Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics
Abstract
1. Introduction
2. Results
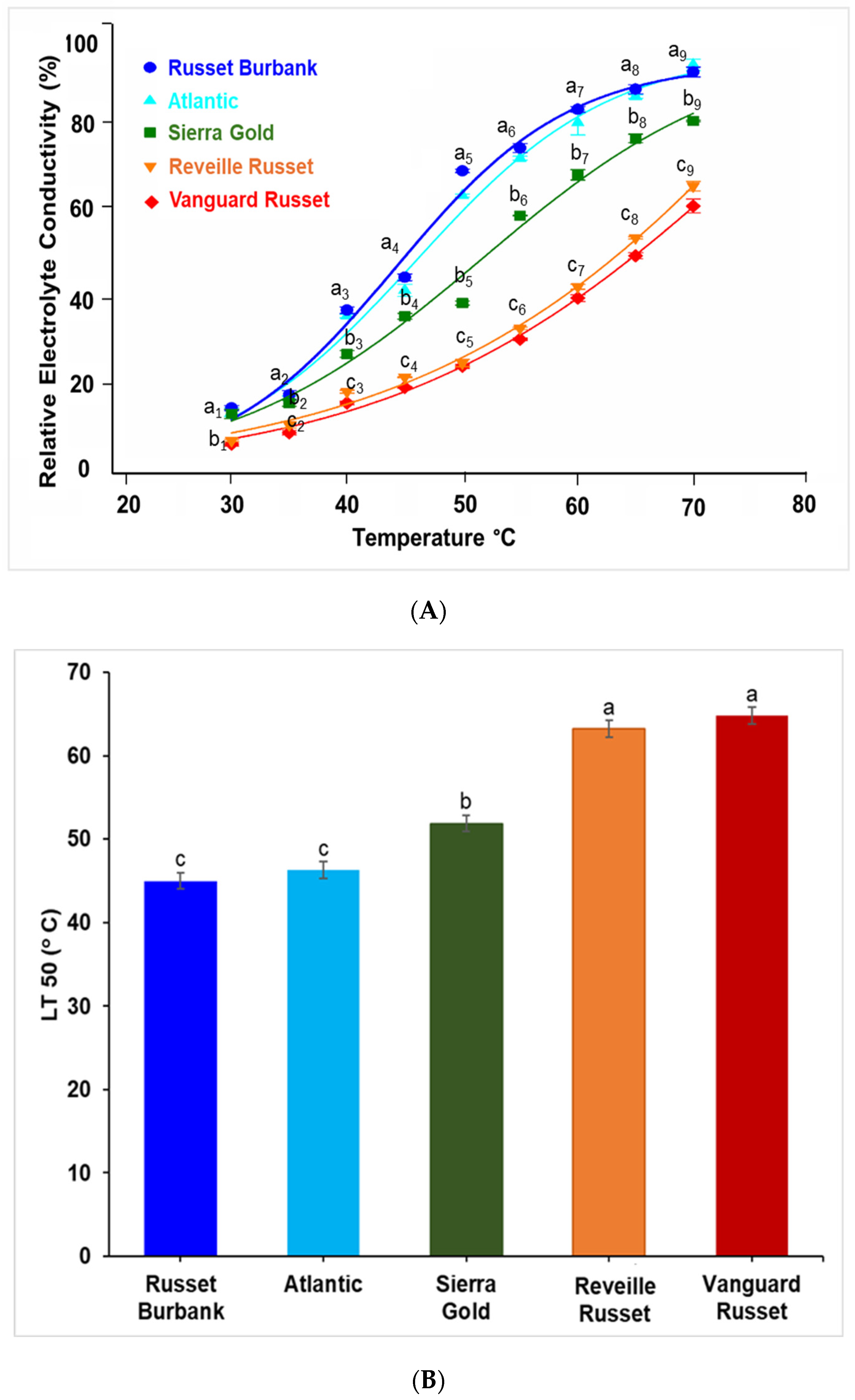
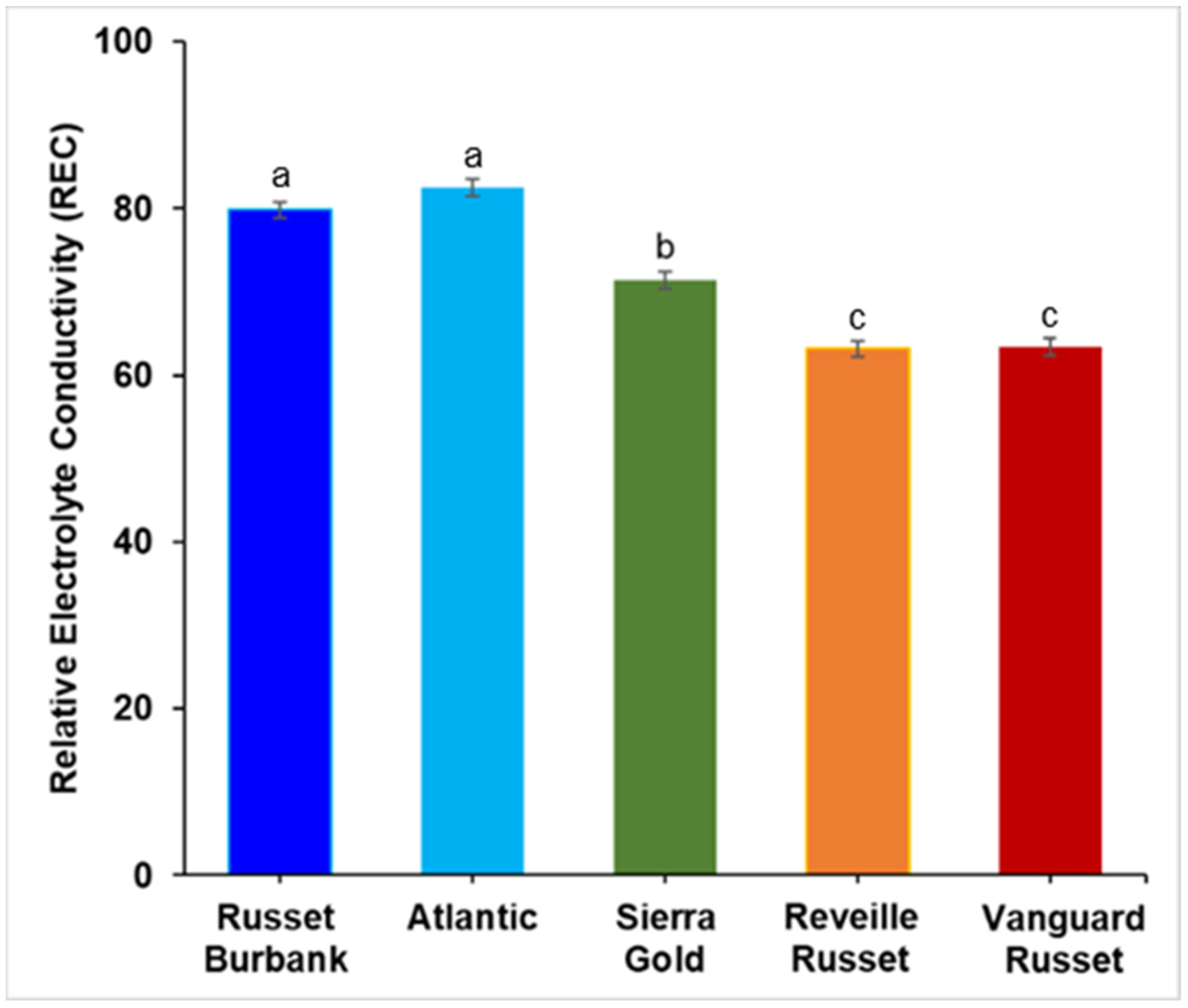
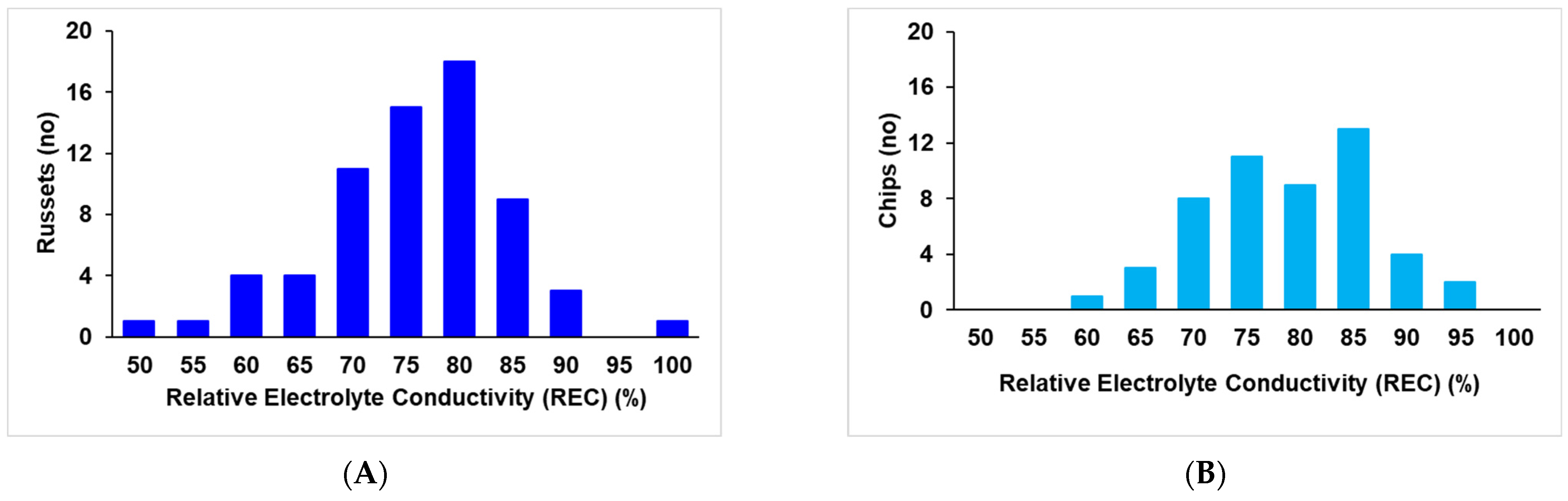
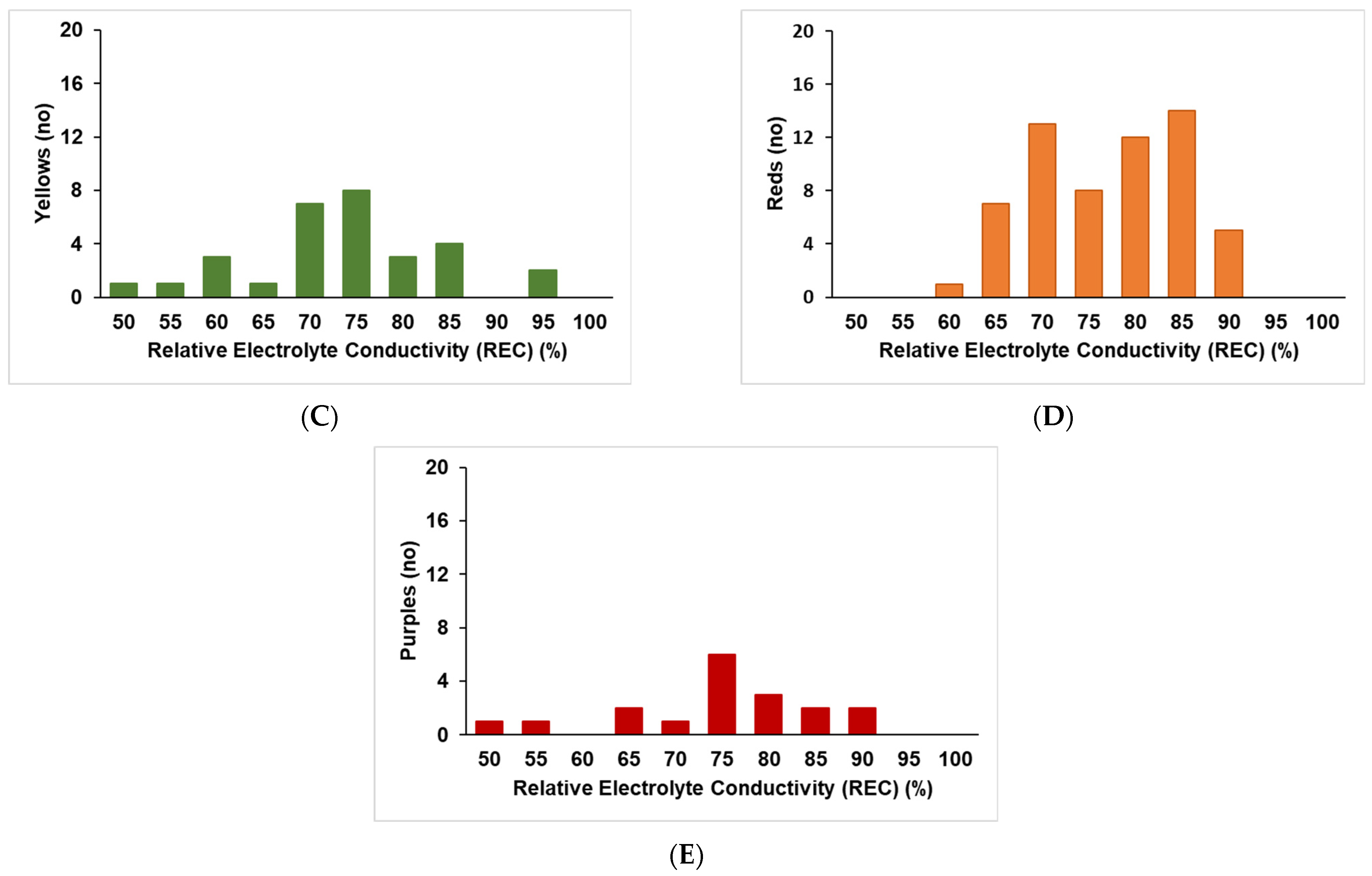
2.1. Extent of Electrolyte Leakage Observed
2.2. REC Significantly Correlated with Tuber Yields, Defects, and Mineral Content
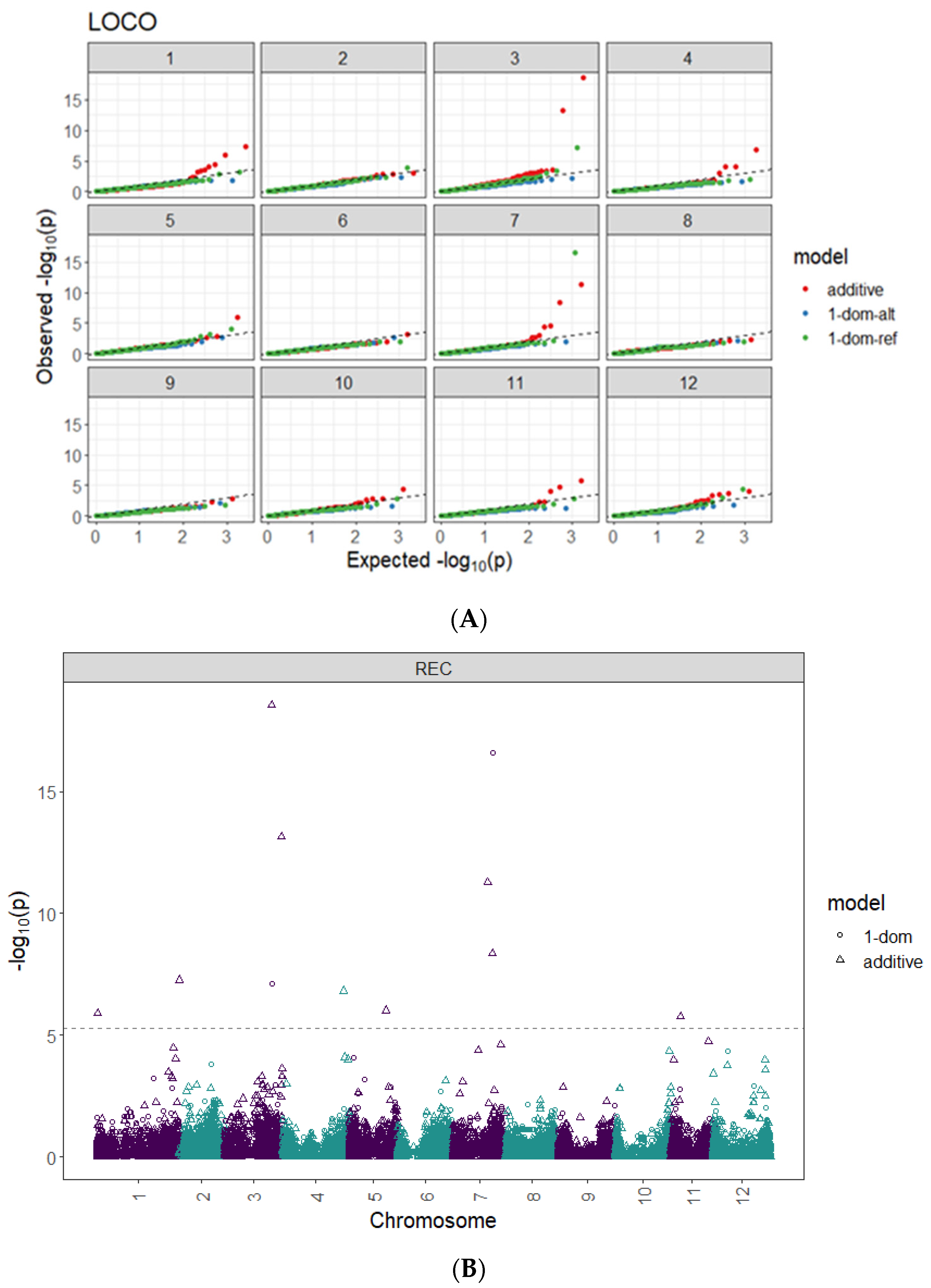
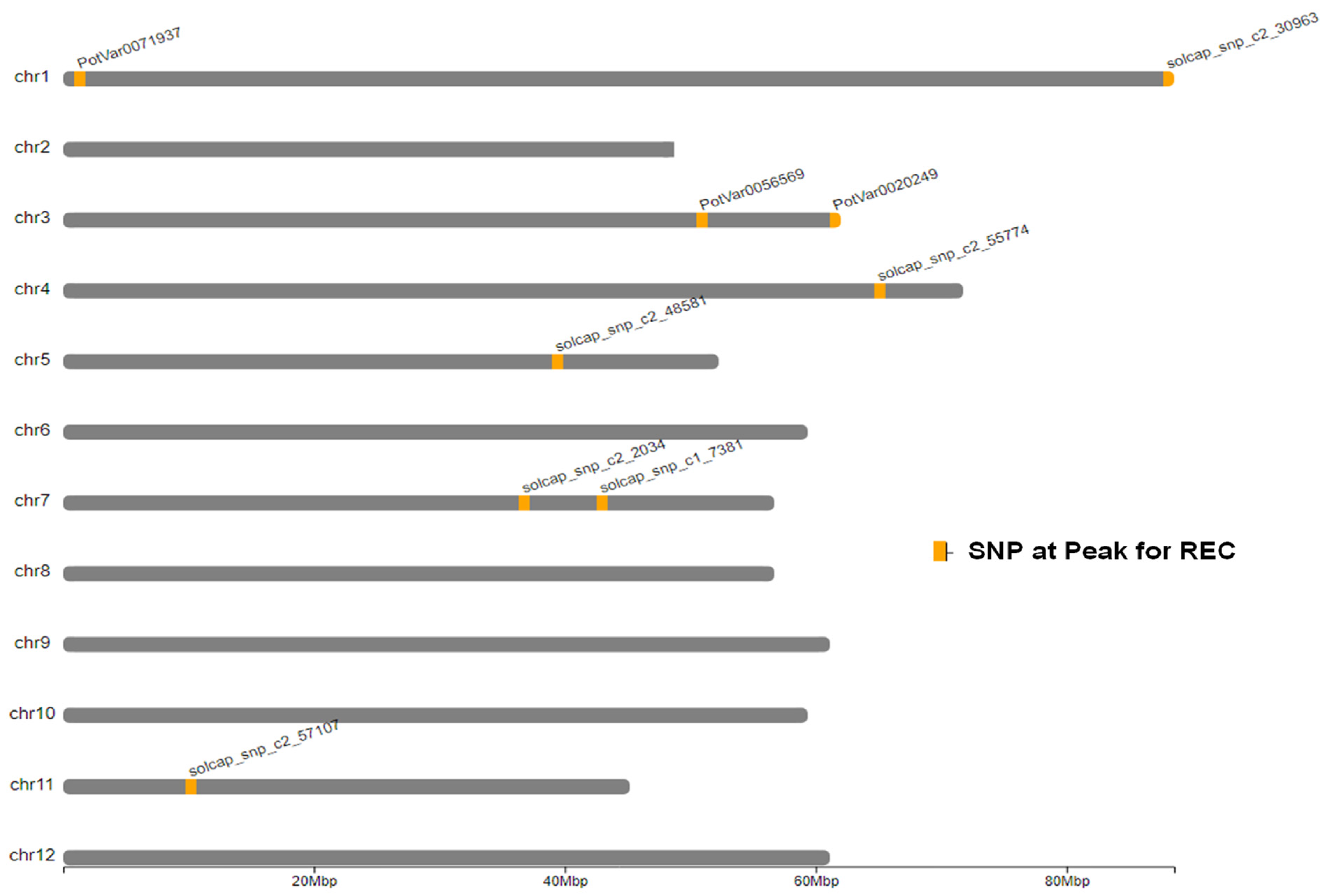
2.3. Genome-Wide Associations
2.4. Heat Shock Protein 18
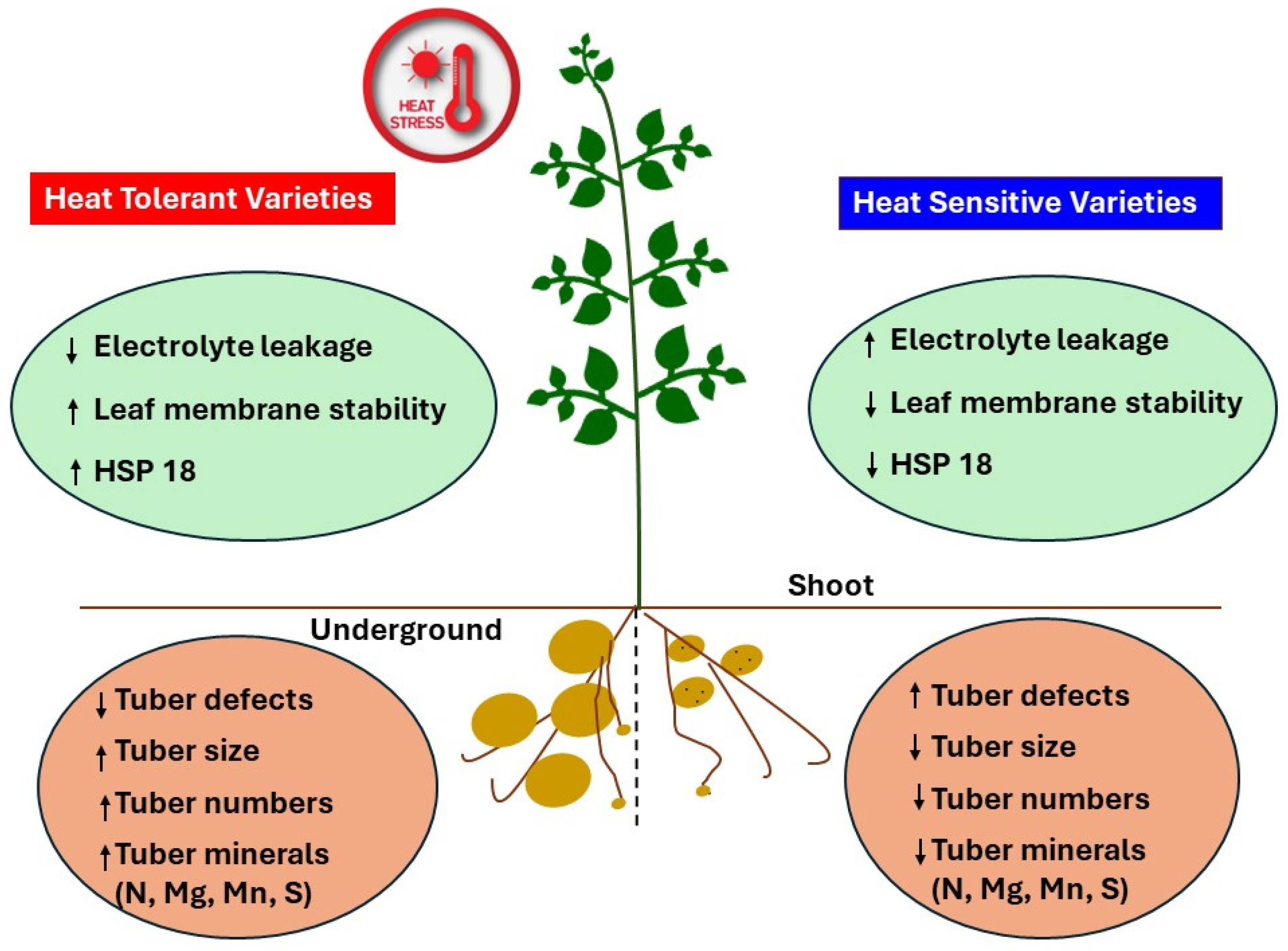
3. Discussion
4. Materials and Methods
4.1. Electrolyte Leakage Assay
4.1.1. Plant Materials and Growing Conditions
4.1.2. Sample Collection and Heat Treatment
4.1.3. Data Analysis for Electrolyte Leakage Assay
4.2. DNA Extraction and Genotyping
4.3. Genome-Wide Association Studies and Correlation Analysis
4.4. Plant Materials and Growing Conditions for Heat Shock Proteins
4.5. Heat Shock Protein Assessment and Immunoblot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef] [PubMed]
- Savic, J.; Dragićević, I.; Pantelic, D.; Oljaca, J.; Momčilović, I. Expression of small heat shock proteins and heat tolerance in potato (Solanum tuberosum L.). Arch. Biol. Sci. 2012, 64, 135–144. [Google Scholar] [CrossRef]
- Tiwari, R.; Lal, M.; Naga, K.; Kumar, R.; Chourasia, K.; Shivaramu, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- ElBasyoni, I.; Saadalla, M.; Baenziger, S.; Bockelman, H.; Morsy, S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability 2017, 9, 1606. [Google Scholar] [CrossRef]
- Raison, J.K.; Berry, J.A.; Armond, P.A.; Pike, C.S. Membrane properties in relation to the adaptation of plants to temperature stress. In Adaptation of Plants to Water and High-Temperature Stress; Turner, N.C., Kramer, P.J., Eds.; Wiley: New York, NY, USA, 1980; pp. 261–273. [Google Scholar]
- Fan, W.; Evans, R.M. Turning up the heat on membrane fluidity. Cell 2015, 161, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Effects of temperature on cell membranes. Symp. Soc. Exp. Biol. 1988, 42, 237–258. [Google Scholar] [PubMed]
- Heins, L.; Mentzel, H.; Schmid, A.; Benz, R.; Schmitz, U.K. Biochemical, molecular, and functional characterization of porin isoforms from potato mitochondria. J. Biol. Chem. 1994, 269, 26402–26410. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Saeed, M.M.; Qureshi, M.J. Tolerance to high temperature in cotton (Gossypium hirsutum L.) at initial growth stages. Environ. Exp. Bot. 1994, 34, 275–283. [Google Scholar] [CrossRef]
- Rahman, H.; Ahmad, S.; Saleem, M. Heat tolerance of upland cotton during the fruiting stage evaluated using cellular membrane thermostability. Field Crops Res. 2004, 85, 149–158. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Klueva, N.; Nguyen, H.T. Wheat cellular thermotolerance is related to yield under heat stress. Euphytica 2001, 117, 117–123. [Google Scholar] [CrossRef]
- Kumar, R. Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H2O2 and transcript of heat shock protein. Int. J. Plant Physiol. Biochem. 2012, 4, 83–91. [Google Scholar] [CrossRef]
- Maheswari, M. Transverse relaxation time of leaf water protons and membrane injury in wheat (Triticum aestivum L.) in response to high temperature. Ann. Bot. 1999, 84, 741–745. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Expression of target gene Hsp70 and membrane stability determine heat tolerance in chili pepper. J. Am. Soc. Hortic. Sci. 2015, 140, 144–150. [Google Scholar] [CrossRef]
- Arvin, M.J.; Donnelly, D.J. Screening potato cultivars and wild species to abiotic stresses using an electrolyte leakage bioassay. J. Agric. Sci. Technol. 2008, 10, 33–42. [Google Scholar]
- Kovaleski, A.P.; Grossman, J.J. Standardization of electrolyte leakage data and a novel liquid nitrogen control improve measurements of cold hardiness in woody tissue. Plant Methods 2021, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.L.; Galal, A.A.; Manninger, K.; Barna, B. Inhibition of the development of leaf rust (Puccinia recondita) by treatment of wheat with allopurinol and production of a hypersensitive-like reaction in a compatible host. Plant Pathol. 2000, 49, 317–323. [Google Scholar] [CrossRef]
- Sriram, S.; Raguchander, T.; Babu, S.; Nandakumar, R.; Shanmugam, V.; Vidhyasekaran, P.; Balasubramanian, P.; Samiyappan, R. Inactivation of phytotoxin produced by the rice sheath blight pathogen Rhizoctonia solani. Can. J. Microbiol. 2000, 46, 520–524. [Google Scholar] [CrossRef]
- FAO. FAO Global Statistical Yearbook, FAO Regional Statistical Yearbooks. FAOSTAT 126. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 May 2023).
- King, J.C.; Slavin, J.L. White potatoes, human health, and dietary guidance. Adv. Nutr. 2013, 4, 393S–401S. [Google Scholar] [CrossRef]
- Levy, D.; Veilleux, R.E. Adaptation of potato to high temperatures and salinity-a review. Am. J. Potato Res. 2007, 84, 487–506. [Google Scholar] [CrossRef]
- Gautam, S.; Pandey, J.; Scheuring, D.; Koym, J.; Vales, M.I. Genetic basis of potato tuber defects and identification of heat-tolerant clones. Plants 2024, 13, 616. [Google Scholar] [CrossRef]
- Haynes, K.G.; Goth, R.W.; Sterrett, S.B.; Christ, B.J.; Halseth, D.E.; Porter, G.A.; Henninger, M.R.; Wilson, D.R.; Webb, R.E.; Hammond, D.F.; et al. Coastal Chip: A chipping potato variety resistant to heat stress. Am. Potato J. 1992, 69, 515–523. [Google Scholar] [CrossRef]
- Khan, M.A.; Munive, S.; Bonierbale, M. Early generation in vitro assay to identify potato populations and clones tolerant to heat. Plant Cell Tiss. Organ Cult. 2015, 121, 45–52. [Google Scholar] [CrossRef]
- Kim, H.; Cho, K.; Cho, J.; Park, Y.; Cho, H. Selection of heat tolerant potato lines via in vitro screening of true potato seeds. Acta Hortic. 2012, 935, 225–230. [Google Scholar] [CrossRef]
- Mienie, A.; De Ronde, J.A. A comparison of drought stress and heat stress in the leaves and tubers of 12 potato cultivars. S. Afr. J. Sci. 2008, 104, 156–159. [Google Scholar]
- Minhas, J.; Kumar, D.; Ta, J.; Raj, B.T.; Khurana, S.; Pandey, S.K.; Singh, S.V.; Singh, B.P.; Naik, P. Kufri surya: A new heat-tolerant Potato variety suitable for early planting in North-Western Plains, Peninsular India and processing into French fries and chips. Potato J. 2006, 33, 35–43. [Google Scholar]
- Zhang, S.; Ye, H.; Kong, L.; Li, X.; Chen, Y.; Wang, S.; Liu, B. Multivariate analysis compares and evaluates heat tolerance of potato germplasm. Plants 2024, 13, 142. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Zinta, R.; Bhatia, N.; Kardile, H.B.; Singh, R.K.; Kumar, M. Germplasm, breeding, and genomics in potato improvement of biotic and abiotic stresses tolerance. Front. Plant Sci. 2022, 13, 805671. [Google Scholar] [CrossRef]
- Gautam, S.; Solis-Gracia, N.; Teale, M.K.; Mandadi, K.; da Silva, J.A.; Vales, M.I. Development of an in vitro microtuberization and temporary immersion bioreactor system to evaluate heat stress tolerance in potatoes (Solanum tuberosum L.). Front. Plant Sci. 2021, 12, 700328. [Google Scholar] [CrossRef]
- Ahn, Y.; Claussen, K.; Lynn Zimmerman, J. Genotypic differences in the heat-shock response and thermotolerance in four potato cultivars. Plant Sci. 2004, 166, 901–911. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Z.; Li, P.H. Adaptability of crop plants to high temperatures stress. Crop Sci. 1982, 22, 719–725. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Alagar Boopathy, L.R.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms tailoring the expression of heat shock proteins to proteostasis challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, B.; Guihur, A. Heat shock signaling in land plants: From plasma membrane sensing to the transcription of small heat shock proteins. Front. Plant Sci. 2021, 12, 710801. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Asselbergh, B.; Rivera-Mejías, P.; Bervoets, S.; Vendredy, L.; De Winter, V.; Spaas, K.; de Rycke, R.; van Isterdael, G.; Impens, F.; et al. Small heat shock proteins operate as molecular chaperones in the mitochondrial intermembrane space. Nat. Cell Biol. 2023, 25, 467–480. [Google Scholar] [CrossRef]
- Bukau, B.; Weissman, J.; Horwich, A. Molecular chaperones and protein quality control. Cell 2006, 125, 443–451. [Google Scholar] [CrossRef]
- Park, C.-J.; Seo, Y.-S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Nakamoto, H.; Vígh, L. The small heat shock proteins and their clients. Cell. Mol. Life Sci. 2007, 64, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Roberts, J.K.; Key, J.L. Acquisition of thermotolerance in soybean seedlings: Synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiol. 1984, 74, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.J.; Skøt, K.P. Detailed characterization of heat shock protein synthesis and induced thermotolerance in seedlings of Sorghum bicolor L. J. Exp. Bot. 1994, 45, 1353–1363. [Google Scholar] [CrossRef]
- Bowen, J.; Lay-Yee, M.; Plummer, K.i.m.; Ferguson, I.a.n. The heat shock response is involved in thermotolerance in suspension-cultured apple fruit cells. J. Plant Physiol. 2002, 159, 599–606. [Google Scholar] [CrossRef]
- Murakami, T.; Matsuba, S.; Funatsuki, H.; Kawaguchi, K.; Saruyama, H.; Tanida, M.; Sato, Y. Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 2004, 13, 165–175. [Google Scholar] [CrossRef]
- Mamedov, T.G.; Shono, M. Molecular chaperone activity of tomato (Lycopersicon esculentum) endoplasmic reticulum-located small heat shock protein. J. Plant Res. 2008, 121, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Süle, A.; Vanrobaeys, F.; Hajós, G.; Van Beeumen, J.; Devreese, B. Proteomic analysis of small heat shock protein isoforms in barley shoots. Phytochemistry 2004, 65, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Rampino, P.; Mita, G.; Pataleo, S.; Pascali, M.D.; Fonzo, N.D.; Perrotta, C. Acquisition of thermotolerance and HSP gene expression in durum wheat (Triticum durum Desf.) cultivars. Environ. Exp. Bot. 2009, 66, 257. [Google Scholar] [CrossRef]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and prospects of genome-wide association studies in plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef]
- Zhu, C.; Gore, M.; Buckler, E.S.; Yu, J. Status and prospects of association mapping in plants. Plant Genome 2008, 1. [Google Scholar] [CrossRef]
- Rosyara, U.R.; De Jong, W.S.; Douches, D.S.; Endelman, J.B. Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Yang, J.; Zaitlen, N.A.; Goddard, M.E.; Visscher, P.M.; Price, A.L. Advantages and pitfalls in the application of mixed-model association methods. Nat. Genet. 2014, 46, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.G.; Paulo, M.J.; Bourke, P.M.; Maliepaard, C.A.; van Eeuwijk, F.A.; Visser, R.G.F.; van Eck, H.J. GWAS in tetraploid potato: Identification and validation of SNP markers associated with glycoalkaloid content. Mol. Breed. 2022, 42, 76. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Scheuring, D.C.; Vales, M.I. Genomic regions associated with tuber traits in tetraploid potatoes and identification of superior clones for breeding purposes. Front. Plant Sci. 2022, 13, 952263. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.A.B.; Demirel, U.; Nadeem, M.A.; Çaliskan, M.E. Genome-wide association study identifies various loci underlying agronomic and morphological traits in diversified potato panel. Physiol. Mol. Biol. Plants 2020, 26, 1003–1020. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.R.; Coombs, J.; Felcher, K.J.; Hammerschmidt, R.; Zuehlke, M.L.; Buell, C.R.; Douches, D. Genome-wide association analysis of common scab resistance and expression profiling of tubers in response to thaxtomin a treatment underscore the complexity of common scab resistance in tetraploid potato. Am. J. Potato Res. 2020, 97, 513–522. [Google Scholar] [CrossRef]
- Klaassen, M.T.; Willemsen, J.H.; Vos, P.G.; Visser, R.G.F.; van Eck, H.J.; Maliepaard, C.; Trindade, L.M. Genome-wide association analysis in tetraploid potato reveals four QTLs for protein content. Mol. Breed. 2019, 39, 151. [Google Scholar] [CrossRef]
- Pandey, J.; Scheuring, D.; Koym, J.; Coombs, J.; Novy, R.; Thompson, A.; Holm, D.; Douches, D.; Miller, J.; Vales, M.I. Genetic diversity and population structure of advanced clones selected over forty years by a potato breeding program in the USA. Sci. Rep. 2021, 11, 8344. [Google Scholar] [CrossRef]
- Pandey, J.; Thompson, D.; Joshi, M.; Scheuring, D.C.; Koym, J.W.; Joshi, V.; Vales, M.I. Genetic architecture of tuber-bound free amino acids in potato and effect of growing environment on the amino acid content. Sci. Rep. 2023, 13, 13940. [Google Scholar] [CrossRef]
- Pandey, J.; Gautam, S.; Scheuring, D.C.; Koym, J.W.; Vales, M.I. Variation and genetic basis of mineral content in potato tubers and prospects for genomic selection. Front Plant Sci. 2023, 14, 1301297. [Google Scholar] [CrossRef]
- Virdi, A.S.; Singh, S.; Singh, P. Abiotic stress responses in plants: Roles of calmodulin-regulated proteins. Front. Plant Sci. 2015, 6, 809. [Google Scholar] [CrossRef]
- Geng, Z.; Dou, H.; Liu, J.; Zhao, G.; An, Z.; Liu, L.; Zhao, N.; Zhang, H.; Wang, Y. GhFB15 is an F-box protein that modulates the response to salinity by regulating flavonoid biosynthesis. Plant Sci. 2024, 338, 111899. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Spear, R.R.; Holden, Z.J.; Pavek, M.J. Fresh market evaluation of six russet-type potato varieties and four Russet Norkotah strains. Am. J. Potato Res. 2017, 94, 437–448. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Kleinhenz, M.D.; Palta, J.P. Application of calcium and nitrogen for mitigating heat stress effects on potatoes. Am. Potato J. 1996, 73, 261–273. [Google Scholar] [CrossRef]
- Janicka-Russak, M. Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; IntechOpen: London, UK, 2011; Available online: https://www.intechopen.com/chapters/18472 (accessed on 11 March 2024).
- Sahoo, S.; Raghuvanshi, R.; Srivastava, A.; Penna, S. Phosphatases: The critical regulator of abiotic stress tolerance in plants. In Protein Phosphatases and Stress Management in Plants; Pandey, G.K., Ed.; Springer: Cham, Switzerland, 2020; pp. 163–201. ISBN 978-3-030-48732-4. [Google Scholar]
- Trapero-Mozos, A.; Morris, W.L.; Ducreux, L.J.M.; McLean, K.; Stephens, J.; Torrance, L.; Bryan, G.J.; Hancock, R.D.; Taylor, M.A. Engineering heat tolerance in potato by temperature-dependent expression of a specific allele of Heat-Shock Cognate 70. Plant Biotechnol. J. 2018, 16, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rudić, J.; Pantelić, D.; Oljača, J.; Momčilović, I. Effects of elevated temperature and salicylic acid on heat shock response and growth of potato microplants. Horticulturae 2022, 8, 372. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, R.; Niu, S.; Si, H.; Yang, Q.; Bizimungu, B.; Regan, S.; Li, X.-Q. Effects of earliness on heat stress tolerance in fifty potato cultivars. Am. J. Potato Res. 2020, 97, 23–32. [Google Scholar] [CrossRef]
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. META-R: A software to analyze data from multi-environment plant breeding trials. Crop J. 2020, 8, 745–756. [Google Scholar] [CrossRef]
- Anand, L.; Rodriguez Lopez, C.M. ChromoMap: An R package for interactive visualization of multi-omics data and annotation of chromosomes. BMC Bioinform. 2022, 23, 33. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, I.; Ristic, Z. Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. J. Plant Physiol. 2007, 164, 90–99. [Google Scholar] [CrossRef] [PubMed]


| Traits | Correlation to REC (%) | Probability | Sig. Level |
|---|---|---|---|
| Total Yield | −0.05 | 0.5710 | ns |
| Yield without culls | −0.09 | 0.3200 | ns |
| Culls | 0.25 | 0.0042 | ** |
| % Tubers with knobs | 0.33 | 0.0001 | *** |
| % Tubers with growth cracks | 0.12 | 0.1830 | ns |
| % Tubers with external defects | 0.35 | 0.0001 | *** |
| % Tubers with hollow heart | 0.15 | 0.0810 | ns |
| % Tubers with vascular discoloration | 0.12 | 0.1640 | ns |
| % Tubers with internal heat necrosis | 0.27 | 0.0070 | ** |
| % Tubers with internal defects | 0.23 | 0.0040 | ** |
| Total Yield (Z) | −0.18 | 0.0380 | * |
| % Tubers with external defects (Z) | 0.40 | 0.0001 | *** |
| % Tubers with internal defects (Z) | 0.34 | 0.0001 | *** |
| Weighted multi-index selection (Z) | −0.64 | 0.0001 | *** |
| Minerals | Correlation to REC (%) | Probability | Sig. Level |
|---|---|---|---|
| Nitrogen | −0.21 | 0.0160 | * |
| Phosphorus | −0.17 | 0.0550 | ns |
| Potassium | −0.08 | 0.3800 | ns |
| Calcium | −0.10 | 0.2830 | ns |
| Magnesium | −0.25 | 0.0051 | ** |
| Sodium | −0.13 | 0.1541 | ns |
| Zinc | −0.08 | 0.3492 | ns |
| Iron | −0.15 | 0.0843 | ns |
| Copper | 0.07 | 0.4391 | ns |
| Manganese | −0.22 | 0.0121 | * |
| Sulfur | −0. 20 | 0.0230 | * |
| Boron | −0.17 | 0.0630 | ns |
| Weighted multi-index selection (Z) | −0.25 | 0.0041 | ** |
| Model | SNP at QTL Peak | Chr | Peak Position (bp) | Score 1 − log10(p) | R2 (%) |
|---|---|---|---|---|---|
| Additive | PotVar0071937 | 1 | 1,158,384 | 5.9 | 2.60 |
| Additive | solcap_snp_c2_30963 | 1 | 88,362,869 | 7.3 | 1.40 |
| Additive | PotVar0056569 | 3 | 50,880,446 | 18.5 | 4.30 |
| Additive | PotVar0020249 | 3 | 61,550,994 | 13.1 | 1.50 |
| Additive | solcap_snp_c2_55774 | 4 | 65,972,478 | 6.8 | 0.01 |
| Additive | solcap_snp_c2_48581 | 5 | 39,349,874 | 6.0 | 1.50 |
| Additive | solcap_snp_c2_2034 | 7 | 37,157,836 | 11.3 | 3.90 |
| Additive | solcap_snp_c1_7381 | 7 | 42,659,912 | 8.4 | 1.20 |
| Additive | solcap_snp_c2_57107 | 11 | 10,505,722 | 5.8 | 0.05 |
| 1-dom-ref | PotVar0056569 | 3 | 50,880,446 | 7.1 | 8.20 |
| 1-dom-ref | solcap_snp_c1_7381 | 7 | 42,659,912 | 16.6 | 13.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ifeduba, A.M.; Zhen, S.; Pandey, J.; Vales, M.I. Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics. Plants 2024, 13, 2175. https://doi.org/10.3390/plants13162175
Ifeduba AM, Zhen S, Pandey J, Vales MI. Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics. Plants. 2024; 13(16):2175. https://doi.org/10.3390/plants13162175
Chicago/Turabian StyleIfeduba, Amaka M., Shuyang Zhen, Jeewan Pandey, and M. Isabel Vales. 2024. "Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics" Plants 13, no. 16: 2175. https://doi.org/10.3390/plants13162175
APA StyleIfeduba, A. M., Zhen, S., Pandey, J., & Vales, M. I. (2024). Leaf Membrane Stability under High Temperatures as an Indicator of Heat Tolerance in Potatoes and Genome-Wide Association Studies to Understand the Underlying Genetics. Plants, 13(16), 2175. https://doi.org/10.3390/plants13162175






