A Link between Species Abundance and Plant Strategies for Semi-Natural Dry Grasslands
Abstract
1. Introduction
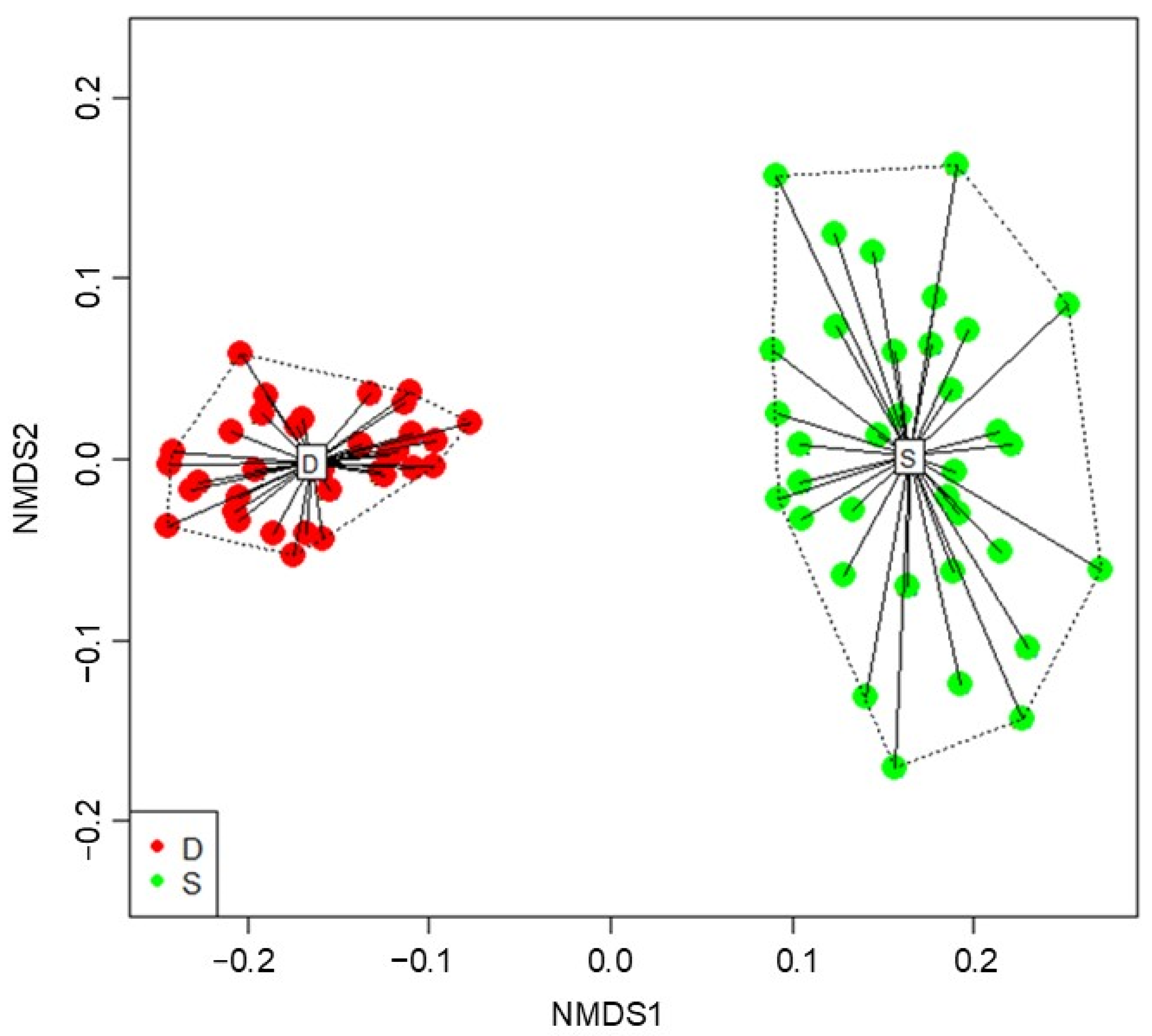
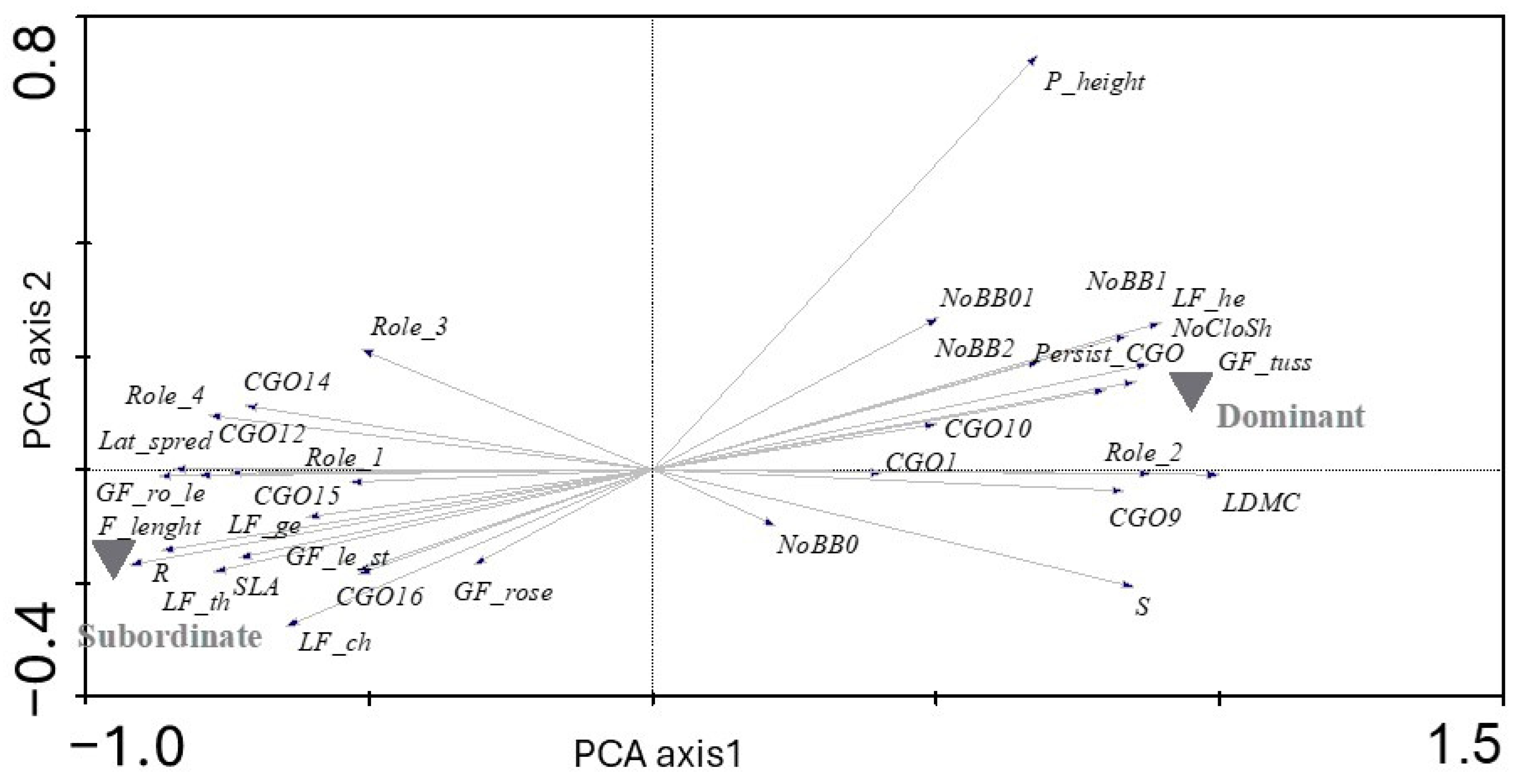
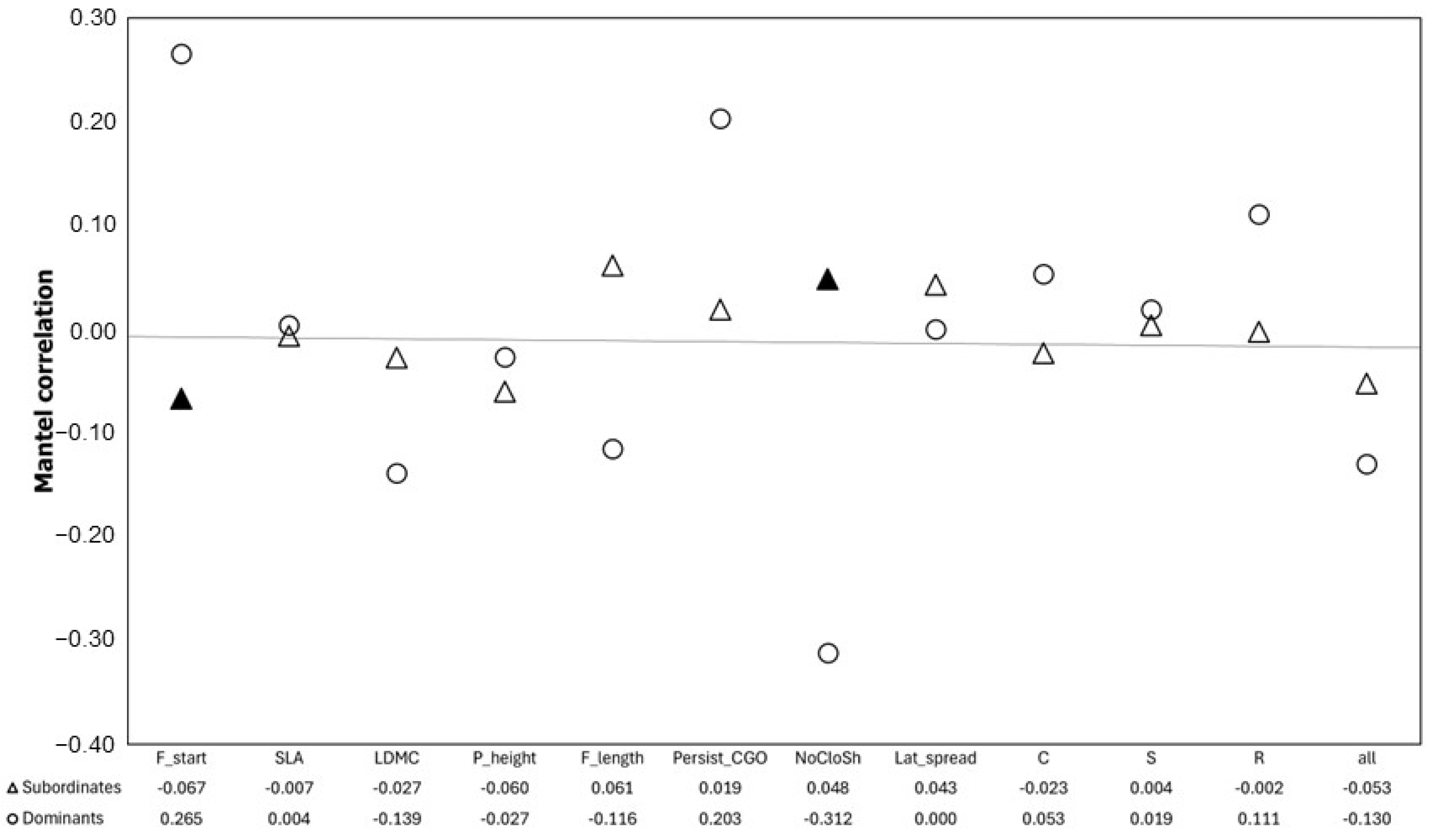
2. Results
3. Discussion
4. Materials and Methods
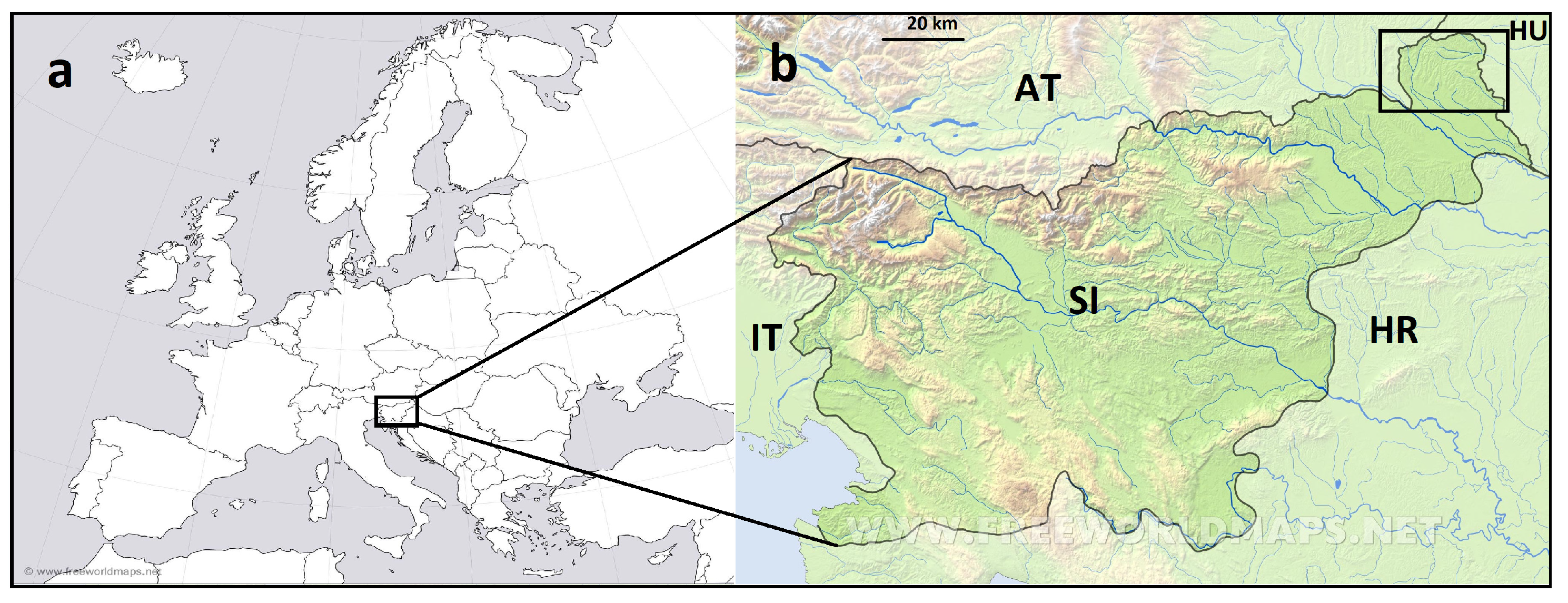
4.1. Study Area and Field Methods
4.2. Selected Plant Functional Traits
4.3. Functional Trait Indices
4.4. Data Analysis
5. Conclusions and Conservation Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Fr (%) |
|---|---|
| Festuca rupicola d | 100 1−4 |
| Luzula campestris | 97 +2 |
| Briza media d | 91 +4 |
| Ranunculus bulbosus | 91 +2 |
| Leontodon hispidus subsp. hastilis | 86 +2 |
| Hieracium bauhinia d | 83 +3 |
| Anthoxanthum odoratum d | 83 +3 |
| Dianthus deltoides | 83 +2 |
| Achillea millefolium | 80 +1 |
| Lotus corniculatus | 80 +1 |
| Trifolium pratense | 80 +2 |
| Centaurea jacea d | 77 +3 |
| Thymus pulegioides | 77 +2 |
| Festuca rubra d | 74 +3 |
| Dianthus armeria | 74 +1 |
| Plantago lanceolata | 71 +2 |
| Hieracium pilosella | 69 +2 |
| Leucanthemum vulgare agg. | 69 +1 |
| Trisetum flavescens | 69 +1 |
| Holcus lanatus | 66 +2 |
| Pimpinella saxifraga | 66 +1 |
| Carex caryophyllea | 63 +1 |
| Galium verum | 60 +1 |
| Tragopogon orientalis | 60 +1 |
| Arrhenatherum elatius | 57 +1 |
| Campanula patula | 57 +1 |
| Euphorbia cyparissias | 57 +2 |
| Danthonia decumbens | 54 +2 |
| Peucedanum oreoselinum d | 51 +3 |
| Orchis morio | 51 +1 |
| Polygala comosa | 51 +2 |
| Agrostis tenuis | 49 +2 |
| Moenchia mantica subsp. mantica | 49 +2 |
| Rhinanthus minor | 49 +2 |
| Avenochloa pubescens | 46 +2 |
| Daucus carota | 46 +1 |
| Hypochoeris radicata | 46 +1 |
| Betonica officinalis | 43 +2 |
| Rumex acetosella | 43 +1 |
| Plantago media | 40 +1 |
| Potentilla erecta | 40 +1 |
| Prunella laciniata | 40 +1 |
| Trifolium repens | 37 +1 |
| Carex pallescens | 31 +1 |
| Trifolium campestre | 31 +1 |
| Silene nutans | 29 +1 |
| Carlina acaulis | 26 +1 |
| Cynosurus cristatus | 26 +1 |
| Helianthemum ovatum | 26 +2 |
| Polygala vulgaris | 26 +1 |
| Sanguisorba officinalis | 26 +1 |
| Sedum sexangulare | 26 +2 |
| Linum catharticum | 23 +1 |
| Knautia drymeia | 20 +1 |
| Poa angustifolia | 20 +1 |
| Potentilla reptans | 20 +1 |
| Scabiosa triandra | 20 +1 |
| Thesium linophyllon | 20 +2 |
| Lychnis viscaria | 17 +2 |
| Carlina vulgaris | 11 +1 |
| Hieracium umbellatum | 11 +1 |
| Dianthus carthusianorum d | 9 13 |
| Chamaecytisus supinus | 9 +1 |
| Chamaespartium sagittale | 9 +2 |
| Ornithogalum umbellatum | 9 +1 |
| Sanguisorba minor | 9 +1 |
| Filipendula vulgaris | 6 +1 |
| Peucedanum cervaria | 6 12 |
| Verbascum austriacum | 6 1 |
Appendix B
| Plant Functional Traits (PFTs) | Abbreviation of Specific PFT | CWM | FD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D | S | p | Sig. | D | S | p | Sig. | ||
| Life form | LF_ch | 0 | 0.07 | *** | <0.001 | 0.000 | 0.317 | *** | <0.001 |
| LF_ge | 0 | 0.03 | *** | <0.001 | |||||
| LF_he | 1 | 0.81 | *** | <0.001 | |||||
| LF_te | 0 | 0.09 | *** | <0.001 | |||||
| Growth form | GF_tuss | 0.66 | 0.25 | *** | <0.001 | 0.512 | 0.719 | *** | <0.001 |
| GF_rose | 0.12 | 0.16 | ** | 0.035 | |||||
| GF_le_st | 0.21 | 0.35 | *** | <0.001 | |||||
| GF_ro_le | 0.01 | 0.24 | *** | <0.001 | |||||
| Plant height | P_height | 42.97 | 32.75 | *** | <0.001 | 0.210 | 0.218 | n.s. | 0.465 |
| Specific leaf area | SLA | 17.69 | 21.36 | *** | <0.001 | 0.189 | 0.169 | n.s. | 0.065 |
| Leaf dry matter content | LDMC | 350.77 | 250.31 | *** | <0.001 | 0.195 | 0.214 | *** | 0.001 |
| Flowering start | F_start | 140.75 | 140.15 | n.s. | 0.700 | 0.401 | 0.456 | *** | <0.001 |
| Flowering length | F_length | 2.95 | 3.91 | *** | <0.001 | ||||
| Type of clonal plant organ | CGO1 | 0.12 | 0.06 | *** | <0.001 | 0.639 | 0.700 | *** | <0.001 |
| CGO9 | 0.46 | 0.22 | *** | <0.001 | |||||
| CGO10 | 0.31 | 0.22 | *** | <0.001 | |||||
| CGO12 | 0 | 0.04 | *** | <0.001 | |||||
| CGO13 | 0 | 0.00 | n.s. | 0.112 | |||||
| CGO14 | 0.10 | 0.28 | *** | <0.001 | |||||
| CGO15 | 0 | 0.06 | *** | <0.001 | |||||
| CGO16 | 0 | 0.02 | *** | <0.001 | |||||
| Persistence of connection in CGO | Persist_CGO | 3.69 | 3.04 | *** | <0.001 | 0.147 | 0.362 | *** | <0.001 |
| Number of clonal offspring shoots | NoCloSh | 2.31 | 1.87 | *** | <0.001 | 0.219 | 0.344 | *** | <0.001 |
| Lateral spreading distance by clonal growth | Lat_spread | 1 | 1.23 | *** | <0.001 | 0.000 | 0.245 | *** | <0.001 |
| Role of clonal growth organs | Role_1 | 0 | 0.02 | *** | <0.001 | 0.139 | 0.471 | *** | <0.001 |
| Role_2 | 0.91 | 0.60 | *** | <0.001 | |||||
| Role_3 | 0 | 0.01 | *** | <0.001 | |||||
| Role_4 | 0.09 | 0.28 | *** | <0.001 | |||||
| Bud bank of the plant | NoBB1 | 5 | 3.53 | *** | <0.001 | 0.000 | 0.143 | *** | <0.001 |
| NoBB2 | 5 | 4.35 | *** | <0.001 | 0.000 | 0.135 | *** | <0.001 | |
| NoBB0 | 6.24 | 5.86 | ** | 0.033 | 0.157 | 0.199 | ** | 0.006 | |
| NoBB01 | 12.55 | 11.10 | *** | <0.001 | 0.275 | 0.367 | *** | <0.001 | |
| CSR strategy | C | 0.38 | 0.38 | n.s. | 0.996 | 0.219 | 0.224 | n.s. | 0.552 |
| S | 0.41 | 0.30 | *** | <0.001 | 0.195 | 0.317 | *** | <0.001 | |
| R | 0.21 | 0.33 | *** | <0.001 | 0.117 | 0.228 | *** | <0.001 | |
References
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S.; Grigulis, K. How fundamental plant functional trait relationships scale-up to trade-offs and synergies in ecosystem services. J. Ecol. 2012, 100, 128–140. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Brigham, C.A.; Hoeksema, J.D.; Lyons, K.G.; Mills, M.H.; VanMantgem, P.J. Linking biodiversity to ecosystem function: Implications for conservation ecology. Oecology 2000, 122, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Sachs, J.D.; Baillie, J.E.M.; Sutherland, W.J.; Armsworth, P.R.; Beddington, J.; Blackburn, et.al. Biodiversity conservation and the millennium development goals. Science 2009, 325, 1502–1503. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Vitousek, P.M. The effects of plant composition and diversity on ecosystem processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Lawler, S.P.; Armesto, J.J.; Kareiva, P. How relevant to conservation are studies linking biodiversity and ecosystem functioning? In The Functional Consequences of Biodiversity; Kinzing, A.P., Pacala, S.W., Tilman, D., Eds.; Princeton University Press: Princeton, NJ, USA, 2001; pp. 294–313. [Google Scholar]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jousseau, C. Effects of biodiversity on the functioning of trophic group sand ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Orwin, K.H.; Ostle, N.; Wilby, A.; Bardgett, R.D. Effects of species evenness and dominant species identity on multiple ecosystem functions in model grassland communities. Oecologia 2014, 174, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.H.; Rosenberg, R. A comparative study of the effects on the marine environment of wastes from cellulose industries in Scotland and Sweden. Ambio 1976, 5, 77–79. [Google Scholar]
- Vitousek, P.M.; Melillo, J.M. Nitrate losses from disturbed forests: Patterns and mechanisms. For. Sci. 1979, 25, 605–619. [Google Scholar] [CrossRef]
- Allan, E.; Weisser, W.; Weigelt, A.; Roscher, C.; Fischer, M.; Hillebrand, H. More diverse plant communities have higher functioning over time due to turnover in complementary dominant species. Proc. Natl. Acad. Sci. USA 2011, 108, 17034–17039. [Google Scholar] [CrossRef]
- Mouillot, D.; Bellwood, D.R.; Baraloto, C.; Chave, J.; Galzin, R.; Harmelin-Vivien, M.; Kulbicki, M.; Lavergne, S.; Lavorel, S.; Mouquet, N.; et al. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 2013, 11, e1001569. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Torrez, V.; Mergeay, J.; Meester, L.D.; Honnay, O.; Helsen, K. Differential effects of dominant and subordinate plant species on the establishment success of target species in a grassland restoration experiment. Appl. Veg. Sci. 2017, 20, 363–375. [Google Scholar] [CrossRef]
- Arnillas, C.A.; Borer, E.T.; Seabloom, E.W.; Alberti, J.; Baez, S.; Bakker, J.D.; Boughton, E.H.; Buckley, Y.M.; Bugalho, M.N.; Donohue, I.; et al. Opposing community assembly patterns for dominant and nondominant plant species in herbaceous ecosystems globally. Ecol. Evol. 2021, 11, 17744–17761. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.L.; Antia, A.; McKone, M.J. The ecosystem impacts of dominant species exclusion in a prairie restoration. Ecol. Appl. 2022, 32, e2592. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: Astochastic theory of resource competition, invasion, and community assembly. Proc. Nat. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Reader, R.J. Relationship between species relative abundance and plant traits for an infertile habitat. Plant Ecol. 1998, 134, 43–51. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. The assembly of experimental wetland plant communities. Oikos 1995, 73, 323–335. [Google Scholar] [CrossRef]
- Fantinato, E.; Del Vecchio, S.; Slaviero, A.; Conti, L.; Acosta, A.T.R.; Buffa, G. Does flowering synchrony contribute to the sustainment of dry grassland biodiversity? Flora 2016, 222, 96–103. [Google Scholar] [CrossRef]
- Wellstein, C.; Campetella, G.; Spada, F.; Chelli, S.; Mucina, L.; Canullo, R.; Bartha, S. Context-dependent assembly rules and the role of dominating grasses in semi-natural abandoned sub-Mediterranean grasslands. Agric. Ecosyst. Environ. 2014, 182, 113–122. [Google Scholar] [CrossRef]
- Richardson, S.J.; Williams, P.A.; Mason, N.W.; Buxton, R.P.; Courtney, S.P.; Rance, B.D.; Clarkson, B.R.; Hoare, R.J.B.; St. John, M.G.; Wiser, S.K. Rare species drive local trait diversity in two geographically disjunct examples of a naturally rare alpine ecosystem in New Zealand. J. Veg. Sci. 2012, 23, 626–639. [Google Scholar] [CrossRef]
- Mariotte, P.; Vandenberghe, C.; Kardol, P.; Hagedorn, F.; Buttler, A. Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J. Ecol. 2013, 101, 763–773. [Google Scholar] [CrossRef]
- Smit, H.J.; Metzger, M.J.; Ewert, F. Spatial distribution of grassland productivity and land use in Europe. Agric. Syst. 2008, 98, 208–219. [Google Scholar] [CrossRef]
- Van Helsdingen, P.J.; Willemse, L.; Speight, M.C.D. Background Information on Invertebrates of the Habitats Directive and the Bern Convention; Part 1: Crustacea, Coleoptera and Lepidoptera; Nature and Environment; Council of Europe Publishing: Strasbourg, France, 1996; Volume 79. [Google Scholar]
- Ssymank, A.; Hauke, U.; Rückriem, C.; Schröder, E. Das europäischeschutzgebietssystem NATURA 2000–BfN Handbuch zur umsetzung derfauna-flora-habitat-richtlinie und der vogelschutz-richtlinie. Schriftenr. Landschaftspfl. Natursch. 1998, 53, 1–560. [Google Scholar]
- Martinčič, A. Mala Flora Slovenije [Slovenian Flora]; Tehniška Založba Slovenije: Ljubljana, Slovenia, 2007. [Google Scholar]
- Kleyer, M.; Bekker, R.M.; Knevely, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Klimešová, J.; Klimeš, L. CLO-PLA3: A Database of Clonal Growth Architecture of Central-European Plants. 2006. Available online: http://clopla.butbn.cas.cz (accessed on 30 June 2024).
- Klimešová, J.; de Bello, F. CLO-PLA: The database of clonal and bud bank traits of Central European flora. J. Veg. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Wilson, P.J.; Hunt, R.; Grime, J.P.; Thompson, K. Allocating C-S-R plant functional types: A soft approach to a hard problem. Oikos 1999, 85, 282–294. [Google Scholar] [CrossRef]
- Škornik, S. Suha travišča reda Brometalia erecti Koch 1926 na Goričkem (SV Slovenija). Hacquetia 2003, 2, 71–90. [Google Scholar]
- Prach, K.; Pyšek, P. How do species dominating in succession differ from others? J. Veg. Sci. 1999, 10, 383–392. [Google Scholar] [CrossRef]
- Falinska, K. Plant Demography in Vegetation Succession; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Schöb, C.; Butterfield, B.J.; Pugnaire, F.I. Foundation species influence trait-based community assembly. New Phytol. 2012, 196, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Ryser, P.; Urbas, P. Ecological significance of leaf life span among Central European grass species. Oikos 2000, 91, 41–50. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Austral. J. Botany 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Grime, J.P.; Wilson, P.J.; Thompson, K.; Band, S.R. The impacts of agricultural change (1963–2003) on the grassland flora of Central England: Processes and prospects. Basic Appl. Ecol. 2005, 6, 107–118. [Google Scholar] [CrossRef]
- Martínková, J.; Klimeš, A.; Puy, J.; Klimešová, J. Response of clonal versus non-clonal herbs to disturbance: Different strategies revealed. Perspect. Plant Ecol. 2020, 44, 125529. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; Wiley: Chichester, UK, 2001. [Google Scholar]
- Paušič, I.; Kaligarič, M.; Bakan, B. Late seasonal mowing enhances central European Spiranthes spiralis (L.) Chevall. (Orchidaceae) population viability. Bot. Lett. 2017, 164, 401–412. [Google Scholar] [CrossRef]
- Šorgo, A.; Špur, N.; Škornik, S. Public attitudes and opinions as dimensions of efficient management with extensive meadows in Natura 2000 area. J. Environ. Manag. 2016, 183, 637–646. [Google Scholar] [CrossRef]
- Kaligarič, M.; Škornik, S. Variety of dry and semi-dry secondary grasslands (Festuco-Brometea) in Slovenia-contact area of different geoelements. Slovenska akademija znanosti in umetnosti. Razprave 2002, 43, 227–246. [Google Scholar]
- Klimešová, J.; Martínková, J.; Pausas, J.G.; de Moraes, M.G.; Herben, T.; Yu, F.-H.; Puntieri, J.; Vesk, P.A.; de Bello, F.; Janeček, Š.; et al. Handbook of standardized protocols for collecting plant modularity traits. Perspect. Plant Ecol. 2019, 40, 125485. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. The advantages of clonal integration under different ecological conditions: A community-wide test. Ecology 2000, 81, 709–716. [Google Scholar] [CrossRef]
- Gao, Y.; Xing, F.; Jin, Y.J.; Nie, D.D.; Wang, Y. Foraging responses of clonal plants to multi-patch environmental heterogeneity: Spatial preference and temporal reversibility. Plant Soil 2012, 359, 137–147. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Ottaviani, G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Kurokawa, H.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Nakashizuka, T. Relationships between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Funct. Ecol. 2016, 30, 1205–1215. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Grime, J.P. Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar] [CrossRef]
- Grubb, P.J. Problems posed by sparse and patchily distributed species in species-rich plant communities. In Community Ecology; Diamond, J.J., Case, T.J., Eds.; Harper & Row Publishers: New York, NY, USA, 1986; pp. 207–225. [Google Scholar]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Pipenbaher, N.; Kaligarič, M.; Mason, N.W.; Škornik, S. Dry calcareous grasslands from two neighboring biogeographic regions: Relationship between plant traits and rarity. Biodivers. Conserv. 2013, 22, 2207–2221. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Paušič, I.; Ivajnšič, D.; Kaligarič, M.; Pipenbaher, N. Relation Between Plant Species Diversity and Landscape Variables in Central-European Dry Grassland Fragments and Their Successional Derivates. Acta Bot. Croat. 2017, 76, 111–119. [Google Scholar] [CrossRef]
- Crimmins, T.M.; Crimmins, M.A.; Bertelsen, C.D. Onset of summer floweringin a ‘Sky Island’ is driven by monsoon moisture. New Phytol. 2011, 191, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.A.; Torezan-Silingardi, H.M.; Del-Claro, K. Conditional outcomes in ant–plant–herbivore interactions influenced by sequential flowering. Flora 2014, 209, 359–366. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M.T. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer Science & Business Media: New York, NY, USA, 2003. [Google Scholar]
- Stubbs, W.J.; Wilson, J.B. Evidence for limiting similarity in a sand dune community. J. Ecol. 2004, 92, 557–567. [Google Scholar] [CrossRef]
- Mason, N.W.H.; de Bello, F.; Dolezal, J.; Lepš, J. Niche overlap reveals the effects of competition, disturbance and contrasting assembly processes in experimental grassland communities. J. Ecol. 2011, 99, 788–796. [Google Scholar] [CrossRef]
- Laland, K.; Matthews, B.; Feldman, M.W. An introduction to niche construction theory. Evol. Ecol. 2016, 30, 191–202. [Google Scholar] [CrossRef]
- Aarssen, L.W.; Schamp, B.S.; Pither, J. Why are there so many small plants? Implications for species coexistence. J. Ecol. 2006, 94, 569–580. [Google Scholar] [CrossRef]
- Rabinowitz, D.; Rapp, J.K.; Dixon, P.M. Competitive abilities of sparse grass species: Means of persistence or cause of abundance. Ecology 1984, 65, 1144–1154. [Google Scholar] [CrossRef]
- Ivajnšič, D.; Cousins, S.A.; Kaligarič, M. Colonization by Robinia pseudoacacia of various soil and habitat types outside woodlands in a traditional Central-European agricultural landscape. Pol. J. Ecol. 2012, 60, 301–309. [Google Scholar]
- Kaligarič, M.; Sedonja, J.; Šajna, N. Traditional agricultural landscape in Goričko Landscape Park (Slovenia): Distribution and variety of riparian stream corridors and patches. Landsc. Urban Plan. 2008, 85, 71–78. [Google Scholar] [CrossRef]
- ARSO, Ministry of the Environment and Spatial Planning of the Republic of Slovenia, Slovenian Environment Agency, 30-Year Climate Data, Veliki Dolenci. 2014. Available online: http://www.arso.gov.si/vreme/napovedi%20in%20podatki/veliki_dolenci.htm (accessed on 9 January 2018).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Zweite, umgearbeitete und vermehrte Auflage; Springer: Wien, Austria, 1964. [Google Scholar]
- Šilc, U.; Čarni, A. Conspectus of vegetation syntaxa in Slovenia. Hacquetia 2012, 11, 113–164. [Google Scholar] [CrossRef]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Lepš, J.; de Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical considerations matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quetier, F.; Thebault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Moretti, M.; de Bello, F.; Roberts, S.P.; Pottsc, S.G. Taxonomical vs. functional responses of bee communities to fi re in two contrasting climatic regions. J. Anim. Ecol. 2009, 78, 98–108. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. Assessing the functional turnover of species assemblages with tailored dissimilarity matrices. Oikos 2010, 119, 1089–1098. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, H.H. Vegan: Community Ecology Package. 2006. Available online: https://cran.r-project.org/ (accessed on 25 June 2024).
- Deepayan, S. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: http://www.R-project.org (accessed on 9 January 2018).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin, Germany, 2002. [Google Scholar]
- Goodall, D.W. Objective methods for the classification of vegetation. III. An essay in the use of factor analysis. Aust. J. Bot. 1954, 2, 304–324. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination [CD-ROM]; Version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
| Plant Functional Traits (PFTs) | Abbreviation and Description | Data Source | |
|---|---|---|---|
| Life form | cat | LF_ch—chamaephytes; LF_ge—geophytes; LF_he—hemicryptophytes; LF_th—therophytes; | [29] |
| Growth form | cat | GF_tuss—tussocks; GF_rose—rosette; GF_le_st—leafy stem; GF_ro_le—rosette and leafy stem; | [29] |
| Plant height | cont | P_height (cm) | Own measurements |
| Specific leaf area | cont | SLA (mm2/mg) | Own measurements; LEDA database [30] |
| Leaf dry matter content | cont | LDMC (mg/g) | Own measurements; LEDA database [30] |
| Flowering start | circ | F_start (°) | [29] |
| Flowering length | cont | F_length (months) | [29] |
| Type of clonal growth organ | cat | CGO1—stolon CGO9—epigeogenous rhizome CGO10—hypogeogenous rhizome CGO12—belowground stem tuber CGO13—bulb CGO14—root splitters CGO15—root with adventitious buds CGO16—root tuber | CLO-PLA3 database [31,32] |
| Persistence of connection in CGO | cont | Persist_CGO (year) | CLO-PLA3 database [31,32] |
| Number of clonal offspring shoots | cont | NoCloSh | CLO-PLA3 database [31,32] |
| Lateral spreading distance by clonal growth | cont | Lat_spread (m) | CLO-PLA3 database [31,32] |
| Role of clonal growth organs | cat | Role_1—additive Role_2—necessary Role_3—regenerative Role_4—none | CLO-PLA3 database [31,32] |
| Bud bank of the plant | cat | NoBB1—bud bank on the plant higher than 10 cm NoBB2—bud bank on the plant 10 to 0 cm NoBB0—bud bank at the soil surface NoBB01—bud bank at a depth of 0 to 10 cm | CLO-PLA3 database [31,32] |
| CSR strategy | cont | C—competitors S—stress tolerators R—ruderals | Own measurements of PFT for CSR determination: plant height, LDMC, flowering length, flowering start, lateral spread, leaf dry mass and SLA (protocol by Hodgson et al. [33]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škornik, S.; Pipenbaher, N. A Link between Species Abundance and Plant Strategies for Semi-Natural Dry Grasslands. Plants 2024, 13, 2260. https://doi.org/10.3390/plants13162260
Škornik S, Pipenbaher N. A Link between Species Abundance and Plant Strategies for Semi-Natural Dry Grasslands. Plants. 2024; 13(16):2260. https://doi.org/10.3390/plants13162260
Chicago/Turabian StyleŠkornik, Sonja, and Nataša Pipenbaher. 2024. "A Link between Species Abundance and Plant Strategies for Semi-Natural Dry Grasslands" Plants 13, no. 16: 2260. https://doi.org/10.3390/plants13162260
APA StyleŠkornik, S., & Pipenbaher, N. (2024). A Link between Species Abundance and Plant Strategies for Semi-Natural Dry Grasslands. Plants, 13(16), 2260. https://doi.org/10.3390/plants13162260


.png)



