.; Hensel, G.; Thiel, T.; Kumlehn, J.; Sharbel, T.F. A Conserved Apomixis-Specific Polymorphism Is Correlated with Exclusive Exonuclease Expression in Premeiotic Ovules of Apomictic Boechera Species. Plant Physiol. 2013, 163, 1660–1672. [Google Scholar] [CrossRef]
- Siena, L.A.; Ortiz, J.P.A.; Calderini, O.; Paolocci, F.; Cáceres, M.E.; Kaushal, P.; Grisan, S.; Pessino, S.C.; Pupilli, F. An Apomixis-Linked ORC3-like Pseudogene Is Associated with Silencing of Its Functional Homolog in Apomictic Paspalum Simplex. J. Exp. Bot. 2016, 67, 1965–1978. [Google Scholar] [CrossRef]
- Conner, J.A.; Goel, S.; Gunawan, G.; Cordonnier-Pratt, M.-M.; Johnson, V.E.; Liang, C.; Wang, H.; Pratt, L.H.; Mullet, J.E.; DeBarry, J.; et al. Sequence Analysis of Bacterial Artificial Chromosome Clones from the Apospory-Specific Genomic Region of Pennisetum and Cenchrus. Plant Physiol. 2008, 147, 1396–1411. [Google Scholar] [CrossRef]
- Conner, J.A.; Ozias-Akins, P. Apomixis: Engineering the Ability to Harness Hybrid Vigor in Crop Plants. In Plant Germline Development; Schmidt, A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1669, pp. 17–34. ISBN 978-1-4939-7285-2. [Google Scholar]
- Grimanelli, D.; Leblanc, O.; Espinosa, E.; Perotti, E.; González De León, D.; Savidan, Y. Mapping Diplosporous Apomixis in Tetraploid Tripsacum: One Gene or Several Genes? Heredity 1998, 80, 33–39. [Google Scholar] [CrossRef]
- Leblanc, O.; Grimanelli, D.; González-de-León, D.; Savidan, Y. Detection of the Apomictic Mode of Reproduction in Maize-Tripsacum Hybrids Using Maize RFLP Markers. Theoret. Appl. Genet. 1995, 90, 1198–1203. [Google Scholar] [CrossRef]
- Leblanc, O.; Grimanelli, D.; Hernandez-Rodriguez, M.; Galindo, P.A.; Soriano-Martinez, A.M.; Perotti, E. Seed Development and Inheritance Studies in Apomictic Maize-Tripsacum Hybrids Reveal Barriers for the Transfer of Apomixis into Sexual Crops. Int. J. Dev. Biol. 2009, 53, 585–596. [Google Scholar] [CrossRef]
- Sokolov, V.A. Independent Inheritance and Expression of Apomeiosis and Parthenogenesis in Maize Hybrids with Tripsacum. Dokl. RAN 2000, 374, 280–282. [Google Scholar]
- Gerlach, D. Botanische Mikrotomtechnik, Eine Einführung, 2. Auflage; Thieme: Stuttgart, Germany, 1984. [Google Scholar]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the Crop Wild Relatives for Breeding Future Crops. Trends Biotechnol. 2022, 40, 412–431. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The Use of Wild Relatives in Crop Improvement: A Survey of Developments over the Last 20 Years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Bergquist, R.R. Transfer from Tripsacum Dactyloides to Corn of a Major Gene Locus Conditioning Resistance to Puccinia Sorghi. Phytopathology 1981, 71, 518–520. [Google Scholar] [CrossRef]
- Scherbak, V.S.; Khatefov, E.V. Mais, Their Phylogenetic Connections and the Significance for the Selection of Corn. Risovodstvo 2017, 37, 57–63. [Google Scholar]
- Abdoul-Raouf, S.M.; Ju, Q.; Jianyu, M.; Zhizhai, L. Utilization of Wild Relatives for Maize (Zea mays L.) Improvement. Afr. J. Plant Sci. 2017, 11, 105–113. [Google Scholar] [CrossRef]
- Kindiger, B. Principles of Plant Genetics and Breeding; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 161–169. [Google Scholar]
- Throne, J.; Eubanks, M. Resistance of Tripsacorn-Introgressed Maize Lines to Sitophilus Zeamais. J. Stored Prod. Res. 2015, 64, 62–64. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Sokolov, V. Maize-Gamagrass Interspecific Hybrid, Zea mays × Tripsacum dactyloides, Shows Better Salinity Tolerance and Higher Na+ Exclusion than Maize and Sorghum. Int. J. Latest Res. Sci. Technol. 2015, 2015, 128–133. [Google Scholar]
- Iqbal, M.Z.; Cheng, M.; Su, Y.; Li, Y.; Jiang, W.; Li, H.; Zhao, Y.; Wen, X.; Zhang, L.; Ali, A.; et al. Allopolyploidization Facilitates Gene Flow and Speciation among Corn, Zea perennis and Tripsacum dactyloides. Planta 2019, 249, 1949–1962. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Wen, X.; Xu, L.; Zhao, Y.; Li, J.; Jiang, W.; Cheng, M.; Li, H.; Li, Y.; Li, X.; et al. Multispecies Polyploidization, Chromosome Shuffling, and Genome Extraction in Zea/Tripsacum Hybrids. Genetics 2023, 223, iyad029. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Xia, J.; Wu, L.; Xu, G.; Wu, W.; Li, D.; Qin, W.; Han, X.; Chen, Q.; et al. Teosinte Ligule Allele Narrows Plant Architecture and Enhances High-Density Maize Yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef]
- Mangelsdorf, P.C.; Reeves, R.G. Hybridization of Maize, Tripsacum, and Euchlaena. J. Hered. 1931, 22, 328–343. [Google Scholar] [CrossRef]
- Garcia-Aguilar, M.; Michaud, C.; Leblanc, O.; Grimanelli, D. Inactivation of a DNA Methylation Pathway in Maize Reproductive Organs Results in Apomixis-Like Phenotypes. Plant Cell 2010, 22, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Delcuve, G.P.; Rastegar, M.; Davie, J.R. Epigenetic Control. J. Cell. Physiol. 2009, 219, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Talbert, L.E.; Doebley, J.F.; Larson, S.; Chandler, V.L. Tripsacum andersonii is a natural hybrid involving Zea and Tripsacum: Molecular evidence. Am. J. Bot. 1990, 77, 722–726. [Google Scholar] [CrossRef]
- Lemos, G.C.S.; Pereira, T.N.S.; Pereira, M.G.; Amaral Júnior, A.T. Biometric Analysis of Tripsacum-Maize Hybrid Populations. CBAB 2005, 5, 64–70. [Google Scholar] [CrossRef]
- Kosterin, O.E.; Onishko, V.V.; Ilyina, E.V.; Chepurnov, G.Y.; Blinov, A.G. The Genus Coenagrion Kirby, 1890 (Odonata: Coenagrionidae) in the Russian Part of the Caucasus. Zootaxa 2024, 5471, 151–190. [Google Scholar] [CrossRef]



| Maize Lines, Pollinated T. dactyloides | Number of Pollinated Flowers, pcs *. | Number of Developed Embryos, pcs. | Number of F1 Grains That Formed Endosperm, pcs. | Number of F1 Hybrid Plants Grown, pcs. |
|---|---|---|---|---|
| 573MB | 103,163 | 28,193 | 909 | 429 |
| 611CB | 68,998 | 29,840 | 31 | 3 |
| Scheme of Backcross 1 | Number of Pollinated Plants, pcs * | Number of Developed Embryos, pcs. | Number of BC1 Grains That Formed Endosperm, pcs. | Percentage of Formed Grains, % |
|---|---|---|---|---|
| ♀(573MB × Td) × ♂573MB | 11 | 2255 | 23 | 1.02 |
| 9 | 1467 | 277 | 18.88 | |
| ♀(573MB × Td) × ♂611CB | 11 | 5359 | 40 | 0.75 |
| 9 | 3412 | 663 | 19.43 | |
| ♀(611CB × Td) × ♂573MB | 2 | 409 | 6 | 1.47 |
| ♀(611CB × Td) × ♂611CB | 2 | 600 | 18 | 3 |
| № | Hybrid Combination F1 Z. mays × T. dactyloides | № Original F1 | Pollinator Line | Number of Grains Received BC1 | Number of Plants Obtained BC1 ♀F1 × ♂Zm | Behavior of 56-ch. Hybrids in BC2 | ||
|---|---|---|---|---|---|---|---|---|
| 46-ch., pcs. | 56-ch., pcs. (% of All Plants) | Of These BC1apo 56-ch., pcs. | ||||||
| 1 | (573MB × Td) | 1/Б10-9 | 573MB 611CB | 24 91 | 14 61 | 1 (6.7%) 1 (1.6%) | 0 0 | No progeny No progeny |
| 2 | (573MB × Td) | 3/Б10-1 | 573MB 611CB | 11 12 | 9 3 | 1 (10%) 3 (50%) | 0 0 | No progeny BIII-hybridization |
| 3 | (573MB × Td) | 3/Б10-8 | 573MB 611CB | 55 233 | 12 136 | 1 (7.7%) 4 (2.9%) | 1 4 | Stable 56-ch. Stable 56-ch. |
| 4 | (573MB × Td) | 3/Б10-9 | 573MB 611CB | 16 11 | 0 0 | 6 (100%) 8 (100%) | 0 0 | BIII-hybridization BIII-hybridization |
| 5 | (573MB × Td) | 4/Б10-9 | 573MB - | 49 - | 37 - | 0 - | 0 - | - - |
| 6 | (611CB × Td) | 26/Б11-1 | 573MB 611CB | 3 11 | 2 6 | 0 1 (14.3%) | 0 1 | - Stable 56-ch. |
| № | Accession or Hybrid | Sample Karyotypes, 2n | Pox3 | trnL |
|---|---|---|---|---|
| 1 | T. dactyloides | 72 | - | Td |
| 2 | Zea mays line 573MB | 20 | 573MB | 573MB |
| 3 | Hybrid F1 573MB × 611CB | 20 | 573MB + 611CB | 573MB |
| 4 | Hybrid F1 573MB × Td | 46 | 573MB | 573MB |
| 5 | Hybrid BC1 (573MB × Td) × 573MB | 56 | 573MB | 573MB |
| 6 | Hybrid BC1 (573MB × Td) × 611CB | 56 | 573MB + 611CB | 573MB |
| 7 | Zea mays line 611CB | 20 | 611CB | 611CB |
| 8 | Hybrid F1 611CB × 573MB | 20 | 611CB + 573MB | 611CB |
| 9 | Hybrid F1 611CB × Td | 46 | 611CB | 611CB |
| 10 | Hybrid BC1 (611CB × Td) × 611CB | 56 | 611CB | 611CB |
| 11 | Hybrid BC1 (611CB × Td) × 573MB | 56 | 611CB + 573MB | 611CB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, V.A.; Panikhin, P.A.; Plotnikov, K.O.; Chepurnov, G.Y.; Blinov, A.G. Development of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids: A Potential Breakthrough in Heterosis Fixation. Plants 2024, 13, 2138. https://doi.org/10.3390/plants13152138
Sokolov VA, Panikhin PA, Plotnikov KO, Chepurnov GY, Blinov AG. Development of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids: A Potential Breakthrough in Heterosis Fixation. Plants. 2024; 13(15):2138. https://doi.org/10.3390/plants13152138
Chicago/Turabian StyleSokolov, Viktor Andreevich, Pavel Alexandrovich Panikhin, Kirill Olegovich Plotnikov, Grigory Yurievich Chepurnov, and Alexander Genadievich Blinov. 2024. "Development of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids: A Potential Breakthrough in Heterosis Fixation" Plants 13, no. 15: 2138. https://doi.org/10.3390/plants13152138
APA StyleSokolov, V. A., Panikhin, P. A., Plotnikov, K. O., Chepurnov, G. Y., & Blinov, A. G. (2024). Development of Apomictic 56-Chromosomal Maize–Tripsacum Hybrids: A Potential Breakthrough in Heterosis Fixation. Plants, 13(15), 2138. https://doi.org/10.3390/plants13152138





