Antidepressant- and Anxiolytic-like Effects of Pomegranate: Is It Acting by Common or Well-Known Mechanisms of Action?
Abstract
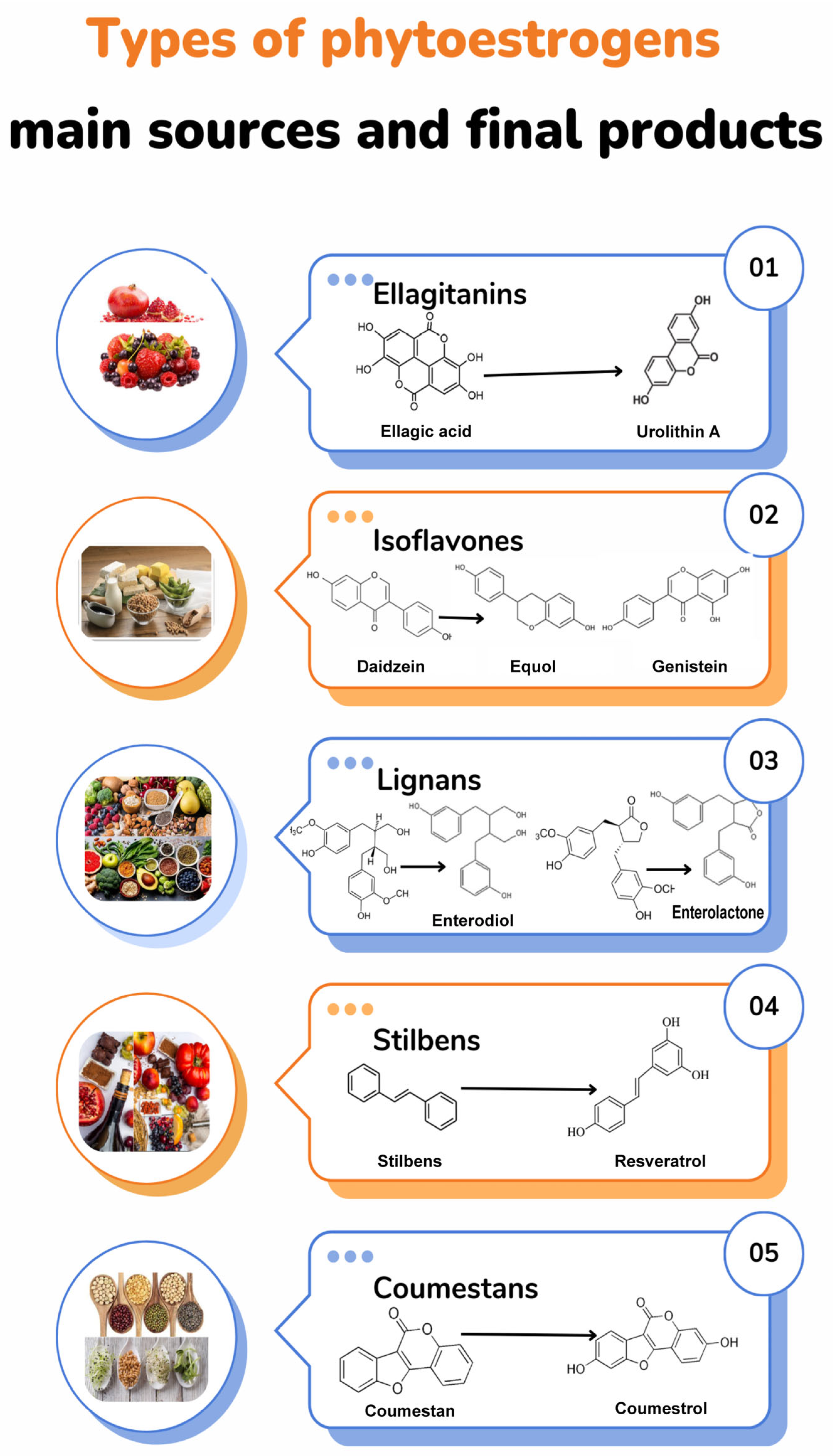
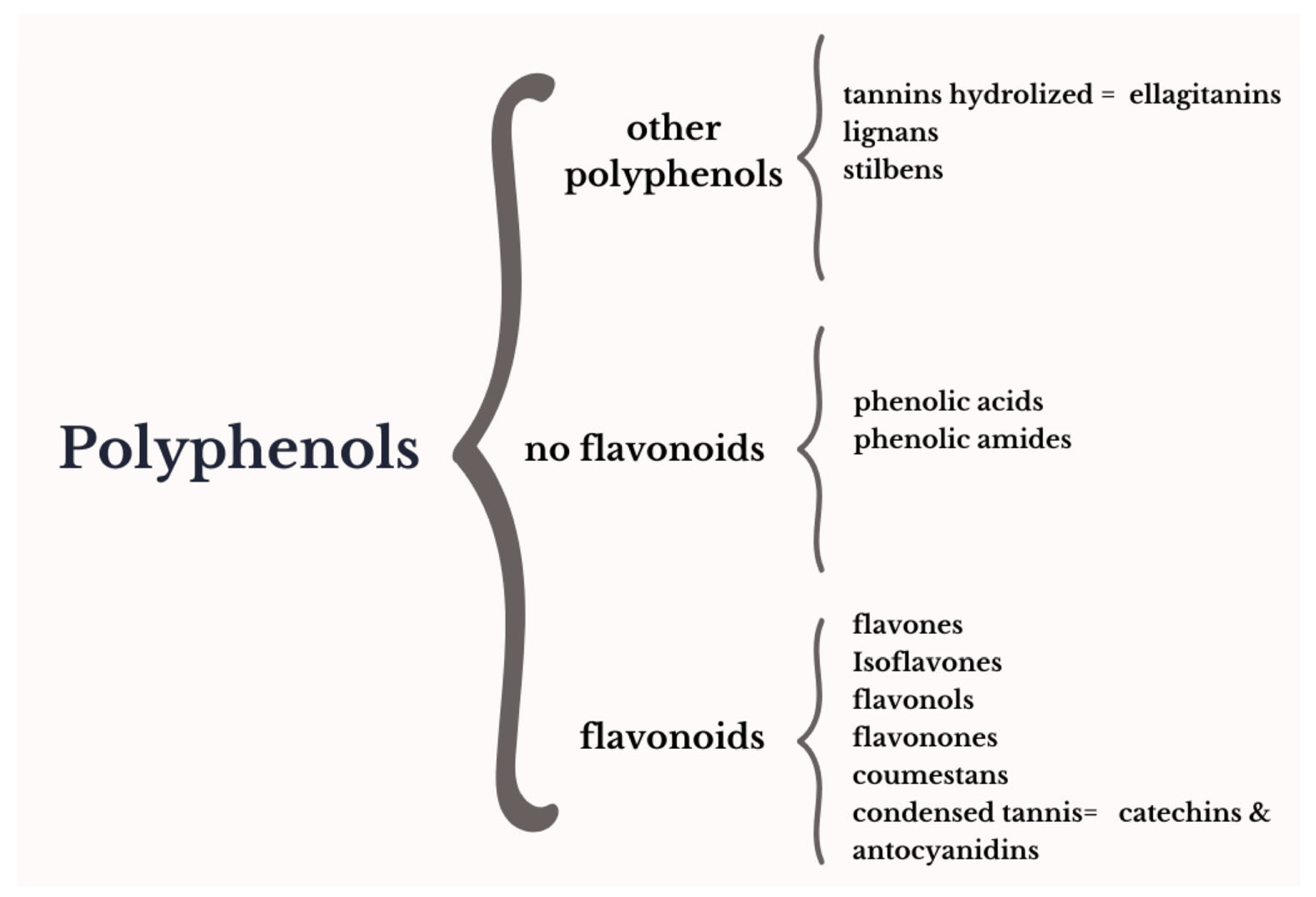
:1. General Description: Botanical Aspects and Bioactive Compounds of Pomegranates
| Phytochemical | Phytoestrogen Group | Metabolite | Estrogen Receptor Activity | References |
|---|---|---|---|---|
| Isoflavones | Genistein | ERa | [5,20] | |
| Daidzein | ER memb | |||
| Equol | ERb | |||
| Flavonols | Quercetin | Rutin (Quercetin-3-O-rutinoside) | ERa/b | [15,17,20] |
| Kaempferol | ER memb ERb | |||
| Myricetin | Era | |||
| Condensed tannins | Anthocyanidins | Cyanidin-3,5 di-O- diglucoside Cyanidin-3-O-glucoside Delphinidin 3,5 di-O- diglucoside Delphinidin 3-O-glucoside Pelargonidin-3,5-di-O-diglucoside Pelargonidin-3-O-glucoside | ERa/b ERa ER memb | [8,15,17,18,20] |
| Catechins | Epicatechin Epicatechin galleate Chysin | ERa, GPR30 ER GPR30 ERa | ||
| Coumestans | Coumestrol | ERa/b ER memb | [5] | |
| Flavonons | Luteolin Apigenin | ERa/b ERa/b | [19] | |
| Lignans | Isolariciresinol Matairesinol | ERa/b | [20] | |
| Stilbenes | Resveratrol | ND | ||
| Flavanones | Baicalein Naringin | ER memb ERa, ER memb | [26] | |
| Hydrolyzed tannins | Ellagitannins | Punicalagin α and β | ERb | [8,15,17,21] |
| Ellagic acid glucoside Ellagic acid | ND | |||
| Phenolic acids | Ferulic acid Cinnamic acid Coumaric acid Gallic acid Caffeic acid | ERa | [13,17,20] |
2. Menopause Is a Natural Process Altering the Endocrine System
3. Antidepressant Effect on Animal Models
Mechanism of Action Proposed on Specific Receptors
4. Anxiolytic-like Action on Animal Models
Mechanism of Action on Specific Receptors
5. Effects on Oxidative Stress Related to Anxiolytic- and Antidepressant-Like Effects
6. Effects on Pain as a Comorbidity of Anxiety and Depression
Mechanism of Action on Specific Receptors
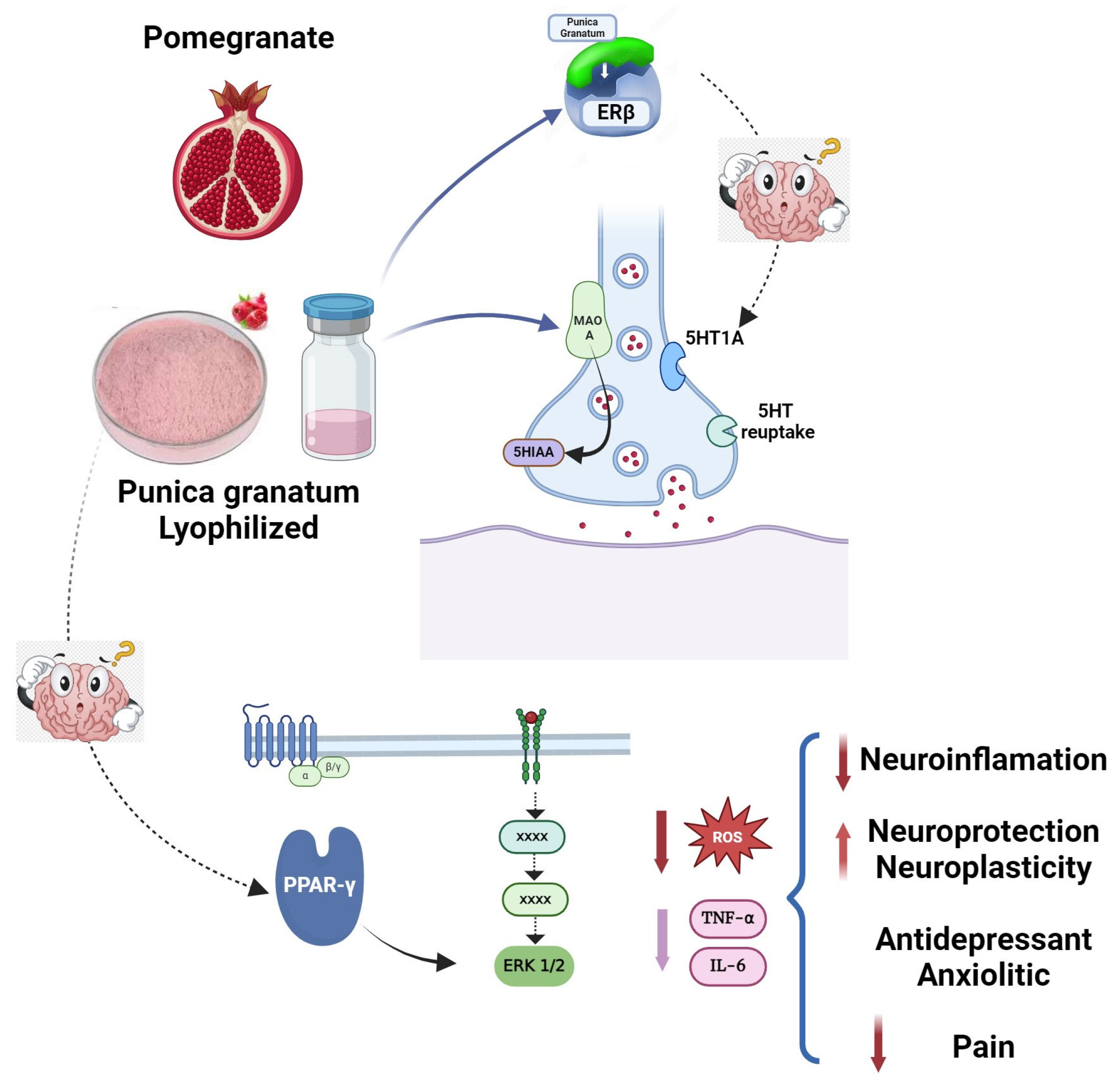
7. Is It Possible to Have a Common Mechanism of Action?
8. Concluding Remarks
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, Horticulture, Breeding. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 127–191. ISBN 978-0-470-59377-6. [Google Scholar]
- Jurenka, J.S. Therapeutic Applications of Pomegranate (Punica granatum L.): A Review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar] [PubMed]
- Shao-bo, W. Research Advances of Pomegranate in China. Heilongjiang Agric. Sci. 2009. [Google Scholar]
- Cervantes-Anaya, N.; Azpilcueta-Morales, G.; Estrada-Camarena, E.; Ramírez Ortega, D.; Pérez de la Cruz, V.; González-Trujano, M.E.; López-Rubalcava, C. Pomegranate and Its Components, Punicalagin and Ellagic Acid, Promote Antidepressant, Antioxidant, and Free Radical-Scavenging Activity in Ovariectomized Rats. Front. Behav. Neurosci. 2022, 16, 836681. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Duo, L.; Wang, J.; Yang, J.; Li, Z.; Tu, Y. A Unique Understanding of Traditional Medicine of Pomegranate, Punica granatum L. and Its Current Research Status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef] [PubMed]
- Al-Muammar, M.N.; Khan, F. Obesity: The Preventive Role of the Pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Camarena, E.M.; López-Rubalcava, C.; Ramírez-Rodríguez, G.B.; Pulido, D.; Cervantes-Anaya, N.; Azpilcueta-Morales, G.; Granados-Juárez, A.; Vega-Rivera, N.M.; Islas-Preciado, D.; Treviño, S.; et al. Aqueous Extract of Pomegranate Enriched in Ellagitannins Prevents Anxiety-like Behavior and Metabolic Changes Induced by Cafeteria Diet in an Animal Model of Menopause. Neurochem. Int. 2020, 141, 104876. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.E.; Pellicer, F.; Mena, P.; Moreno, D.A.; García-Viguera, C. Antinociceptive and Anti-Inflammatory Activities of a Pomegranate (Punica granatum L.) Extract Rich in Ellagitannins. Int. J. Food Sci. Nutr. 2015, 66, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.G.; Smyser, C.D.; Cherkerzian, S.; Alexopoulos, D.; Kenley, J.; Tuuli, M.G.; Nelson, D.M.; Inder, T.E. Maternal Pomegranate Juice Intake and Brain Structure and Function in Infants with Intrauterine Growth Restriction: A Randomized Controlled Pilot Study. PLoS ONE 2019, 14, e0219596. [Google Scholar] [CrossRef] [PubMed]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate Extract Improves a Depressive State and Bone Properties in Menopausal Syndrome Model Ovariectomized Mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Sustaita, B.; López-Rubalcava, C.; González-Trujano, M.E.; García-Viguera, C.; Estrada-Camarena, E. Aqueous Extract of Pomegranate Alone or in Combination with Citalopram Produces Antidepressant-Like Effects in an Animal Model of Menopause: Participation of Estrogen Receptors. Int. J. Mol. Sci. 2017, 18, 2643. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Sustaita, B.; Estrada-Camarena, E.; González-Trujano, M.E.; López-Rubalcava, C. Estrogen Receptors-β and Serotonin Mediate the Antidepressant-like Effect of an Aqueous Extract of Pomegranate in Ovariectomized Rats. Neurochem. Int. 2021, 142, 104904. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hontecillas, R.; Lu, P.; Bassaganya-Riera, J. Preventive and Prophylactic Mechanisms of Action of Pomegranate Bioactive Constituents. Evid. Based Complement. Altern. Med. 2013, 2013, 789764. [Google Scholar] [CrossRef]
- Middha, S.K.; Usha, T.; Pande, V. HPLC Evaluation of Phenolic Profile, Nutritive Content, and Antioxidant Capacity of Extracts Obtained from Punica granatum Fruit Peel. Adv. Pharmacol. Sci. 2013, 2013, 296236. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of Phenolic Compounds in Different Parts of Pomegranate (Punica granatum) Fruit by HPLC-PDA-ESI/MS and Evaluation of Their Antioxidant Activity: Application to Different Italian Varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of Phenolic Compounds from Pomegranate (Punica granatum L.) by-Product with Significant Antioxidant and Therapeutic Effects: A Narrative Review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef]
- van Elswijk, D.A.; Schobel, U.P.; Lansky, E.P.; Irth, H.; van der Greef, J. Rapid Dereplication of Estrogenic Compounds in Pomegranate (Punica granatum) Using on-Line Biochemical Detection Coupled to Mass Spectrometry. Phytochemistry 2004, 65, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, M.; Yu, G.; Pu, J.; Tian, K.; Tang, X.; Du, Y.; Wu, H.; Hu, J.; Luo, X.; et al. Comparative Analysis of the Phenolic Contents and Antioxidant Activities of Different Parts of Two Pomegranate (Punica granatum L.) Cultivars: “Tunisia” and “Qingpi.”. Front. Plant Sci. 2023, 14, 1265018. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Camarena, E.; López-Rubalcava, C.; Valdés-Sustaita, B.; Azpilcueta-Morales, G.S.; MaríaGonzález-Trujano, E.; Estrada-Camarena, E.; López-Rubalcava, C.; Valdés-Sustaita, B.; Azpilcueta-Morales, G.S.; MaríaGonzález-Trujano, E. Use of Phytoestrogens for the Treatment of Psychiatric Symptoms Associated with Menopause Transition. In A Multidisciplinary Look at Menopause; IntechOpen: London, UK, 2017; ISBN 978-953-51-3406-0. [Google Scholar]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors Alpha and Beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Estrogenic Flavonoids and Their Molecular Mechanisms of Action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef] [PubMed]
- Zava, D.T.; Blen, M.; Duwe, G. Estrogenic Activity of Natural and Synthetic Estrogens in Human Breast Cancer Cells in Culture. Env. Health Perspect. 1997, 105 (Suppl. S3), 637–645. [Google Scholar] [CrossRef]
- Puranik, N.V.; Srivastava, P.; Bhatt, G.; John Mary, D.J.S.; Limaye, A.M.; Sivaraman, J. Determination and Analysis of Agonist and Antagonist Potential of Naturally Occurring Flavonoids for Estrogen Receptor (ERα) by Various Parameters and Molecular Modelling Approach. Sci. Rep. 2019, 9, 7450. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Phytonutrients as Therapeutic Agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lei, Y.; Lu, C.; Liu, D.; Ma, W.; Lu, H.; Wang, Y. Punicic Acid Ameliorates Obesity-Related Hyperlipidemia and Fatty Liver in Mice via Regulation of Intestinal Flora and Lipopolysaccharide-Related Signaling Pathways. Food Funct. 2024, 15, 5012–5025. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Wall-Medrano, A.; González-Aguilar, G.A.; López-Díaz, J.A.; Álvarez-Parrilla, E.; de la Rosa, L.A.; Ramos-Jimenez, A. Taninos Hidrolizables: Bioquímica, Aspectos Nutricionales y Analíticos y Efectos En La Salud. Nutr. Hosp. 2015, 31, 55–66. [Google Scholar]
- Iqbal, J.; Huang, G.-D.; Xue, Y.-X.; Yang, M.; Jia, X.-J. Role of Estrogen in Sex Differences in Memory, Emotion and Neuropsychiatric Disorders. Mol. Biol. Rep. 2024, 51, 415. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. Associations of Hormones and Menopausal Status with Depressed Mood in Women with No History of Depression. Arch. Gen. Psychiatry 2006, 63, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Eisenlohr-Moul, T.A.; Rubinow, D.R.; Schrubbe, L.; Girdler, S.S. Naturally Occurring Changes in Estradiol Concentrations in the Menopause Transition Predict Morning Cortisol and Negative Mood in Perimenopausal Depression. Clin. Psychol. Sci. 2016, 4, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Rubinow, D.R.; Eisenlohr-Moul, T.A.; Leserman, J.; Girdler, S.S. Estradiol Variability, Stressful Life Events, and the Emergence of Depressive Symptomatology during the Menopausal Transition. Menopause 2016, 23, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Peltier, A.; Grummisch, J.A.; Sykes Tottenham, L. Estradiol Fluctuation, Sensitivity to Stress, and Depressive Symptoms in the Menopause Transition: A Pilot Study. Front. Psychol. 2019, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Liu, L.; Gracia, C.R.; Nelson, D.B.; Hollander, L. Hormones and Menopausal Status as Predictors of Depression in Women in Transition to Menopause. Arch. Gen. Psychiatry 2004, 61, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kuck, M.J.; Hogervorst, E. Stress, Depression, and Anxiety: Psychological Complaints across Menopausal Stages. Front. Psychiatry 2024, 15, 1323743. [Google Scholar] [CrossRef] [PubMed]
- Perlman, W.R.; Tomaskovic-Crook, E.; Montague, D.M.; Webster, M.J.; Rubinow, D.R.; Kleinman, J.E.; Weickert, C.S. Alteration in Estrogen Receptor Alpha mRNA Levels in Frontal Cortex and Hippocampus of Patients with Major Mental Illness. Biol. Psychiatry 2005, 58, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.Ö.; Kurutaş, E.B.; Doğaner, A.; Türker, E.; Özcü, S.Ş.T.; Güngör, M.; Çakmak, S. Serum Levels of GPER-1 in Euthymic Bipolar Patients. Neuropsychiatr. Dis. Treat. 2018, 14, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. A Review and Update of Mechanisms of Estrogen in the Hippocampus and Amygdala for Anxiety and Depression Behavior. Neuropsychopharmacology 2006, 31, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Ciriza, I.; Garcia-Segura, L.M.; Frye, C.A. Antisense Oligodeoxynucleotides for Estrogen Receptor-Beta and Alpha Attenuate Estradiol’s Modulation of Affective and Sexual Behavior, Respectively. Neuropsychopharmacology 2008, 33, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. Administration of Estrogen Receptor Beta-Specific Selective Estrogen Receptor Modulators to the Hippocampus Decrease Anxiety and Depressive Behavior of Ovariectomized Rats. Pharmacol. Biochem. Behav. 2007, 86, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Numakawa, T.; Chiba, S.; Ninomiya, M.; Kajiyama, Y.; Adachi, N.; Akema, T.; Kunugi, H. Estrogen, Predominantly via Estrogen Receptor α, Attenuates Postpartum-Induced Anxiety- and Depression-like Behaviors in Female Rats. Endocrinology 2013, 154, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Fleischer, R.; Schaeffer, J.M.; Rohrer, S.P.; Hickey, G.J. 17 Beta-Estradiol-Induced Antidepressant-like Effect in the Forced Swim Test Is Absent in Estrogen Receptor-Beta Knockout (BERKO) Mice. Psychopharmacology 2005, 179, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef] [PubMed]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.Å.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front. Endocrinol. 2022, 13, 828780. [Google Scholar] [CrossRef] [PubMed]
- Tongta, S.; Daendee, S.; Kalandakanond-Thongsong, S. Effects of Estrogen Receptor β or G Protein-Coupled Receptor 30 Activation on Anxiety-like Behaviors in Relation to GABAergic Transmission in Stress-Ovariectomized Rats. Neurosci. Lett. 2022, 789, 136885. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, N.; Guo, Y.; Zhao, R.; Shi, T.; Feng, S.; Wang, S.; Yang, Q.; Li, X.; Wu, Y.; et al. G-Protein-Coupled Receptor 30 Mediates Rapid Neuroprotective Effects of Estrogen via Depression of NR2B-Containing NMDA Receptors. J. Neurosci. 2012, 32, 4887–4900. [Google Scholar] [CrossRef] [PubMed]
- Moeini, R.; Shirafkan, H.; Gorji, N. Pomegranate Effects on the Health Aspects of Women during Peri- and Postmenopause: A Systematic Review and Meta-Analysis. Phytother. Res. 2024, 38, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Coyoy, A.; Guerra-Araiza, C.; Camacho-Arroyo, I. Metabolism Regulation by Estrogens and Their Receptors in the Central Nervous System Before and After Menopause. Horm. Metab. Res. 2016, 48, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, Y.; Jin, B.; Shu, J. Role of Estrogen in the Regulation of Central and Peripheral Energy Homeostasis: From a Menopausal Perspective. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231199359. [Google Scholar] [CrossRef] [PubMed]
- Bardhi, O.; Dubey, P.; Palmer, B.F.; Clegg, D.J. Oestrogens, Adipose Tissues and Environmental Exposures Influence Obesity and Diabetes across the Lifecycle. Proc. Nutr. Soc. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Lelong, M.; Bourgine, G.; Efstathiou, T.; Saligaut, C.; Pakdel, F. Assessment of the Potential Activity of Major Dietary Compounds as Selective Estrogen Receptor Modulators in Two Distinct Cell Models for Proliferation and Differentiation. Toxicol. Appl. Pharmacol. 2017, 325, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Zhuang, X.; Luan, F.; Zhao, C.; Cordeiro, M.N.D.S. Interaction of Coumarin Phytoestrogens with ERα and ERβ: A Molecular Dynamics Simulation Study. Molecules 2020, 25, 1165. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, L.; Rakus, J.; Bauer, C.; Gerner, C.; Ullmann, R.; Wimmer, H.; Huber, J. Pomegranate Seed Oil in Women with Menopausal Symptoms: A Prospective Randomized, Placebo-Controlled, Double-Blinded Trial. Menopause 2012, 19, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Mohamed, I.N. Pomegranate Use to Attenuate Bone Loss in Major Musculoskeletal Diseases: An Evidence-Based Review. Curr. Drug Targets 2013, 14, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Gminski, R.; Tang, T.; Weinert, T.; Schulz, S.; Linke-Cordes, M.; Martin, I.; Fischer, H. Pomegranate (Punica granatum) Seed Oil for Treating Menopausal Symptoms: An Individually Controlled Cohort Study. Altern. Ther. Health Med. 2017, 23, 28–34. [Google Scholar] [PubMed]
- Al-Jeborry, M.M.; Ameen, W.A. Treatment of Sweating, Hot Flushing and Sleep Disturbance in Peri and Post Menopausal Women with Oral Pometone. J. Univ. Babylon. Pure Appl. Sci. 2017, 25, 1793–1799. [Google Scholar]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A.S. Intake of Polyphenol-Rich Pomegranate Pure Juice Influences Urinary Glucocorticoids, Blood Pressure and Homeostasis Model Assessment of Insulin Resistance in Human Volunteers. J. Nutr. Sci. 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Seely, D.; Singh, R. Adaptogenic Potential of a Polyherbal Natural Health Product: Report on a Longitudinal Clinical Trial. Evid.-Based Complement. Altern. Med. 2007, 4, 275389. [Google Scholar] [CrossRef] [PubMed]
- López-Ríos, L.; Barber, M.A.; Wiebe, J.; Machín, R.P.; Vega-Morales, T.; Chirino, R. Influence of a New Botanical Combination on Quality of Life in Menopausal Spanish Women: Results of a Randomized, Placebo-Controlled Pilot Study. PLoS ONE 2021, 16, e0255015. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Murray, J.R.; Jorge, P.; Fogel, T.G.; Kim, M.; Wallace, D.R.; Hartman, R.E. Pomegranate Supplementation Improves Cognitive and Functional Recovery Following Ischemic Stroke: A Randomized Trial. Nutr. Neurosci. 2019, 22, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Bekku, N.; Yoshimura, H. Animal Model of Menopausal Depressive-like State in Female Mice: Prolongation of Immobility Time in the Forced Swimming Test Following Ovariectomy. Psychopharmacology 2005, 183, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Erika, E.-C.; López-Rubalcava, C. Can Animal Models Resemble a Premenstrual Dysphoric Condition? Front. Neuroendocr. 2022, 66, 101007. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Molendijk, M.L. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016, 2016, 6503162. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Forced Swim Stressor: Trends in Usage and Mechanistic Consideration. Eur. J. Neurosci. 2022, 55, 2813–2831. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Coping with the Forced Swim Stressor: Current State-of-the-Art. Behav. Brain Res. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Vega-Rivera, N.-M.; González-Trujano, M.E.; Luna-Angula, A.; Sánchez-Chapul, L.; Estrada-Camarena, E. Antidepressant-like Effects of the Punica granatum and Citalopram Combination Are Associated with Structural Changes in Dendritic Spines of Granule Cells in the Dentate Gyrus of Rats. Front. Pharmacol. 2023, 14, 1211663. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, Ellagic Acid and Their Derived Metabolites: A Review about Source, Metabolism, Functions and Health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Naveen, S.; Siddalingaswamy, M.; Singsit, D.; Khanum, F. Anti-Depressive Effect of Polyphenols and Omega-3 Fatty Acid from Pomegranate Peel and Flax Seed in Mice Exposed to Chronic Mild Stress. Psychiatry Clin. Neurosci. 2013, 67, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Lucki, I. Detection of Serotonergic and Noradrenergic Antidepressants in the Rat Forced Swimming Test: The Effects of Water Depth. Behav. Brain Res. 1996, 73, 43–46. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Rhodes, M.E.; Frye, C.A. Antidepressant Effects of ERbeta-Selective Estrogen Receptor Modulators in the Forced Swim Test. Pharmacol. Biochem. Behav. 2004, 78, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tao, J.; Xu, L.; Zhao, N.; Chen, J.; Chen, W.; Zhu, Y.; Qiu, J. Estradiol Decreases Rat Depressive Behavior by Estrogen Receptor Beta but Not Alpha: No Correlation with Plasma Corticosterone. Neuroreport 2014, 25, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Arroyo, L.D.; Frazer, A. Comparison of the Antidepressant-Like Effects of Estradiol and That of Selective Serotonin Reuptake Inhibitors in Middle-Aged Ovariectomized Rats. Front. Aging Neurosci. 2016, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C. Monoamine Oxidase Isoenzymes: Genes, Functions and Targets for Behavior and Cancer Therapy. J. Neural Transm. (Vienna) 2018, 125, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Zhao, J.; Yu, H.; Chen, X.; He, Y.; Tian, Y.; Wang, Y.; Chen, C.; Cheng, K.; et al. Neurotransmitter and Related Metabolic Profiling in the Nucleus Accumbens of Chronic Unpredictable Mild Stress-Induced Anhedonia-Like Rats. Front. Behav. Neurosci. 2022, 16, 862683. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Lappas, S.; Cheeta, S.; Muscat, R. Reversal of Stress-Induced Anhedonia by the Dopamine Receptor Agonist, Pramipexole. Psychopharmacology 1994, 115, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.-C.; Finkelstein, J.; Kim, S.-Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine Neurons Modulate Neural Encoding and Expression of Depression-Related Behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Woroń, J.; Wrzosek, A.; Gupało, J.; Chrobak, A.A. Harder, Better, Faster, Stronger? Retrospective Chart Review of Adverse Events of Interactions between Adaptogens and Antidepressant Drugs. Front. Pharmacol. 2023, 14, 1271776. [Google Scholar] [CrossRef] [PubMed]
- Rafieirad, M.; Abbaszadeh, H. Pomegranate Seed Extract Reduces Ischemia Induced Anxiety in Male Rats. J. Herbmed Pharmacol. 2017, 6, 85–89. [Google Scholar]
- Gadouche, L.; Djebli, N.; Khayra, Z. Algerian pomegranate peel decreases lead concentration in brain and improves neurological disorders. Pol. J. Nat. Sci. 2020, 35, 97–107. [Google Scholar]
- Sahebkar, A.; Simental-Mendía, L.E.; Giorgini, P.; Ferri, C.; Grassi, D. Lipid Profile Changes after Pomegranate Consumption: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytomedicine 2016, 23, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.; Rezaiian, F.; Goudarzi, K.; Nooshan Mirmohammadali, S.; Asbaghi, O.; Sadat Hosseini kolbadi, K.; Naderian, M.; Hosseini, A. The Effects of Pomegranate Consumption on Lipid Profile in Adults: A Systematic Review and Meta-Analysis. J. Funct. Foods 2023, 108, 105727. [Google Scholar] [CrossRef]
- Zare, H.; Amiri Ardekani, E.; Tavakoli, A.; Bradley, R.; Tavakoli, F.; Pasalar, M. Reporting of Adverse Effects of Pomegranate in Clinical Studies: A Systematic Review. J. Complement. Integr. Med. 2024, 21, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, K.; Bardees, R.; Alkhawaja, B.; Mallah, E.; AbuQatouseh, L.; Schmidt, M.; Matalka, K. Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review. Molecules 2023, 28, 2117. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.; Oleson, L.E.; Zhao, Y.; Harmatz, J.S.; Zinny, M.A.; Court, M.H.; Greenblatt, D.J. Pomegranate Juice Does Not Impair Clearance of Oral or Intravenous Midazolam, a Probe for Cytochrome P450-3A Activity: Comparison with Grapefruit Juice. J. Clin. Pharmacol. 2007, 47, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Abdlekawy, K.S.; Donia, A.M.; Elbarbry, F. Effects of Grapefruit and Pomegranate Juices on the Pharmacokinetic Properties of Dapoxetine and Midazolam in Healthy Subjects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, D.; Sahu, A.K.; Rathod, R.; Sengupta, P.; Kate, A.S. Investigation of the Impact of Grapefruit Juice, Pomegranate Juice and Tomato Juice on Pharmacokinetics of Brexpiprazole in Rats Using UHPLC-QTOF-MS. Biomed. Chromatogr. 2021, 35, e5201. [Google Scholar] [CrossRef] [PubMed]
- Alnaqeeb, M.; Mansor, K.A.; Mallah, E.M.; Ghanim, B.Y.; Idkaidek, N.; Qinna, N.A. Critical Pharmacokinetic and Pharmacodynamic Drug-Herb Interactions in Rats between Warfarin and Pomegranate Peel or Guava Leaves Extracts. BMC Complement. Altern. Med. 2019, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Alblooshi, S.; Taylor, M.; Gill, N. Does Menopause Elevate the Risk for Developing Depression and Anxiety? Results from a Systematic Review. Australas. Psychiatry 2023, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N. Perimenopause: From Research to Practice. J. Womens Health 2016, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Stute, P.; Lozza-Fiacco, S. Strategies to Cope with Stress and Anxiety during the Menopausal Transition. Maturitas 2022, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blümel, J.E.; Chedraui, P.; Aedo, S.; Fica, J.; Mezones-Holguín, E.; Barón, G.; Bencosme, A.; Benítez, Z.; Bravo, L.M.; Calle, A.; et al. Obesity and Its Relation to Depressive Symptoms and Sedentary Lifestyle in Middle-Aged Women. Maturitas 2015, 80, 100–105. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The Menopause Transition and Women’s Health at Midlife: A Progress Report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Zhao, R.; Zhang, S.; Yu, X.-Y.; Li, Y. The Influence of Sex on Cardiac Physiology and Cardiovascular Diseases. J. Cardiovasc. Transl. Res. 2020, 13, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Dulcich, M.S.; Hartman, R.E. Pomegranate Supplementation Improves Affective and Motor Behavior in Mice after Radiation Exposure. Evid. Based Complement. Altern. Med. 2013, 2013, 940830. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taweel, G.M.; Al-Mutary, M.G. Pomegranate Juice Moderates Anxiety- and Depression-like Behaviors in AlCl3-Treated Male Mice. J. Trace Elem. Med. Biol. 2021, 68, 126842. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bazán, T.; Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Negrete-Díaz, J.V.; German-Ponciano, L.J.; Olivo-Ramírez, D. Pomegranate (Punica granatum L.) and Its Phytochemicals as Anxiolytic; an Underreported Effect with Therapeutic Potential: A Systematic Review. Brain Res. 2023, 1820, 148554. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M. Chapter Seven—The Roles of Peroxisome Proliferator-Activated Receptors in the Metabolic Syndrome. In Progress in Molecular Biology and Translational Science; Tao, Y.-X., Ed.; Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 217–266. [Google Scholar]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR Gamma and Alpha by Punicic Acid Ameliorates Glucose Tolerance and Suppresses Obesity-Related Inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.W.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Anti-Diabetic Action of Punica granatum Flower Extract: Activation of PPAR-Gamma and Identification of an Active Component. Toxicol. Appl. Pharmacol. 2005, 207, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, R.; Abad, N.; Ghorbanzadeh, B.; Dolatshahi, M.; Mansouri, M.T. Simvastatin Exerts Antidepressant-like Activity in Mouse Forced Swimming Test: Role of NO-cGMP-KATP Channels Pathway and PPAR-Gamma Receptors. Pharmacol. Biochem. Behav. 2019, 180, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, X.; Yan, S.; Xie, X.; Fan, Y.; Zhang, J.; Peng, C.; You, Z. The Antidepressant-like Effects of Pioglitazone in a Chronic Mild Stress Mouse Model Are Associated with PPARγ-Mediated Alteration of Microglial Activation Phenotypes. J. Neuroinflammation 2016, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yin, J.; Wang, C.; Liao, J.; Liu, G.; Chen, L. Lack of Seipin in Neurons Results in Anxiety- and Depression-like Behaviors via down Regulation of PPARγ. Hum. Mol. Genet. 2014, 23, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Khan, R.A. Behavioral Effects of Citrus Limon and Punica granatum Combinations in Rats. Metab. Brain Dis. 2017, 32, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Khan, R. Effect of Punica granatum on Behavior in Rats. Afr. J. Pharm. Pharmacol. 2014, 8, 1118–1126. [Google Scholar]
- Hernández-Vázquez, L.; Cassani, J.; Heyerdahl-Viau, I.; Martínez-Casares, R.M.; Luna, H.; Dorantes-Barrón, A.M.; Arrieta-Báez, D.; Estrada-Reyes, R. Recovery of Naringin-Rich Flavonoid Extracts from Agroresidues with Anxiolytic- and Antidepressant-like Effects in Mice. Molecules 2022, 27, 8507. [Google Scholar] [CrossRef] [PubMed]
- Cameron, O.G. Anxious-Depressive Comorbidity: Effects on HPA Axis and CNS Noradrenergic Functions. Essent. Psychopharmacol. 2006, 7, 24–34. [Google Scholar] [PubMed]
- Arango-Dávila, C.A.; Rincón-Hoyos, H.G. Depressive Disorder, Anxiety Disorder and Chronic Pain: Multiple Manifestations of a Common Clinical and Pathophysiological Core. Rev. Colomb. Psiquiatr. (Engl. Ed.) 2018, 47, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Malek, Z.; Dara, S.M.; Jahromy, M.H. Antinociceptive Effects of Pomegranate (Punica granatum L.) Juice and Seed Extracts on Acute Corneal Pain in Mice. World J. Neurosci. 2014, 2014, 44976. [Google Scholar] [CrossRef]
- Sarker, M.; Das, S.C.; Saha, S.K.; Mahmud, Z.A.; Bachar, S.C. Analgesic and Anti-Inflammatory Activities of Flower Extracts of Punica granatum Linn. (Punicaceae). J. App Pharm. Sci. 2012, 2, 133–136. [Google Scholar] [CrossRef]
- Salwe, K.J.; Sachdev, D. Evaluation of antinociceptive and anti-inflammatory effect of the hydroalcoholic extracts of leaves and fruit peel of p. granatum in experimental animals. Asian J. Pharm. Clin. Res. 2014, 7, 137–141. [Google Scholar]
- Saad, L.B.; Hwi, K.K.; Quah, T. Evaluation of the Antinociceptive Effect of the Ethanolic Extract of Punica granatum. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 228–233. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.-T. Ellagitannin Metabolites, Urolithin A Glucuronide and Its Aglycone Urolithin A, Ameliorate TNF-α-Induced Inflammation and Associated Molecular Markers in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Solano, J.A.; Bautista, M.; Espinosa-Juárez, J.V.; Moreno-Rocha, L.A.; Betanzos-Cabrera, G.; Salanță, L.C.; De la O Arciniega, M.; Olvera-Hernández, E.G.; Jaramillo-Morales, O.A. Differential Antinociceptive Efficacy of Peel Extracts and Lyophilized Juices of Three Varieties of Mexican Pomegranate (Punica granatum L.) in the Formalin Test. Plants 2022, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Q.; Ye, Z.; Wang, E.; Zou, W.; Sun, Z.; He, Z.; Zhong, T.; Weng, Y.; Pan, Y. PPAR γ Prevents Neuropathic Pain by Down-Regulating CX3CR1 and Attenuating M1 Activation of Microglia in the Spinal Cord of Rats Using a Sciatic Chronic Constriction Injury Model. Front. Neurosci. 2021, 15, 620525. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential Therapeutic Effects of the Simultaneous Targeting of the Nrf2 and NF-κB Pathways in Diabetic Neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Liou, C.-J.; Shen, S.-C.; Hu, S.; Chao, J.C.-J.; Huang, C.-H.; Wu, S.-J. Punicalagin from Pomegranate Ameliorates TNF-α/IFN-γ-Induced Inflammatory Responses in HaCaT Cells via Regulation of SIRT1/STAT3 Axis and Nrf2/HO-1 Signaling Pathway. Int. Immunopharmacol. 2024, 130, 111665. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Imbesi, C.; Bitto, A.; Minutoli, L.; Squadrito, F.; D’Angelo, T.; Booz, C.; Pallio, G.; Irrera, N. Anti-Oxidant and Anti-Inflammatory Effects of Ellagic and Punicic Acid in an in Vitro Model of Cardiac Fibrosis. Biomed. Pharmacother. 2023, 162, 114666. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis by Plants and Phytonutrients: A Systematic Review of Human Trials. Nutr. Neurosci. 2022, 25, 1704–1730. [Google Scholar] [CrossRef] [PubMed]
- Slowik, A.; Lammerding, L.; Hoffmann, S.; Beyer, C. Brain Inflammasomes in Stroke and Depressive Disorders: Regulation by Oestrogen. J. Neuroendocr. 2018, 30, e12482. [Google Scholar] [CrossRef]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Rantamäki, T.; Yalcin, I. Antidepressant Drug Action--From Rapid Changes on Network Function to Network Rewiring. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 285–292. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Estrada-Camarena, E.; Azpilcueta-Morales, G.; Cervantes-Anaya, N.; Treviño, S.; Becerril-Villanueva, E.; López-Rubalcava, C. Chronic Variable Stress and Cafeteria Diet Combination Exacerbate Microglia and C-Fos Activation but Not Experimental Anxiety or Depression in a Menopause Model. Int. J. Mol. Sci. 2024, 25, 1455. [Google Scholar] [CrossRef] [PubMed]
- Grès, S.; Gomez-Zorita, S.; Gomez-Ruiz, A.; Carpéné, C. 5-Hydroxytryptamine Actions in Adipocytes: Involvement of Monoamine Oxidase-Dependent Oxidation and Subsequent PPARγ Activation. J. Neural Transm. 2013, 120, 919–926. [Google Scholar] [CrossRef]
- Wnuk, A.; Rzemieniec, J.; Lasoń, W.; Krzeptowski, W.; Kajta, M. Apoptosis Induced by the UV Filter Benzophenone-3 in Mouse Neuronal Cells Is Mediated via Attenuation of Erα/Pparγ and Stimulation of Erβ/Gpr30 Signaling. Mol. Neurobiol. 2018, 55, 2362–2383. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, E.-J.; Choi, Y.Y.; Hong, J.; Yang, W.M. Lycium Chinense Improves Post-Menopausal Obesity via Regulation of PPAR-γ and Estrogen Receptor-α/β Expressions. Am. J. Chin. Med. 2017, 45, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Wagner, H. Plant Adaptogens. III. Earlier and More Recent Aspects and Concepts on Their Mode of Action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Understanding Adaptogenic Activity: Specificity of the Pharmacological Action of Adaptogens and Other Phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Dose | Sample | Outcomes | Type of Study | Effect | Reference |
|---|---|---|---|---|---|---|
| Pomegranate formulation | 77.82 mg/12 w | 100 (51, 49), from 45 to 60 y, healthy women | Menopause rating scale | RCT | Improved vegetative symptoms vs. placebo group. Overall, MRS was not significant. | [55] |
| Pomegranate formulation | 500 mg/twice a day/8 w | 78 from 45 to 65 y, healthy women | Menopause rating scale | Pre–post | Sleep disturbances and hot flashes decreased. Other symptoms were not reported individually. | [57] |
| Pomegranate formulation | 500 mg/twice a day/4 w | 44, from 40 to 65 y, healthy women | Menopause rating scale | Pre–post | Sleep disturbances and hot flashes decreased. | [58] |
| Pomegranate juice | 500 mL/once a day/4 w | 28, from 40 to 65 y, healthy women | Glucocorticoid levels, insulin, and blood pressure | Pre–post | Decreased ratio of cortisol/cortisone urine and saliva, insulin, and blood pressure. | [59] |
| OCTA® W. somnifera, Lagerstroemiaspeciosa, Bacopamonniera, Zizyphusjujuba, Morindacitrifolia, Punicagranatum, Shisandraechinensis and Lyciumbarbarum. | 30 mL/once a day/3 months | 17 (10 females), healthy participants | Quality of life, perceived stress, state/trait anxiety, and depression | Pre–post | Improvement in all scales from 40 to 50%. | [60] |
| Isoflavane pomegranate | Aframomummelegueta (100 mg) + Punicagranatum (100 mg)/250 mg/once a day/8 w | 34 (17–17) from 45 to 65 y, healthy women | Global health related to the quality-of-life score according to the Cervantes scale | RCT | Global quality of life, according to the Cervantes Scale, improved. No significant effect on psychic domains. | [61] |
| Pomegranate pills | 1 g/once a day/2 w | 11 (6–5), 2 females), 58 y, post-stroke participants | Mini-Mental State Examination, Repeatable Battery for the Assessment of Neuropsychological Status | CT | Neuropsychological status improved, state and trait anxiety (10%), Beck score decreased, and self-care increased (15%). | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Camerena, E.; López-Rubalcava, C.; Vega-Rivera, N.M.; González-Trujano, M.E. Antidepressant- and Anxiolytic-like Effects of Pomegranate: Is It Acting by Common or Well-Known Mechanisms of Action? Plants 2024, 13, 2205. https://doi.org/10.3390/plants13162205
Estrada-Camerena E, López-Rubalcava C, Vega-Rivera NM, González-Trujano ME. Antidepressant- and Anxiolytic-like Effects of Pomegranate: Is It Acting by Common or Well-Known Mechanisms of Action? Plants. 2024; 13(16):2205. https://doi.org/10.3390/plants13162205
Chicago/Turabian StyleEstrada-Camerena, Erika, Carolina López-Rubalcava, Nelly Maritza Vega-Rivera, and María Eva González-Trujano. 2024. "Antidepressant- and Anxiolytic-like Effects of Pomegranate: Is It Acting by Common or Well-Known Mechanisms of Action?" Plants 13, no. 16: 2205. https://doi.org/10.3390/plants13162205






