Environmental Filters Structure Cushion Bogs’ Floristic Composition along the Southern South American Latitudinal Gradient
Abstract
1. Introduction
2. Results
2.1. Beta Diversity and Environment
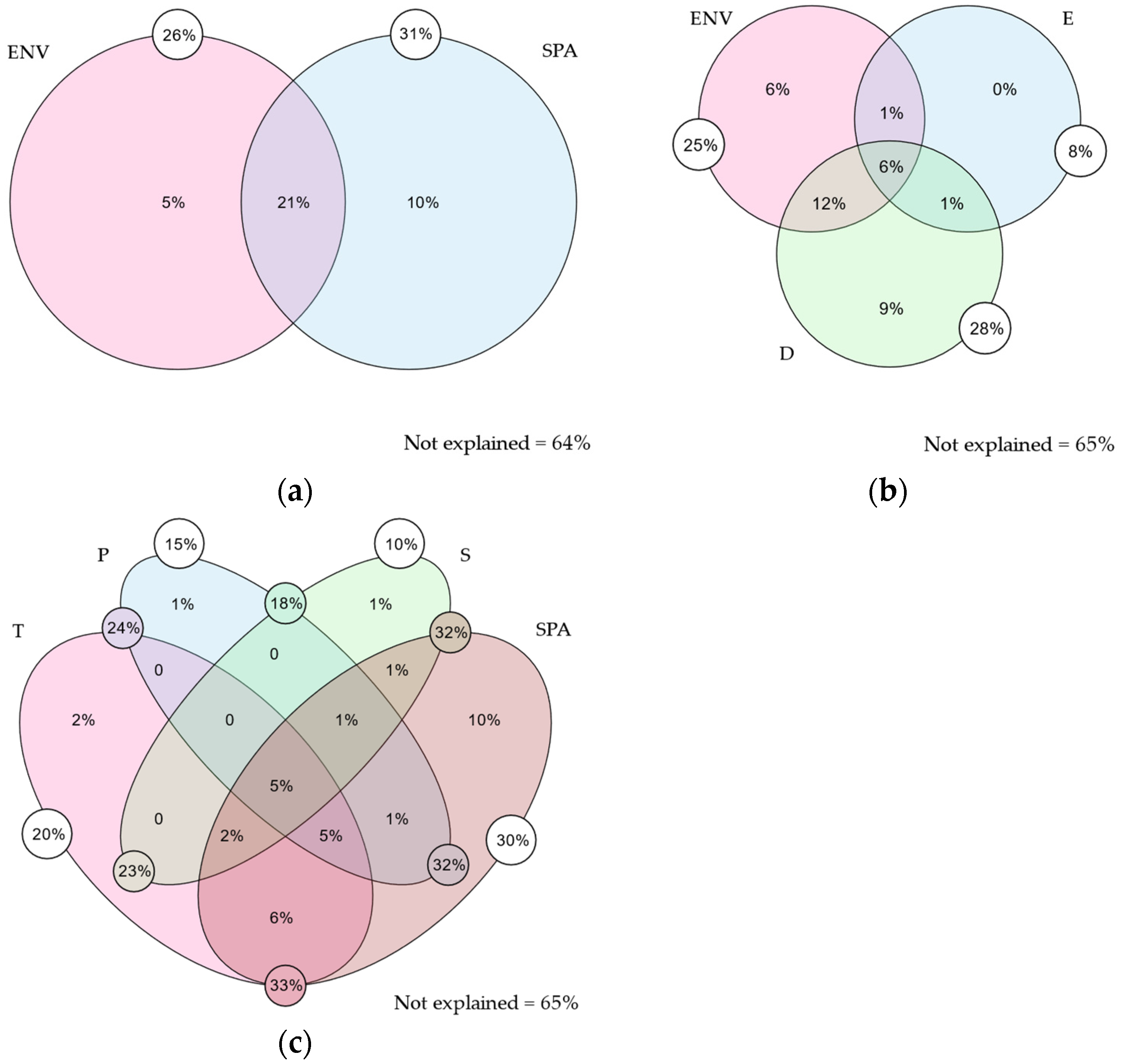
2.1.1. Variation Partitioning
2.1.2. Beta Diversity and Its Components
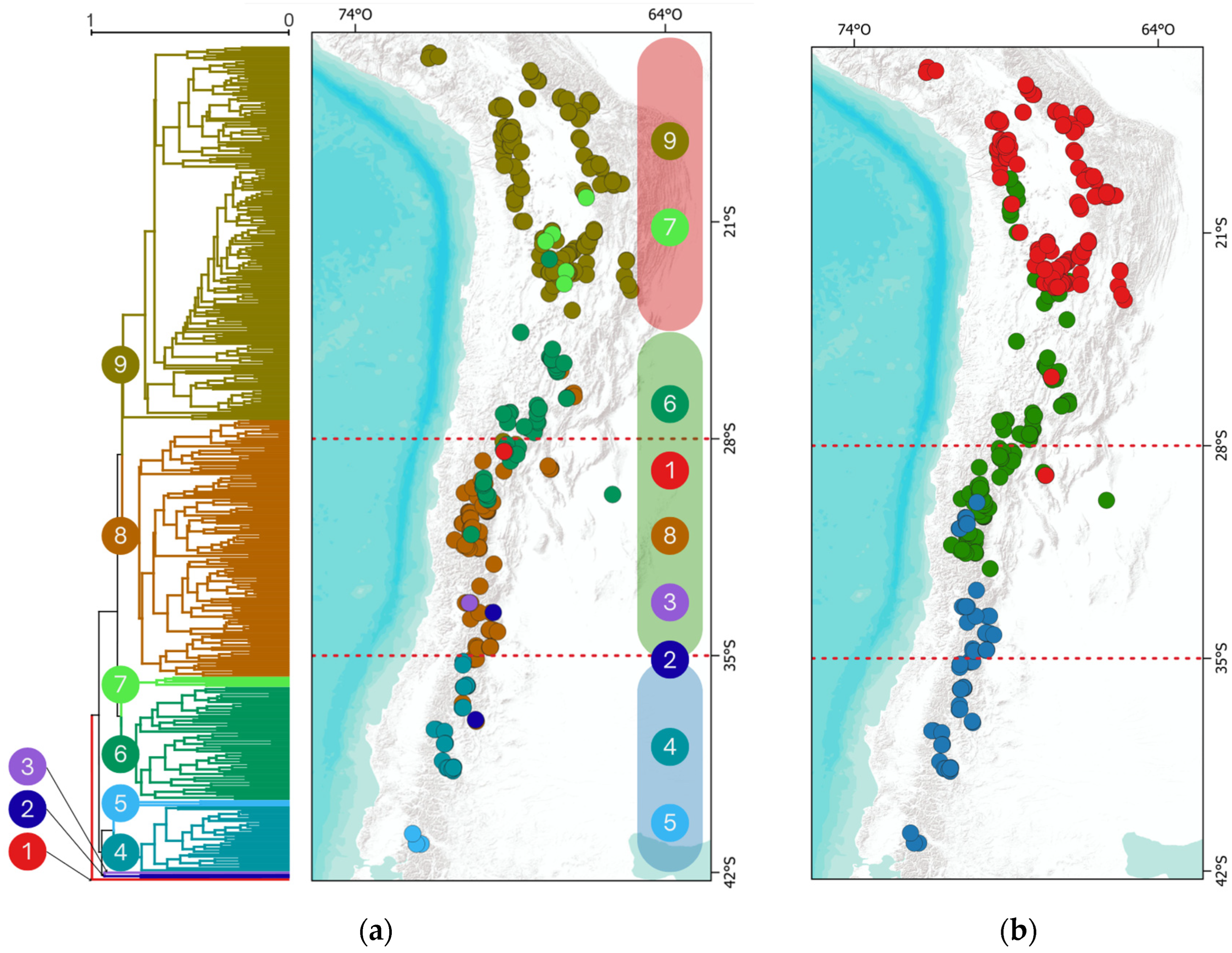
2.1.3. Clustering and Regionalization
2.2. Niche Overlap
2.3. Phylogenetic Diversity
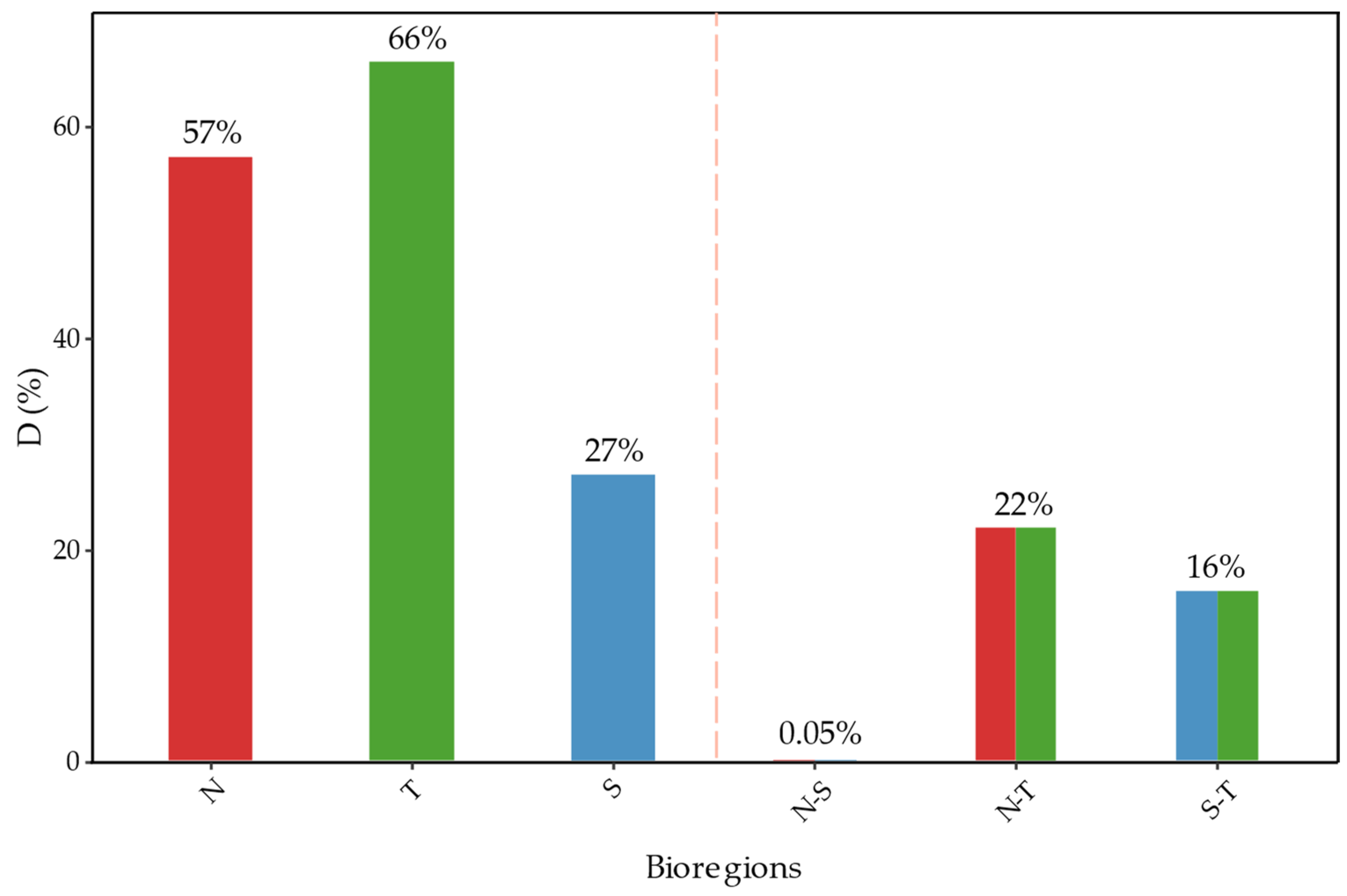
2.3.1. Bioregions
2.3.2. Latitudinal Bands
3. Discussion
3.1. Beta Diversity and Environment
3.1.1. Variation Partitioning
3.1.2. Beta Diversity and Its Components
3.1.3. Clustering and Regionalization
3.2. Niche Overlap
3.3. Phylogenetic Diversity
3.4. Environmental Filtering
4. Materials and Methods
4.1. Study Area
4.2. Data Preparation
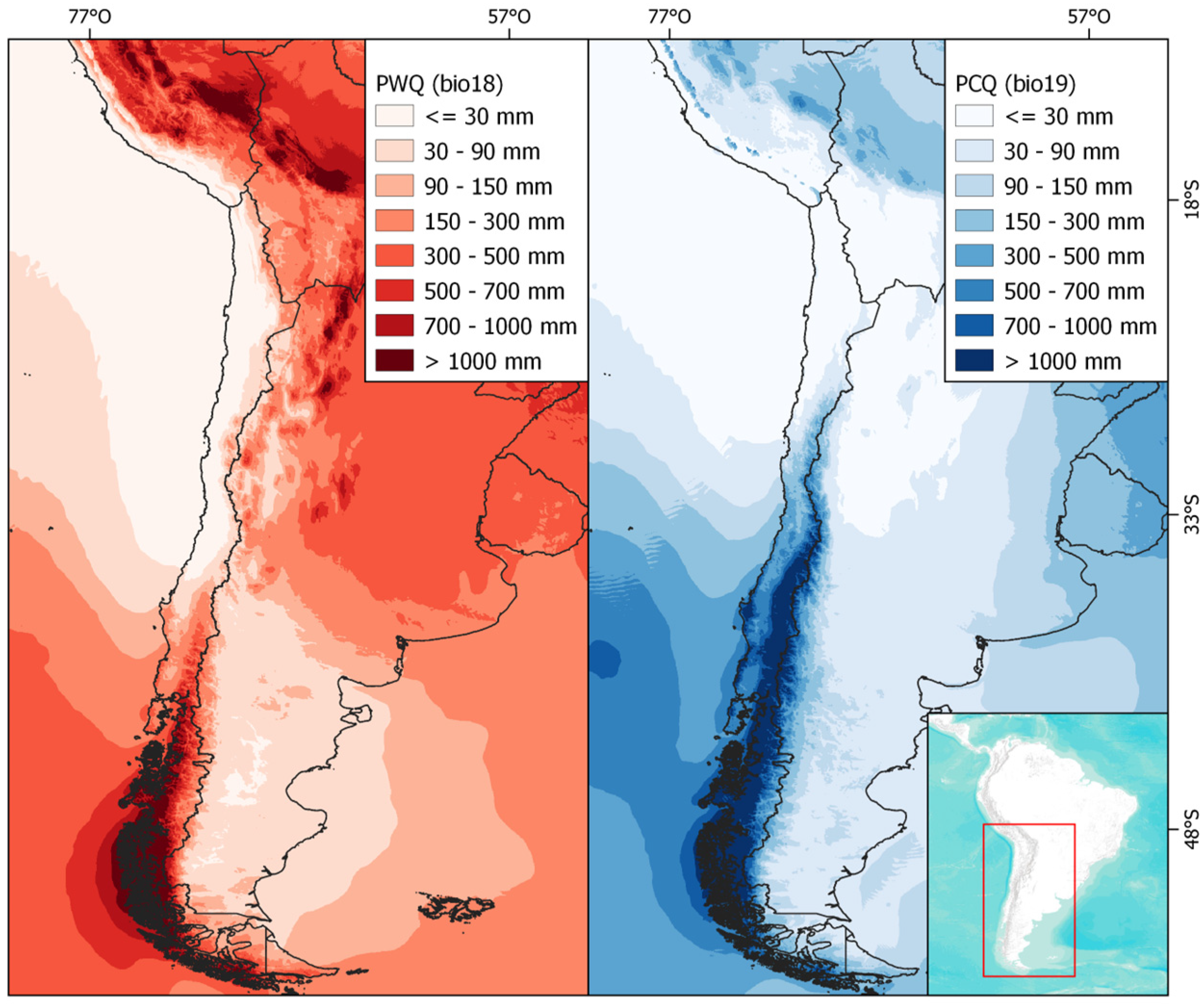
4.3. Environmental Variables
4.4. Data Analysis
4.4.1. Variation Partitioning
4.4.2. Beta Diversity and Its Components
4.4.3. Clustering and Regionalization
4.4.4. Niche Overlap
4.4.5. Phylogenetic Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Woodward, F.I.; Diament, A.D. Functional Approaches to Predicting the Ecological Effects of Global Change. Funct. Ecol. 1991, 5, 202–212. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. Assembly Rules, Null Models, and Trait Dispersion: New Questions from Old Patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef]
- Kraft, N.J.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Gross, N.; Maestre, F.T.; Maire, V.; de Bello, F.; Fonseca, C.R.; Kattge, J.; Valencia, E.; Leps, J.; Liancourt, P. Testing the environmental filtering concept in global drylands. J. Ecol. 2017, 105, 1058–1069. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Tucker, C.M. Should environmental filtering be abandoned? Trends Ecol. Evol. 2017, 32, 429–437. [Google Scholar] [CrossRef]
- Zobel, M. The relative of species pools in determining plant species richness: An alternative explanation of species coexistence? Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Tang, Z. Local and regional processes control species richness of plant communities: The species pool hypothesis. Biodivers. Sci. 2009, 17, 605–612. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Dobrovolski, R.; Melo, A.S.; Cassemiro, F.A.S.; Diniz-Filho, J.A.F. Climatic history and dispersal ability explain the relative importance of turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2011, 21, 191–197. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, L.; Hou, Z.; Zhang, J.; Wang, H.; Lv, G. Environmental filtration and dispersal limitation explain different aspects of beta diversity in desert plant communities. Glob. Ecol. Conserv. 2022, 33, e01956. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Santos, A.; Saraiva, D.; Müller, S.; Overbeck, G. Interactive effects of environmental filtering predict beta-diversity patterns in a subtropical forest metacommunity. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 96–106. [Google Scholar] [CrossRef]
- Jamoneau, A.; Passy, S.; Soininen, J.; Leboucher, T.; Tison-Rosebery, J. Beta diversity of diatom species and ecological guilds: Response to environmental and spatial mechanisms along the stream watercourse. Freshw. Biol. 2018, 63, 62–73. [Google Scholar] [CrossRef]
- Rocha, M.; Bini, L.; Grönroos, M.; Hjort, J.; Lindholm, M.; Karjalainen, S.; Tolonen, K.; Heino, J. Correlates of different facets and components of beta diversity in stream organisms. Oecologia 2019, 191, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O. Exploring the Phylogenetic Structure of Ecological Communities: An Example for Rain Forest Trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kellar, P.R.; Ahrendsen, D.L.; Aust, S.K.; Jones, A.R.; Pires, J.C. Biodiversity comparison among phylogenetic diversity metrics and between three North American prairies. Appl. Plant Sci. 2015, 3, 1400108. [Google Scholar] [CrossRef]
- Horner-Devine, M.C.; Bohannan, B.J.M. Phylogenetic Clustering and Overdispersion in Bacterial Communities. Ecology 2006, 87, S100–S108. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Ackerly, D.D.; Baum, D.A.; Bazzaz, F.A. Phylogenetic Overdispersion in Floridian Oak Communities. Am. Nat. 2004, 163, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Verdú, M.; Pausas, J.G. Fire drives phylogenetic clustering in Mediterranean Basin woody plant communities. J. Ecol. 2007, 95, 1316–1323. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Cavieres, L. High-Elevation Andean Ecosystems. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2013; pp. 96–110. [Google Scholar]
- Garreaud, R.D. The Andes climate and weather. Adv. Geosci. 2009, 22, 3–11. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Squeo, F.A.; Armesto, J.J.; Villagran, C. Effects of Aridity on Plant Diversity in the Northern Chilean Andes: Results of a Natural Experiment. Ann. Mo. Bot. Gard. 1988, 75, 55–78. [Google Scholar] [CrossRef]
- Villagrán, C.; Arroyo, M.T.K.; Marticorena, C. Effectos de la desertización en la distribución de la flora andina de Chile. Rev. Chil. Hist. Nat. 1983, 56, 137–157. [Google Scholar]
- Martínez-Carretero, E. La puna Argentina: Delimitación general y división en distritos florísticos. Bol. Soc. Argent. Bot. 1995, 31, 27–40. [Google Scholar]
- Alatalo, J.M.; Jägerbrand, A.K.; Molau, U. Impacts of different climate change regimes and extreme climatic events on an alpine meadow community. Sci. Rep. 2016, 6, 21720. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Squeo, F.A.; Warner, B.G.; Aravena, R.; Espinoza, D. Bofedales: High altitude peatlands of the central Andes. Rev. Chil. Hist. Nat. 2006, 79, 245–255. [Google Scholar] [CrossRef]
- Ruthsatz, B. Vegetation and ecology of the high Andean peatlands of Bolivia. Phytocoenologia 2012, 42, 133–179. [Google Scholar] [CrossRef]
- Monge-Salazar, M.J.; Tovar, C.; Cuadros-Adriazola, J.; Baiker, J.R.; Montesinos-Tubée, D.B.; Bonnesoeur, V.; Antiporta, J.; Román-Dañobeytia, F.; Fuentealva, B.; Ochoa-Tocachi, B.F.; et al. Ecohydrology and ecosystem services of a natural and an artificial bofedal wetland in the central Andes. Sci. Total Environ. 2022, 838, 155968. [Google Scholar] [CrossRef] [PubMed]
- Cleef, A.M. The Vegetation of the Páramos of the Colombian Cordillera Oriental; Cramer: Vaduz, Fürstentum Liechtenstein, 1981; p. 320. [Google Scholar]
- Ruthsatz, B.; Villagran, C. Vegetation pattern and soil nutrients of a Magellanic moorland on the Cordillera de Piuchué, Chiloé Island, Chile. Rev. Chil. Hist. Nat. 1991, 64, 461–478. [Google Scholar]
- Badano, E.I.; Jones, C.G.; Cavieres, L.A.; Wright, J.P. Assessing impacts of ecosystem engineers on community organ-ization: A general approach illustrated by effects of a high-Andean cushion plant. Oikos 2006, 115, 369–385. [Google Scholar] [CrossRef]
- Izquierdo, A.E.; Aragón, M.R.; Navarro, C.J.; Casagranda, M.E. Humedales de la Puna: Principales proveedores de servicios ecosistémicos de la región. In Puna Argentina: Naturaleza Y Cultura, 1st ed.; Grau, H.R., Babot, M.J., Izquierdo, A.E., Grau, A., Eds.; Fundación Miguel Lillo: Tucumán, Argentina, 2018; pp. 96–111. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana. Botánica 2018, 75, 1–430. [Google Scholar] [CrossRef]
- Carilla, J.; Grau, A.; Cuello, A.S. Vegetación de la Puna Argentina. In Puna Argentina: Naturaleza Y Cultura, 1st ed.; Grau, H.R., Babot, M.J., Izquierdo, A.E., Grau, A., Eds.; Fundación Miguel Lillo: Tucumán, Argentina, 2018; pp. 146–156. [Google Scholar]
- Maldonado-Fonkén, M.S. An introduction to the bofedales of the Peruvian High Andes. Mires Peat 2014, 15, 1–13. [Google Scholar]
- Polk, M.H.; Young, K.R.; Cano, A.; León, B. Vegetation of Andean wetlands (bofedales) in Huascarán National Park, Peru. Mires Peat 2019, 24, 1–26. [Google Scholar] [CrossRef]
- Izquierdo, A.E.; Carilla, J.; Nieto, C.; Osinaga Acosta, O.; Martin, E.; Grau, H.R.; Reynaga, M.C. Multi-taxon patterns from high Andean peatlands: Assessing climatic and landscape variables. Community Ecol. 2020, 21, 317–332. [Google Scholar] [CrossRef]
- Ruthsatz, B. Die Hartpolstermoore der Hochanden und ihre Artenvielfalt. Ber. d. Reinh.-Tüxen-Ges. 2000, 12, 351–371. [Google Scholar]
- Ruthsatz, B. Hartpolstermoore der Hochanden NW-Argentiniens als Indikatoren für Klimagradienten. Mitteilungen Arbeitsgemeinschaft Geobot. Schleswig-Holst. 2008, 65, 209–238. [Google Scholar]
- Méndez, E. La vegetación de los Altos Andes II: Las Vegas del flanco oriental del Cordón del Plata (Mendoza, Argentina). Boletín La Soc. Argent. Botánica 2007, 42, 273–294. [Google Scholar]
- Casagranda, M.E.; Izquierdo, A.E. Modeling the potential distribution of floristic assemblages of high Andean wetlands dominated by Juncaceae and Cyperaceae in the Argentine Puna. Veg. Classif. Surv. 2023, 4, 47–58. [Google Scholar] [CrossRef]
- Ruthsatz, B. Flora und ökologische Bedingungen hochandiner Moore Chiles zwischen 18°00′ (Arica) und 40°30′ (Osorno) suPdl. Br. Phytocoenologia 1993, 23, 157–199. [Google Scholar] [CrossRef]
- Ruthsatz, B.; Schittek, K.; Backes, B. The vegetation of cushion peatlands in the Argentine Andes and changes in their floristic composition across a latitudinal gradient from 39° S to 22° S. Phytocoenologia 2020, 50, 249–278. [Google Scholar] [CrossRef]
- Jones, M.M.; Tuomisto, H.; Borcard, D.; Legendre, P.; Clark, D.B.; Olivas, P.C. Explaining variation in tropical plant community composition: Influence of environmental and spatial data quality. Oecologia 2008, 155, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Blundo, C.; González-Espinosa, M.; Malizia, L.R. Relative contribution of niche and neutral processes on tree species turnover across scales in seasonal forests of NW Argentina. Plant Ecol. 2016, 217, 359–368. [Google Scholar] [CrossRef]
- Zheng, J.; Arif, M.; He, X.; Ding, D.; Zhang, S.; Ni, X.; Li, C. Plant community assembly is jointly shaped by environmental and dispersal filtering along elevation gradients in a semiarid area, China. Front. Plant Sci. 2022, 13, 1041742. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Mendoza, M.M.; Drenkhan, F.; Montoya, N.; Baiker, J.R.; Mackay, J.D.; Hannah, D.M.; Buytaert, W. Seasonal water storage and release dynamics of bofedal wetlands in the Central Andes. Hydrol. Process. 2023, 37, e14940. [Google Scholar] [CrossRef]
- Guo, Y.; Xiang, W.; Wang, B.; Li, D.; Mallik, A.U.; Chen, H.Y.H.; Huang, F.; Ding, T.; Wen, S.; Lu, S.; et al. Partitioning beta diversity in a tropical karst seasonal rainforest in Southern China. Sci. Rep. 2018, 8, 17408. [Google Scholar] [CrossRef]
- Villagrán, C.; Armesto, J.J.; Hinojosa, L.F.; Cuvertino, J.; Pérez, C.; Medina, C. El enigmático origen del bosque relicto de Fray Jorge. In Historia Natural Del Parque Nacional Bosque Fray Jorge, 1st ed.; Squeo, F.A., Gutiérrez, J.R., Hernández, I.R., Eds.; Ediciones Universidad de La Serena: La Serena, Chile, 2004; Volume 1, pp. 3–43. [Google Scholar]
- Cheng, D.; Zhu, Q.; Huang, J.; Wu, Q.; Yang, L. A local cores-based hierarchical clustering algorithm for data sets with complex structures. Neural Comput. Appl. 2019, 31, 8051–8068. [Google Scholar] [CrossRef]
- Almeida, J.A.S.; Barbosa, L.M.S.; Pais, A.A.C.C.; Formosinho, S.J. Improving hierarchical cluster analysis: A new method with outlier detection and automatic clustering. Chemom. Intell. Lab. Syst. 2007, 87, 208–217. [Google Scholar] [CrossRef]
- Carta, A.; Peruzzi, L.; Ramírez-Barahona, S. A global phylogenetic regionalization of vascular plants reveals a deep split between Gondwanan and Laurasian biotas. New Phytol. 2022, 233, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, H.; Boufford, D.E.; Bartholomew, B.; Fritsch, P.W.; Chen, J.; Deng, T.; Ree, R.H. Grade of Membership models reveal geographical and environmental correlates of floristic structure in a temperate biodiversity hotspot. New Phytol. 2021, 232, 1424–1435. [Google Scholar] [CrossRef]
- Biganzoli, F.; Oyarzabal, M.; Teillier, S.; Zuloaga, F.O. Fitogeografía de la provincia altoandina del cono sur de sudamérica. Darwiniana Nueva Ser. 2022, 10, 537–574. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A. Plant Geography of Chile; Springer: Santiago, Chile, 2011; pp. 87–150. [Google Scholar]
- Lörch, M.; Mutke, J.; Weigend, M.; Luebert, F. Historical biogeography and climatic differentiation of the Fulcal-dea-Archidasyphyllum-Arnaldoa clade of Barnadesioideae (Asteraceae) suggest a Miocene, aridity-mediated Andean disjunction associated with climatic niche shifts. Glob. Planet Chang. 2021, 201, 103495. [Google Scholar] [CrossRef]
- Luebert, F.; Lörch, M.; Acuña, R.; Mello-Silva, R.; Weigend, M.; Mutke, J. Clade-Specific Biogeographic History and Climatic Niche Shifts of the Southern Andean-Southern Brazilian Disjunction in Plants. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 661–682. [Google Scholar]
- Scherson, R.A.; Thornhill, A.H.; Urbina-Casanova, R.; Freyman, W.A.; Pliscoff, P.A.; Mishler, B.D. Spatial phylogenetics of the vascular flora of Chile. Mol. Phylogenetics Evol. 2017, 112, 88–95. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, J.; Jiang, M. Global patterns of taxonomic and phylogenetic diversity of flowering plants: Biodiversity hotspots and coldspots. Plant Divers. 2023, 45, 265–271. [Google Scholar] [CrossRef]
- Qian, H.; Jin, Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 2016, 9, 233–239. [Google Scholar] [CrossRef]
- Brožová, V.; Proćków, J.; Záveská Drábková, L. Toward finally unraveling the phylogenetic relationships of Juncaceae with respect to another cyperid family, Cyperaceae. Mol. Phylogenetics Evol. 2022, 177, 107588. [Google Scholar] [CrossRef]
- Elliott, T.L.; Larridon, I.; Barrett, R.L.; Bruhl, J.J.; Costa, S.M.; Escudero, M.; Hipp, A.L.; Jiménez-Mejías, P.; Kirschner, J.; Luceño, M.; et al. Addressing inconsistencies in Cyperaceae and Juncaceae taxonomy: Comment on Brožová et al. (2022). Mol. Phylogenetics Evol. 2023, 179, 107665. [Google Scholar] [CrossRef]
- Leigh, E.G., Jr.; Rosindell, J.; Etienne, R.S. Unified neutral theory of biodiversity and biogeography. Scholarpedia 2010, 5, 8822. [Google Scholar] [CrossRef]
- Chase, J.M. Community assembly: When should history matter? Oecologia 2003, 136, 489–498. [Google Scholar] [CrossRef]
- De Bello, F.; Lavergne, S.; Meynard, C.N.; Lepš, J.; Thuiller, W. The partitioning of diversity: Showing Theseus a way out of the labyrinth. J. Veg. Sci. 2010, 21, 992–1000. [Google Scholar] [CrossRef]
- Wiens, J.J. Speciation and ecology revisited: Phylogenetic niche conservatism and the origin of species. Evolution 2004, 58, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.B. An Historical Phytogeography of the High Andean Flora. Rev. Chil. Hist. Nat. 1983, 56, 109–122. [Google Scholar]
- Luebert, F.; Weigend, M. Phylogenetic insights into Andean plant diversification. Front. Ecol. Evol. 2014, 2, 27. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Wiens, J. The niche, biogeography and species interactions. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2336–2350. [Google Scholar] [CrossRef]
- Zuloaga, F.O.; Belgrano, M.J.; Zanotti, C.A. Actualización del catálogo de las plantas vasculares del cono sur. Darwiniana Nueva Ser. 2019, 7, 208–278. [Google Scholar] [CrossRef]
- Jørgensen, P.M.; Nee, M.; Beck, S.G.; Arrázola, S.; Saldias, M.; Hirth, S.; Swift, V.; Penagos, J.C.; Romero, C. Catálogo De Las Plantas Vasculares De Bolivia; Missouri Botanical Garden Press: St. Louis, MO, USA, 2014. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.; Lange, S.; Hari, C.; Reyer, C.; Conrad, O.; Zimmermann, N.; Frieler, K. CHELSA-W5E5: Daily 1 km meteoro-logical forcing data for climate impact studies. Earth Syst. Sci. Data 2023, 15, 2445–2464. [Google Scholar] [CrossRef]
- Bobrowski, M.; Weidinger, J.; Schickhoff, U. Is New Always Better? Frontiers in Global Climate Datasets for Modeling Treeline Species in the Himalayas. Atmosphere 2021, 12, 543. [Google Scholar] [CrossRef]
- Poggio, L.; De Sousa, L.M.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing soil information for the globe with quantified spatial uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 1 March 2023).
- Hijmans, J.R.; Karney, C.; Williams, E.; Vennes, C. geosphere: Spherical Trigonometry, R package version 1.5-18; 2022. Available online: https://cran.r-project.org/web/packages/geosphere/index.html (accessed on 1 April 2023).
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Adespatial: Multivariate Multiscale Spatial Analysis, R package version 0.3-23; 2023. Available online: https://cran.r-project.org/web/packages/adespatial/index.html (accessed on 1 April 2023).
- Legendre, P.; Fortin, M.-J. Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol. Ecol. Resour. 2010, 10, 831–844. [Google Scholar] [CrossRef]
- Frasconi, C.; Ceia-Hasse, A.; Nunes, A.; Verble, R.; Santini, G.; Boieiro, M.; Branquinho, C. Local environmental variables are key drivers of ant taxonomic and functional beta-diversity in a Mediterranean dryland. Sci. Rep. 2021, 11, 2292. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Smith, T.W.; Lundholm, J.T. Variation partitioning as a tool to distinguish between niche and neutral processes. Ecography 2010, 33, 648–655. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package, R package version 2.5-6; 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 April 2023).
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. The Comparison of Dendrograms by Objective Methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Salvador, S.; Chan, P. Determining the number of clusters/segments in hierarchical clustering/segmentation algorithms. In Proceedings of the 16th IEEE International Conference on Tools with Artificial Intelligence, Boca Raton, FL, USA, 15–17 November 2004; pp. 576–584. [Google Scholar]
- Vavrek, M.J. A comparison of clustering methods for biogeography with fossil datasets. PeerJ 2016, 4, e1720. [Google Scholar] [CrossRef]
- Daru, B.H.; Karunarathne, P.; Schliep, K. phyloregion: R package for biogeographical regionalization and macroecology. Methods Ecol. Evol. 2020, 11, 1483–1491. [Google Scholar] [CrossRef]
- White, A.E.; Dey, K.K.; Mohan, D.; Stephens, M.; Price, T.D. Regional influences on community structure across the tropical-temperate divide. Nat. Commun. 2019, 10, 2646. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2023. Available online: https://www.gbif.org (accessed on 1 November 2023).
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Petitpierre, B.; Breiner, F.; Scherrer, D.; D’Amen, M.; Randin, C.; Engler, R.; Hordijk, W.; Mod, H.; et al. Ecospat: Spatial Ecology Miscellaneous Methods, R package version 4.0.0; 2023. Available online: https://cran.r-project.org/web/packages/ecospat/index.html (accessed on 1 December 2023).
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]

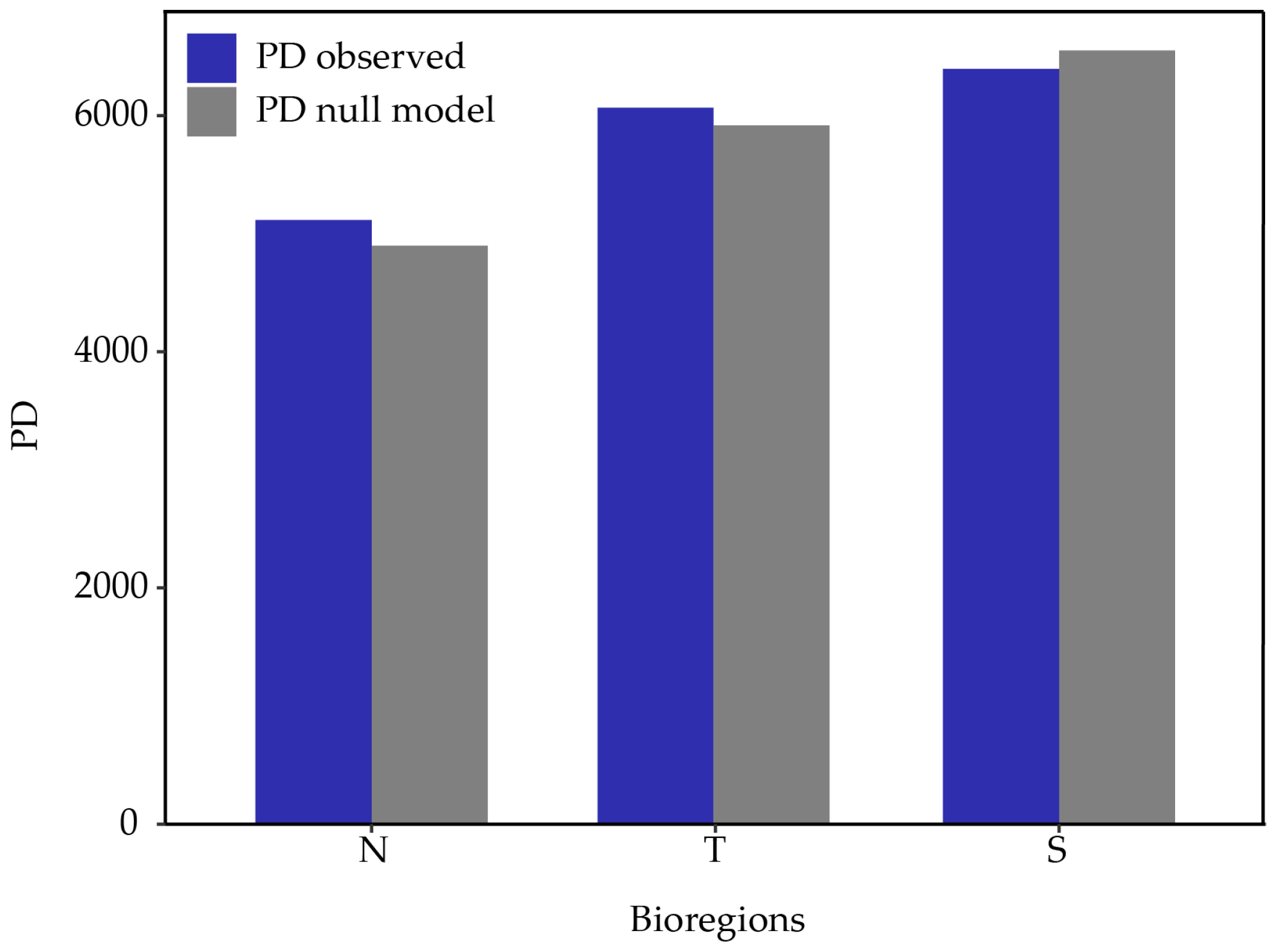
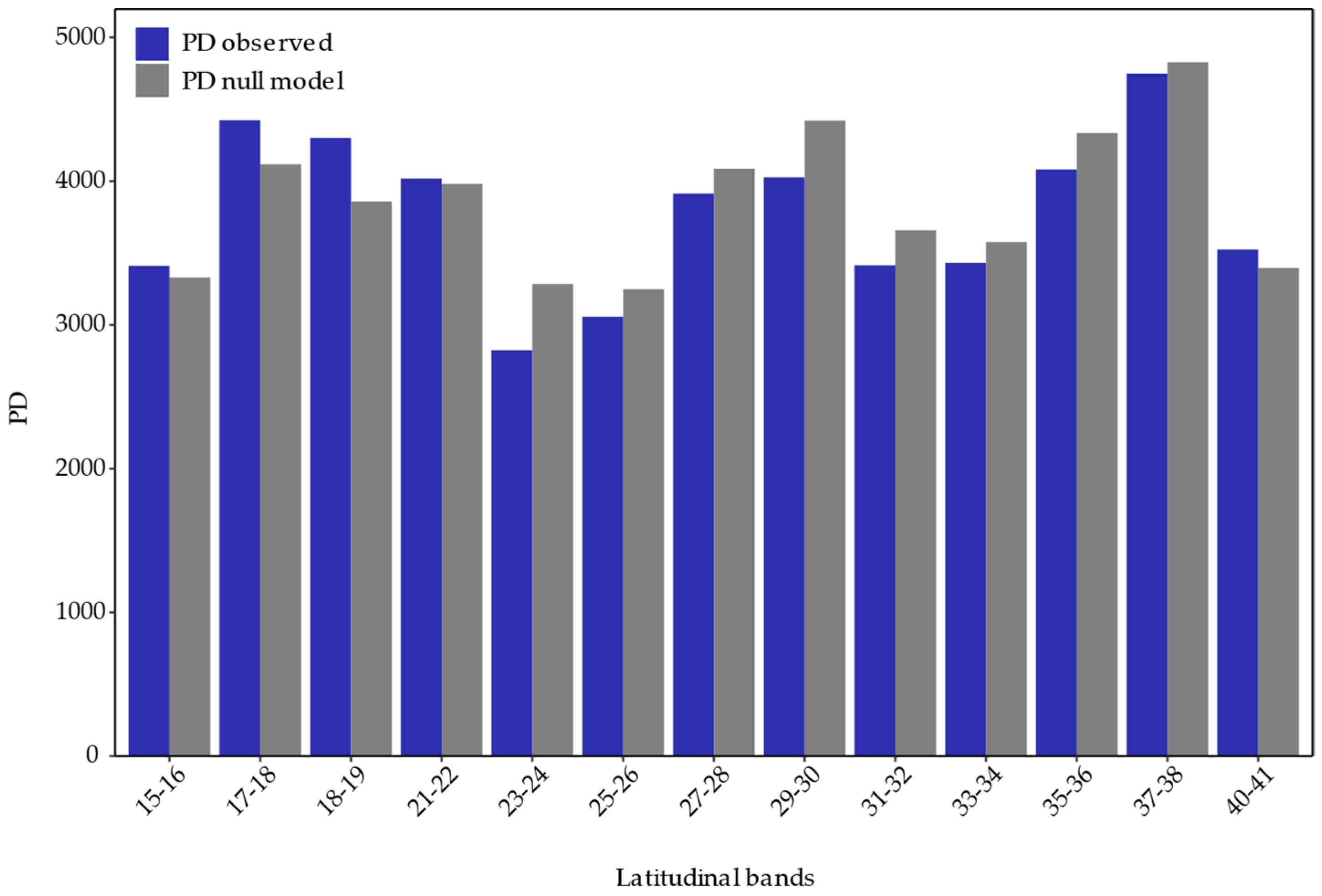
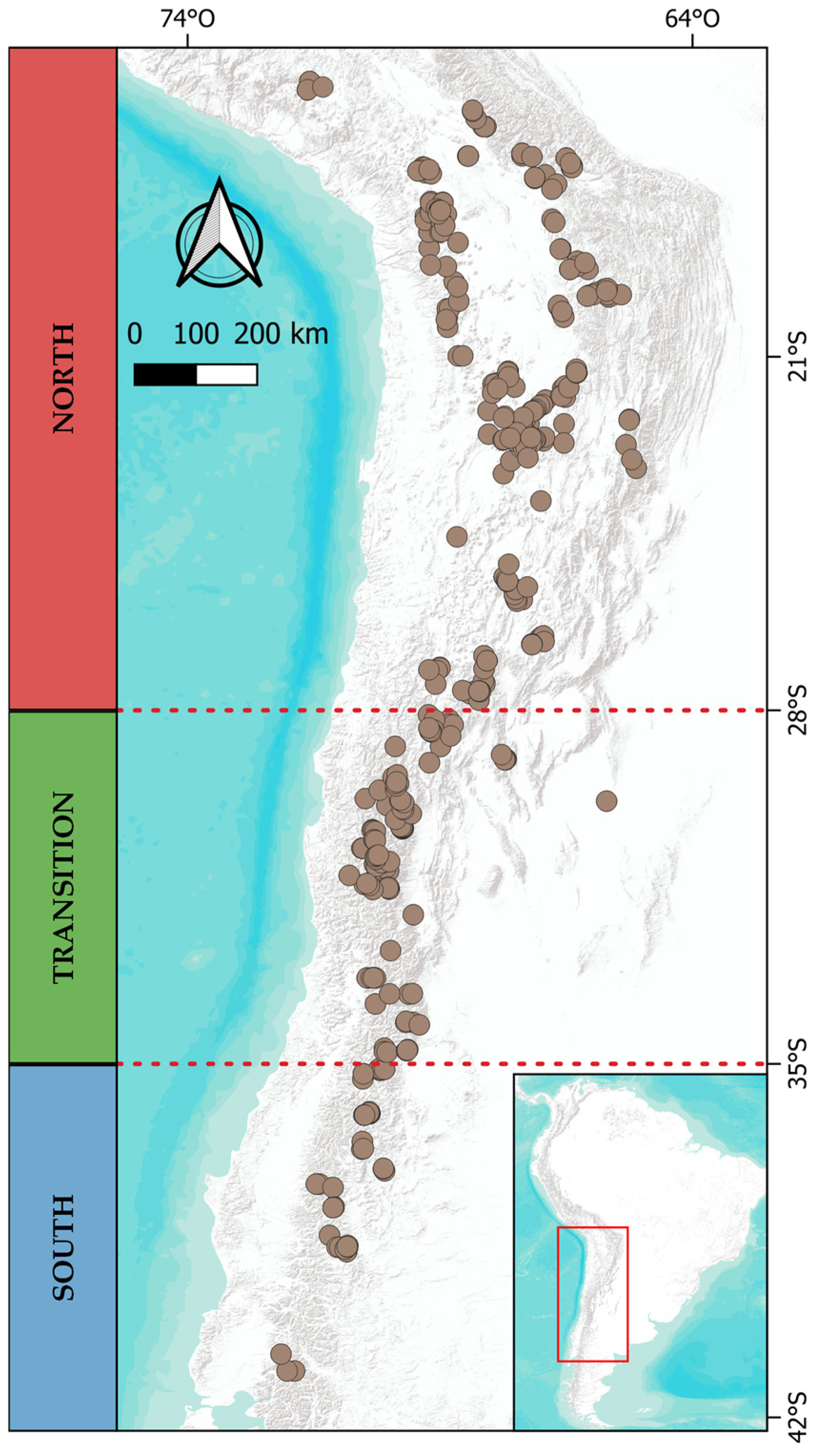
| Bioregion | PD z-Score | αPD | MPD z-Score | αMPD | MNTD z-Score | αMNTD |
|---|---|---|---|---|---|---|
| N | 0.801 | 0.783 | 1.865 | 0.987 | 0.481 | 0.687 |
| T | 0.612 | 0.703 | 1.504 | 0.961 | 0.239 | 0.593 |
| S | −0.521 | 0.312 | −1.250 | 0.148 | −0.564 | 0.282 |
| Band | PD z-Score | αPD | MPD z-Score | αMPD | MNTD z-Score | αMNTD |
|---|---|---|---|---|---|---|
| 15–16 | 0.318 | 0.636 | 1.437 | 0.843 | 0.368 | 0.647 |
| 17–18 | 1.129 | 0.860 | 1.791 | 0.940 | 1.192 | 0.872 |
| 18–19 | 1.728 | 0.951 | 2.125 | 0.974 | 1.742 | 0.950 |
| 21–22 | 0.042 | 0.542 | 1.838 | 0.949 | 0.183 | 0.602 |
| 23–24 | −1.825 | 0.015 | −0.890 | 0.134 | −1.327 | 0.091 |
| 25–26 | −0.717 | 0.251 | −0.480 | 0.340 | −0.489 | 0.325 |
| 27–28 | −0.565 | 0.296 | −0.635 | 0.300 | −0.746 | 0.244 |
| 29–30 | −1.408 | 0.082 | −0.294 | 0.502 | −1.448 | 0.065 |
| 31–32 | −0.793 | 0.226 | −0.852 | 0.173 | −0.292 | 0.411 |
| 33–34 | −0.514 | 0.306 | −0.109 | 0.609 | −0.789 | 0.222 |
| 35–36 | −0.885 | 0.193 | −0.575 | 0.319 | −1.293 | 0.089 |
| 37–38 | −0.309 | 0.404 | −0.745 | 0.258 | 0.196 | 0.581 |
| 40–41 | 0.485 | 0.693 | −0.335 | 0.436 | 0.610 | 0.730 |
| Type | Variable (Abbreviation) | |
|---|---|---|
| Climate | Annual Mean Temperature (Bio1) | Mean Temperature of Coldest Quarter (Bio11) |
| Mean Diurnal Range (Bio2) | Annual Precipitation (Bio12) | |
| Isothermality (Bio3) | Precipitation of Wettest Month (Bio13) | |
| Temperature Seasonality (Bio4) | Precipitation of Driest Month (Bio14) | |
| Max Temperature of Warmest Month (Bio5) | Precipitation Seasonality (Bio15) | |
| Min Temperature of Coldest Month (Bio6) | Precipitation of Wettest Quarter (Bio16) | |
| Temperature Annual Range (Bio7) | Precipitation of Driest Quarter (Bio17) | |
| Mean Temperature of Wettest Quarter (Bio8) | Precipitation of Warmest Quarter (Bio18) | |
| Mean Temperature of Driest Quarter (Bio9) | Precipitation of Coldest Quarter (Bio19) | |
| Mean Temperature of Warmest Quarter (Bio10) | ||
| Edaphic | Soil organic carbon in fine earth (Soc) | Total nitrogen (Nitrogen) |
| Bulk density of the fine earth fraction (Bdod) | Vol. fraction of coarse fragments (>2 mm) (Cfvo) | |
| pH H2O (Phh2o) | Organic Carbon density (Ocd) | |
| Silt (Silt) | Sand (Sand) | |
| Clay (Clay) | ||
| Elevación | Digital elevation model (Elev) | |
| Type | Variables | |||
|---|---|---|---|---|
| Environment | Temperature | Bio2 | ||
| Bio7 | ||||
| Bio9 | ||||
| Bio10 | ||||
| Bio11 | ||||
| Precipitation | Bio15 | |||
| Bio18 | ||||
| Edaphic | Bdod | |||
| Phh2o | ||||
| Cfvo | ||||
| Spatial | Elevation | Elev | ||
| MEM | 4 | 1 | 3 | |
| 5 | 9 | 8 | ||
| 2 | 12 | 51 | ||
| 19 | 17 | 10 | ||
| 32 | 59 | 28 | ||
| 11 | 37 | 6 | ||
| 16 | 40 | 24 | ||
| 23 | 20 | 33 | ||
| 22 | 52 | 42 | ||
| 31 | 36 | |||
| Index | Equation | Source | |
|---|---|---|---|
| S (Sorensen) | (Chao et al., 2006) [90] | (1) | |
| BDtotal | (Legendre, 2013) [89] | (2) | |
| Turnover (ReplS) | (Legendre, 2014) [12] | (3) | |
| Nestedness (RichDiffS) | (Legendre, 2014) [12] | (4) |
| Type | S | Turnover | Nestedness |
|---|---|---|---|
| Temperature | Bio2 | Bio2 | Bio11 |
| Bio7 | Bio7 | ||
| Bio9 | Bio9 | ||
| Bio10 | Bio10 | ||
| Bio11 | Bio11 | ||
| Precipitation | Bio15 | Bio14 | Bio16 |
| Bio16 | Bio15 | ||
| Bio18 | Bio16 | ||
| Edaphic | Bdod | Bio18 | Silt |
| Phh2o | Bdod | Bdod | |
| Silt | Phh2o | Soc | |
| Sand | Silt | ||
| Cfvo | Cfvo | ||
| Elevation | Elev | Elev |
| North (N) | Transition (T) | South (S) | |
|---|---|---|---|
| 1 | Plantago tubulosa | Deschampsia eminens | Ochetophila nana |
| 2 | Distichia muscoides | Cinnagrostis velutina | Ranunculus peduncularis |
| 3 | Hypochaeris taraxacoides | Eleocharis pseudoalbibracteata | Hordeum comosum |
| Climate | Edaphic | |
|---|---|---|
| Bio2 | Bio11 | Bdod |
| Bio3 | Bio12 | Cfvo |
| Bio5 | Bio15 | Clay |
| Bio7 | Bio19 | Nitrogen |
| Bio9 | Silt | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Ponce, F.; Hinojosa, L.F. Environmental Filters Structure Cushion Bogs’ Floristic Composition along the Southern South American Latitudinal Gradient. Plants 2024, 13, 2202. https://doi.org/10.3390/plants13162202
Figueroa-Ponce F, Hinojosa LF. Environmental Filters Structure Cushion Bogs’ Floristic Composition along the Southern South American Latitudinal Gradient. Plants. 2024; 13(16):2202. https://doi.org/10.3390/plants13162202
Chicago/Turabian StyleFigueroa-Ponce, Felipe, and Luis Felipe Hinojosa. 2024. "Environmental Filters Structure Cushion Bogs’ Floristic Composition along the Southern South American Latitudinal Gradient" Plants 13, no. 16: 2202. https://doi.org/10.3390/plants13162202
APA StyleFigueroa-Ponce, F., & Hinojosa, L. F. (2024). Environmental Filters Structure Cushion Bogs’ Floristic Composition along the Southern South American Latitudinal Gradient. Plants, 13(16), 2202. https://doi.org/10.3390/plants13162202






