Contribution of Litter and Root to Soil Nutrients in Different Rocky Desertification Grasslands in a Karst Area
Abstract
:1. Introduction
2. Results
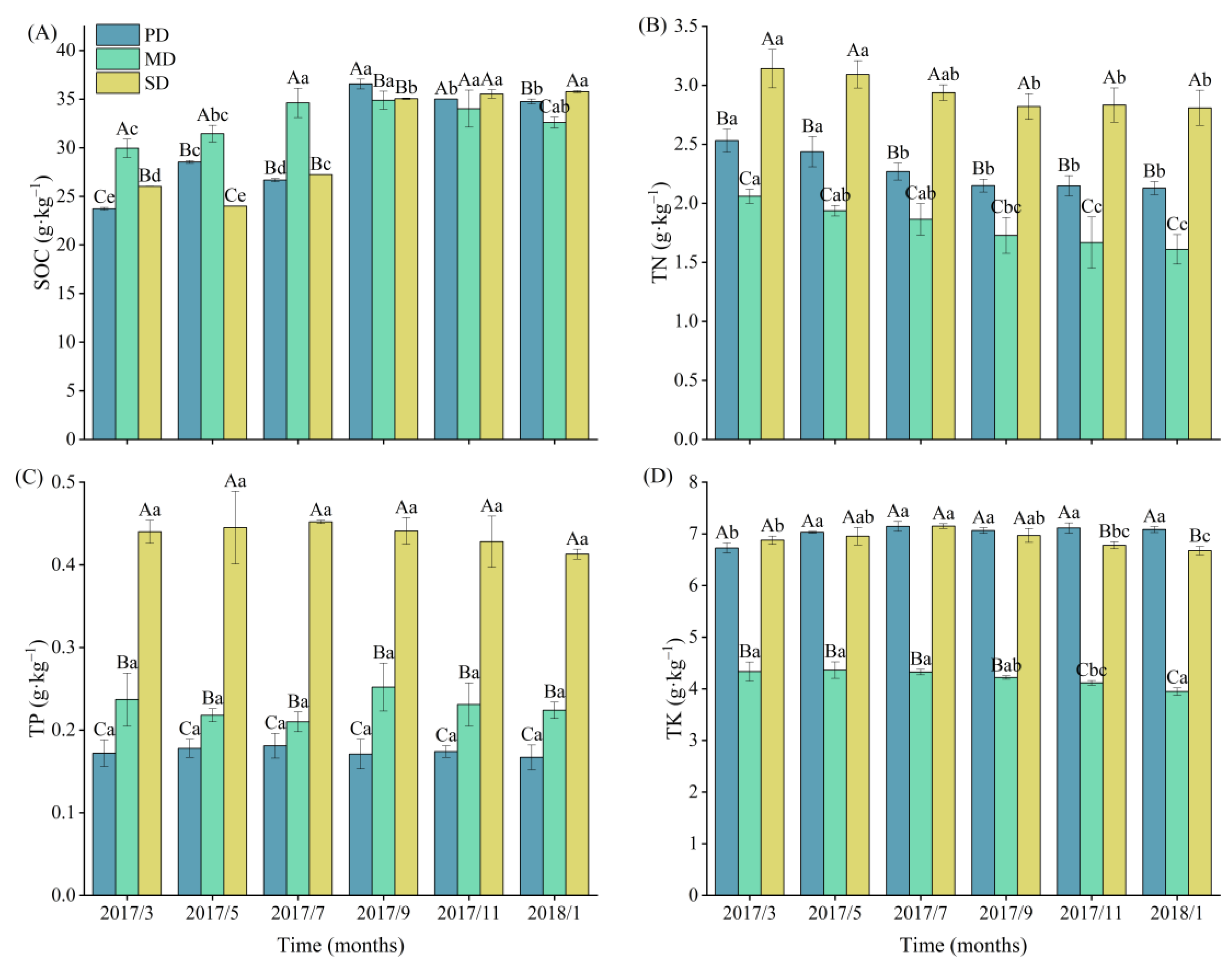
2.1. Soil Nutrients
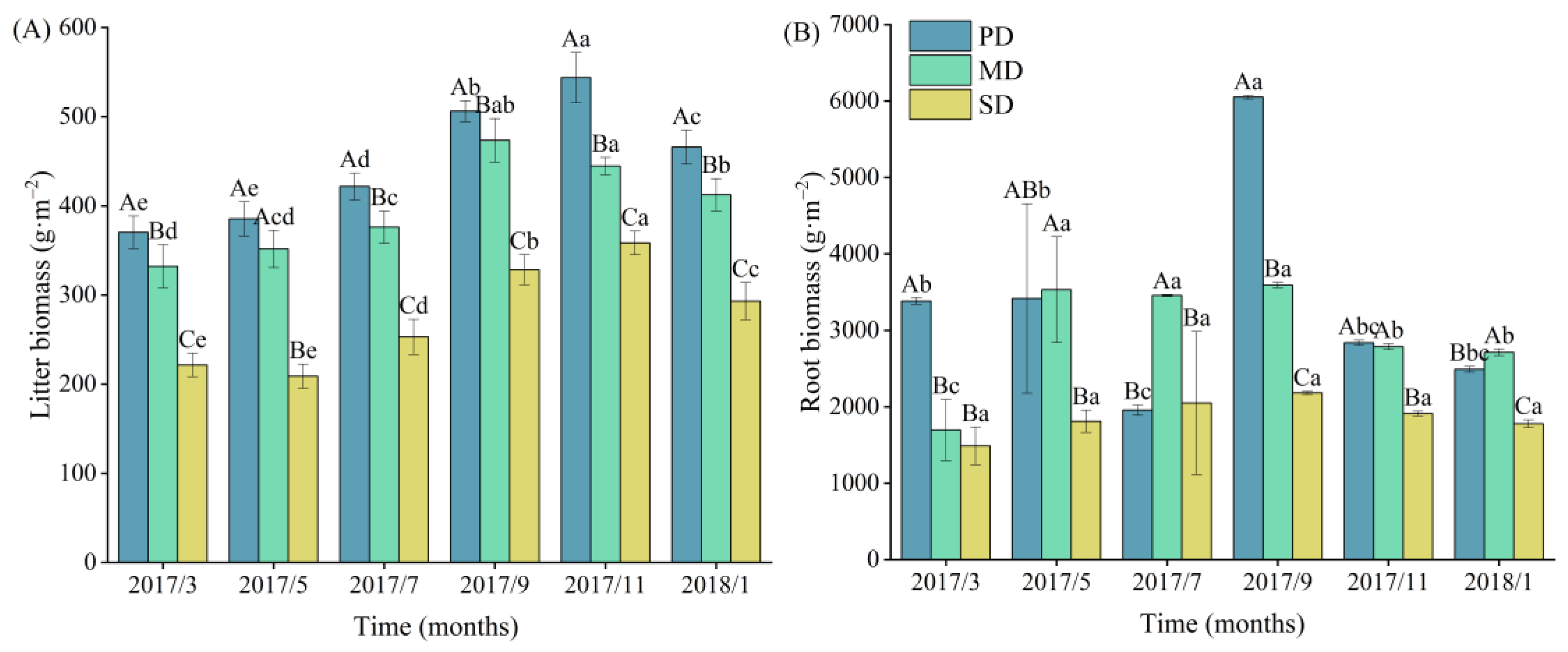
2.2. Litter and Root Biomass and Dynamics
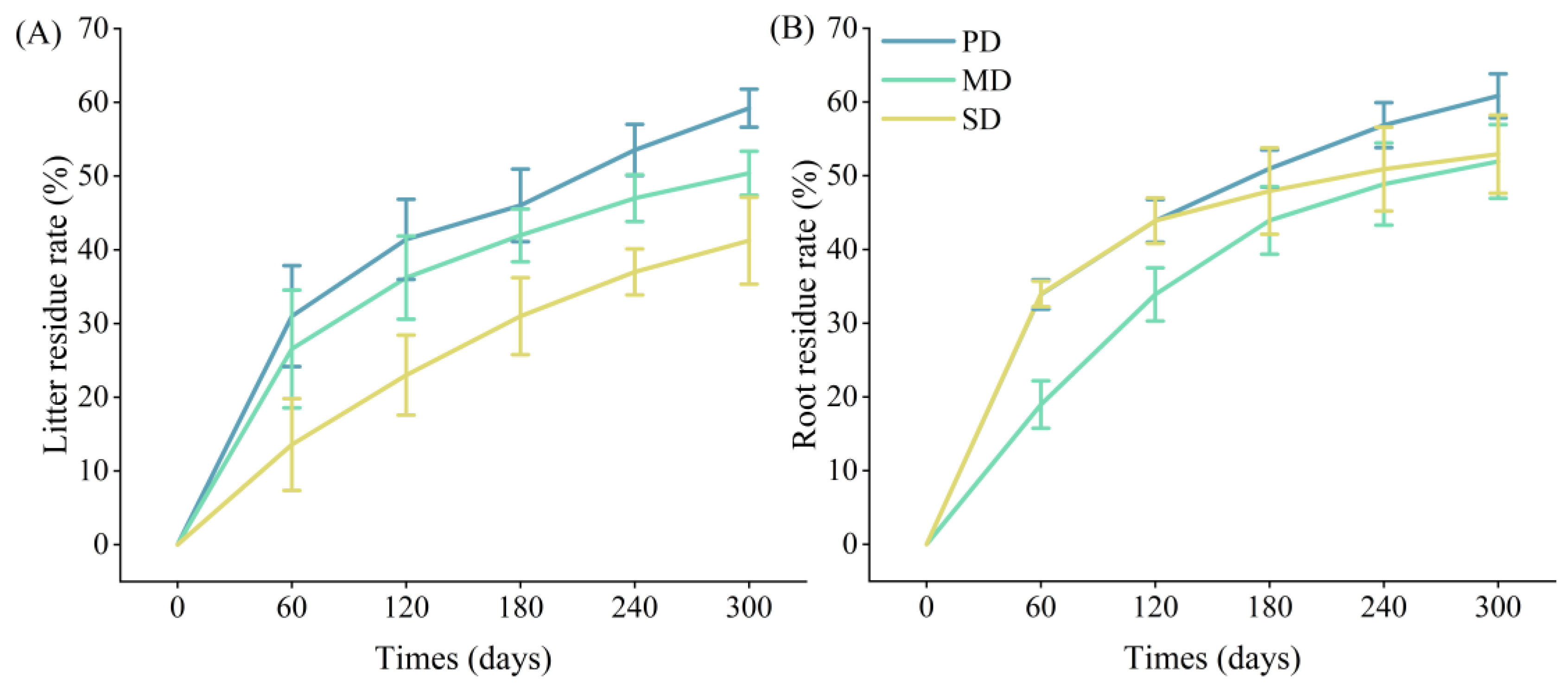
2.3. Decomposition of Litter and Root
2.4. Nutrient Release from Litter and Root
3. Discussion
3.1. Decomposition of Litter and Root in the Different Rocky Desertification Grasslands
3.2. Contribution of Litter and Root Decomposition in Different Rocky Desertification Grasslands to Soil Nutrients
4. Materials and Methods
4.1. Overview of the Study Area
4.2. Experimental Design
4.2.1. Litter and Root Biomass
4.2.2. Soil Sampling
4.2.3. Litter and Root Decomposition
4.3. Laboratory Analysis
4.4. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, T.; Hobbie, S.E.; Berg, B.; Zhang, H.; Httenschwiler, S. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. USA 2018, 115, 10392–10397. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Deng, M.F.; Yang, S.; Liu, W.X.; Wang, X.; Wang, J.; Liu, L.L. The coordination between leaf and fine root litter decomposition and the difference in their controlling factors. Glob. Ecol. Biogeogr. 2021, 30, 2286–2296. [Google Scholar] [CrossRef]
- Loranger, G.; Ponge, J.F.; Imbert, D.; Lavelle, P. Leaf decomposition in two semi-evergreen tropical forests: Influence of litter quality. Biol. Fertil. 2002, 35, 247–252. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; Mckie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer Press: New York, NY, USA, 2012; pp. 229–258. [Google Scholar]
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above-and belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar] [CrossRef]
- Ruess, R.W.; Cleve, K.V.; Yarie, J.; Viereck, L.A. Contributions of fine root production and turnover to the carbon and nitrogen cycling in taiga forests of the Alaskan interior. Can. J. For. Res. 1996, 26, 1326–1336. [Google Scholar] [CrossRef]
- Hyvönen, R.; Olsson, B.A.; Lundkvist, H.; Staaf, H. Decomposition and nutrient release from Picea abies (L.) Karst. and Pinus sylvestris L. logging residues. For. Ecol. Manag. 2000, 126, 97–112. [Google Scholar] [CrossRef]
- Penner, J.F.; Frank, D.A. Litter decomposition in Yellowstone grasslands: The roles of large herbivores, litter quality, and climate. Ecosystems 2019, 22, 929–937. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Gao, J.F.; Su, X.L.; Xiong, K.L. Grasslands eco-environment and stockbreeding development in the karst areas of Guizhou province. Acta Pratacul. Sin. 2011, 20, 279–286. [Google Scholar]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Zhu, X.A.; Zou, X.; Lu, E.F.; Deng, Y.; Luo, Y.; Chen, H.; Liu, W.J. Litterfall biomass and nutrient cycling in karst and nearby non-karst forests in tropical China: A 10-year comparison. Sci. Total Environ. 2021, 758, 143619. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Tian, J.; Lv, X.Y.; Wang, R.; Wang, D.X.; Zhang, F.F. Contrasting dynamics and factor controls in leaf compared with different-diameter fine root litter decomposition in secondary forests in the Qinling Mountains after 5 years of whole-tree harvesting. Sci. Total Environ. 2022, 838, 156194. [Google Scholar] [CrossRef]
- Lv, C.; Saba, T.; Wang, J.Y.; Hui, W.K.; Kang, X.K.; Xie, Y.X.; Wang, H.L.; Gong, W. Conversion effects of farmland to Zanthoxylum bungeanum plantations on soil organic carbon fractions in the arid valley of the upper reaches of the yangtze river, China. Catena 2022, 217, 106523. [Google Scholar] [CrossRef]
- Contos, P.; Murphy, N.P.; Kayll, Z.J.; Morgan, T.; Vido, J.J.; Decker, O.; Gibb, H. Rewilding soil and litter invertebrates and fungi increases decomposition rates and alters detritivore communities. Ecol. Evol. 2024, 14, e11128. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, K.B.; Dong, L.B.; Li, J.W.; Wang, X.Z.; Shangguan, Z.P.; Qu, B.D.; Deng, L. Dynamics of litter decomposition rate and soil organic carbon sequestration following vegetation succession on the Loess Plateau, China. Catena 2023, 229, 107225. [Google Scholar] [CrossRef]
- Glass, N.; Oliveira, E.D.D.; Molano-Flores, B.; Matamala, R.; Whelan, C.J.; Gonzalez-Meler, M.A. Root litter decomposition rates and impacts of drought are regulated by ecosystem legacy. Appl. Soil Ecol. 2023, 189, 104903. [Google Scholar] [CrossRef]
- Fu, Y.M.; Feng, F.J.; Zhang, X.Y.; Qi, D.D. Changes in fine root decomposition of primary Pinus koraiensis forest after clear cutting and restoration succession into secondary broad-leaved forest. Appl. Soil Ecol. 2021, 158, 103785. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Mun, S.; Lee, E.J. Litter decomposition rate and nutrient dynamics of giant ragweed (Ambrosia trifida L.) in the non-native habitat of South Korea. Plant Soil 2020, 449, 373–388. [Google Scholar] [CrossRef]
- Ni, J.; Luo, D.H.; Xia, J.; Zhang, Z.H.; Hu, G. Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 2015, 6, 799–810. [Google Scholar] [CrossRef]
- Zhu, X.A.; Shen, Y.X.; He, B.B.; Zhao, Z.M. Humus soil as a critical driver of flora conversion on karst rock outcrops. Sci. Rep. 2017, 7, 12611. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Y.X.; Zhu, X.A.; Zhao, G.J.; Zhao, Z.M.; Li, Z.J. Spatial distribution patterns of rock fragments and their underlying mechanism of migration on steep hillslopes in a karst region of Yunnan Province, China. Environ. Sci. Pollut. Res. 2019, 26, 24840–24849. [Google Scholar] [CrossRef]
- Song, X.W.; Gao, Y.; Wen, X.F.; Guo, D.L.; Yu, G.R.; He, N.P.; Zhang, J.Z. Carbon sequestration potential and its eco-service function in the karst area, China. J. Geogr. Sci. 2017, 27, 967–980. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Mo, J. Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 2010, 335, 289–298. [Google Scholar] [CrossRef]
- Berenstecher, P.; Araujo, P.I.; Austin, A.T. Worlds apart: Location above-or below-ground determines plant litter decomposition in a semi-arid Patagonian steppe. J. Ecol. 2021, 109, 2885–2896. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Xiong, Y.M.; Liu, X.; Guan, W.; Liao, B.W.; Chen, Y.J.; Li, M.; Zhong, C.R. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil 2017, 413, 83–95. [Google Scholar] [CrossRef]
- Guo, Y.L.; Chen, H.Y.H.; Mallik, A.U.; Wang, B.; Li, D.X.; Xiang, W.S.; Li, X.K. Predominance of abiotic drivers in the relationship between species diversity and litterfall production in a tropical karst seasonal rainforest. For. Ecol. Manag. 2019, 449, 117452. [Google Scholar] [CrossRef]
- Yan, Y.J.; Dai, Q.H.; Hu, G.; Jiao, Q.; Mei, L.N.; Fu, W.B. Effects of vegetation type on the microbial characteristics of the fissure soil-plant systems in karst rocky desertification regions of SW China. Sci. Total Environ. 2020, 712, 136543. [Google Scholar] [CrossRef]
- Xin, W.D.; Yin, X.Q.; Song, B. Contribution of soil fauna to litter decomposition in Songnen sandy lands in northeastern China. J. Arid. Environ. 2012, 77, 90–95. [Google Scholar] [CrossRef]
- Dai, X.Y.; Tang, J.; Song, L.H. A review on ecological studies of soil fauna in karst region, Southwest China. Chin. J. Ecol. 2019, 38, 3189–3194. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Ruiz-Peinado, R.; Onrubia, R.; del Río, M. Thinning alters the early-decomposition rate and nutrient immobilization-release pattern of foliar litter in Mediterranean oak-pine mixed stands. For. Ecol. Manag. 2017, 391, 309–320. [Google Scholar] [CrossRef]
- Fujii, S.; Makita, N.; Mori, A.S.; Takeda, H. A stronger coordination of litter decomposability between leaves and fine roots for woody species in a warmer region. Trees 2016, 30, 395–404. [Google Scholar] [CrossRef]
- Zhuang, L.Y.; Yang, W.Q.; Wu, F.Z.; Tan, B.; Zhang, L.; Yang, K.J.; He, R.Y.; Li, Z.J.; Xu, Z.F. Diameter-related variations in root decomposition of three common subalpine tree species in southwestern China. Geoderma 2018, 311, 1–8. [Google Scholar] [CrossRef]
- Alvafritz, L.; Hertel, D. Impacts of land use history on leaf litter input, chemical composition, decomposition and related nutrient cycling in young and old secondary tropical lowland rainforests (Sumatra, Indonesia). Plant Soil 2024, 495, 359–370. [Google Scholar] [CrossRef]
- Guo, L.B.; Wang, M.B.; Gifford, R.M. The change of soil carbon stocks and fine root dynamics after land use change from a native pasture to a pine plantation. Plant Soil 2007, 299, 251–262. [Google Scholar] [CrossRef]
- Lin, C.F.; Yang, Y.S.; Guo, J.F.; Chen, G.S.; Xie, J.S. Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical China: Dynamics of dry mass, nutrient and organic fractions. Plant Soil 2011, 338, 311–327. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; Spohn, M. Microbial substrate stoichiometry governs nutrient effects on nitrogen cycling in grassland soils. Soil Biol. Biochem. 2021, 155, 108168. [Google Scholar] [CrossRef]
- Sheng, M.Y.; Liu, Y.; Xiong, K.N. Response of soil physical and chemical properties to karst rocky desertification succession in southern South China Karst. Acta Ecol. Sin. 2013, 33, 6303–6313. [Google Scholar] [CrossRef]
- Persson, H.A.; Stadenberg, I. Fine root dynamics in a Norway spruce forest (Picea abies (L.) Karst) in eastern Sweden. Plant Soil 2010, 330, 329–344. [Google Scholar] [CrossRef]




| Grassland Type | Productivity (g·m−2·yr−1) | Decomposition Amount (g·yr−1) | Turnover Rate (yr−1) | |
|---|---|---|---|---|
| Litter | PD | 222 ± 6.14 a | 454 ± 25.7 a | 0.50 ± 0.01 b |
| MD | 186 ± 3.85 b | 318 ± 27.8 b | 0.47 ± 0.02 b | |
| SD | 157 ± 7.27 c | 269 ± 21.6 b | 0.57 ± 0.05 a | |
| Root | PD | 4386 ± 532 a | 294 ± 59.3 b | 1.51 ± 0.18 a |
| MD | 2985 ± 251 b | 549 ± 20.5 a | 1.39 ± 0.07 a | |
| SD | 1167 ± 141 c | 273 ± 32.6 b | 0.80 ± 0.08 b |
| Grassland Types | Fitted Equation | R2 | 50% Decomposition Time (yr) | 95% Decomposition Time (yr) | Annual Decomposition Rate (%) | |
|---|---|---|---|---|---|---|
| PD | y = 4.64e−1.13t | 0.90 | 0.55 | 2.59 | 69.98 | |
| Litter | MD | y = 4.64e−0.90t | 0.88 | 0.69 | 3.26 | 62.14 |
| SD | y = 4.89e−0.67t | 0.98 | 1.01 | 4.46 | 49.79 | |
| PD | y = 2.77e−1.25t | 0.89 | 0.49 | 2.33 | 73.64 | |
| Root | MD | y = 2.69e−1.02t | 0.78 | 0.57 | 2.84 | 67.61 |
| SD | y = 2.89e−0.99t | 0.96 | 0.66 | 3.00 | 64.09 |
| Grassland Types | OC (g·m−2) | TN (g·m−2) | TP (g·m−2) | TK (g·m−2) | |
|---|---|---|---|---|---|
| Litter | PD | 66.35 ± 3.75 a | 0.68 ± 0.04 a | 0.10 ± 0.01 a | 0.71 ± 0.04 a |
| MD | 42.45 ± 3.71 b | 0.52 ± 0.05 b | 0.04 ± 0.00 c | 0.09 ± 0.01 c | |
| SD | 38.69 ± 3.11 b | 0.47 ± 0.04 b | 0.07 ± 0.01 b | 0.28 ± 0.02 b | |
| Root | PD | 196.67 ± 39.58 b | 2.94 ± 0.59 b | 0.31 ± 0.06 b | 1.02 ± 0.2 b |
| MD | 314.64 ± 11.75 a | 5.07 ± 0.19 a | 0.43 ± 0.02 a | 3.61 ± 0.13 a | |
| SD | 110.24 ± 13.13 c | 1.32 ± 0.16 c | 0.11 ± 0.01 c | 0.38 ± 0.04 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Wang, Y.; Wang, X.; Jin, B.; Chen, C.; Zhao, X. Contribution of Litter and Root to Soil Nutrients in Different Rocky Desertification Grasslands in a Karst Area. Plants 2024, 13, 2329. https://doi.org/10.3390/plants13162329
Wang Y, Wang J, Wang Y, Wang X, Jin B, Chen C, Zhao X. Contribution of Litter and Root to Soil Nutrients in Different Rocky Desertification Grasslands in a Karst Area. Plants. 2024; 13(16):2329. https://doi.org/10.3390/plants13162329
Chicago/Turabian StyleWang, Yuefeng, Jigao Wang, Yini Wang, Xiaojing Wang, Baocheng Jin, Chao Chen, and Xuechun Zhao. 2024. "Contribution of Litter and Root to Soil Nutrients in Different Rocky Desertification Grasslands in a Karst Area" Plants 13, no. 16: 2329. https://doi.org/10.3390/plants13162329





