Characteristics of Rhizosphere Microbiome, Soil Chemical Properties, and Plant Biomass and Nutrients in Citrus reticulata cv. Shatangju Exposed to Increasing Soil Cu Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Soil Chemical Properties, Enzyme Activities Analysis, and Plant Nutrient Concentrations
2.3. DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.4. Data Analysis
3. Results
3.1. Soil Chemical Properties and Extracellular Enzymes
3.2. Plant Growth and Nutrients Uptake
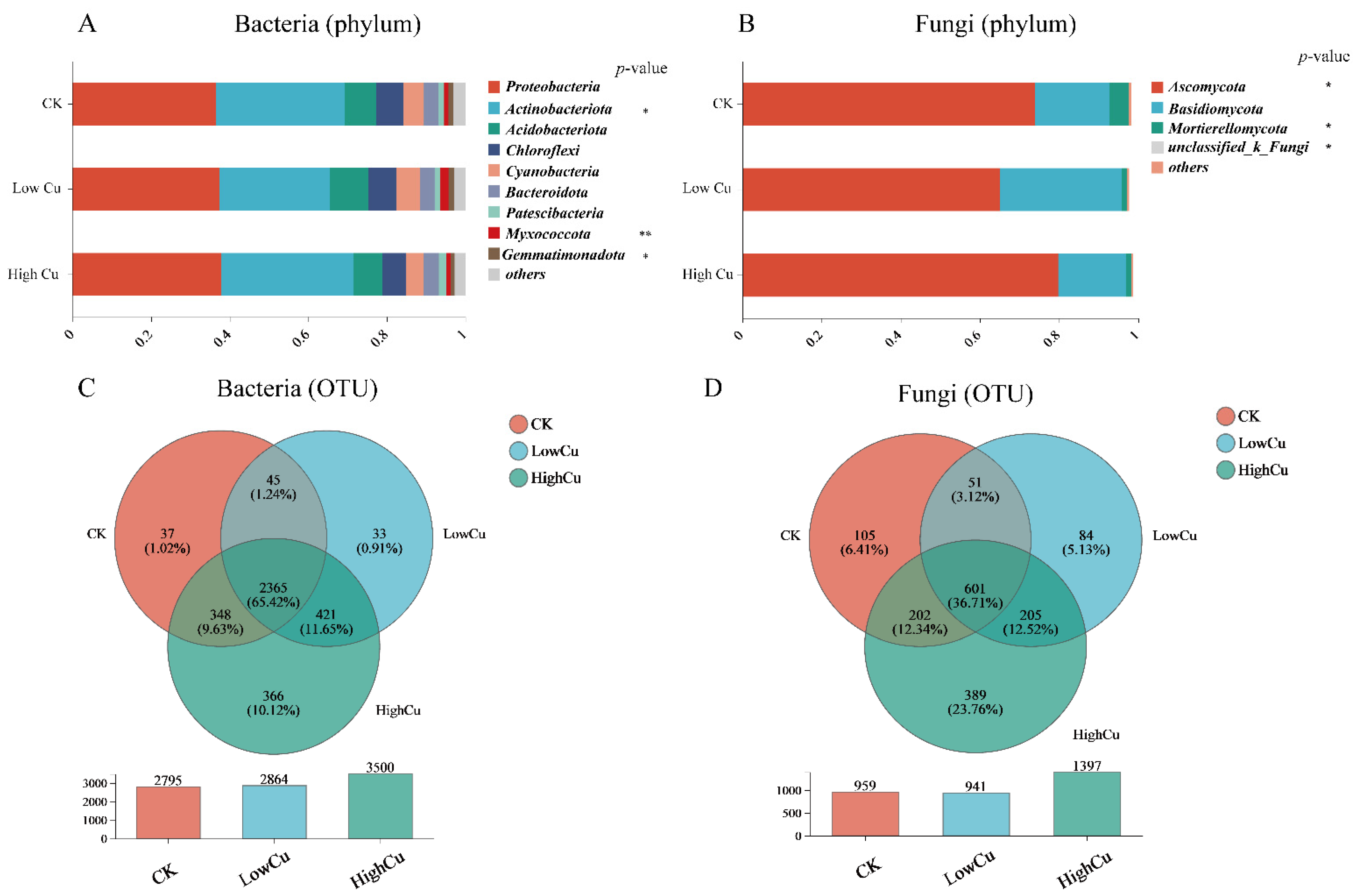
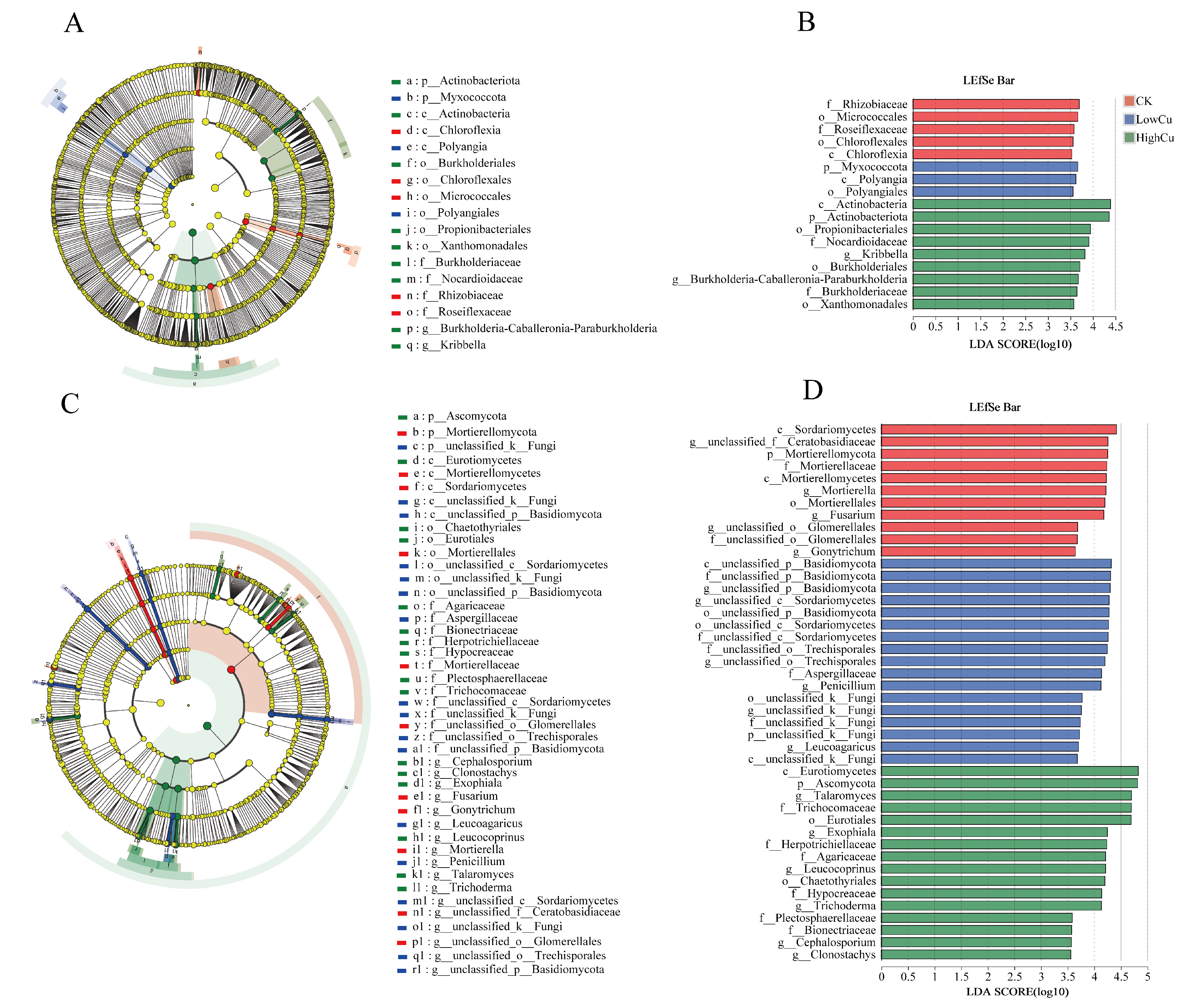
3.3. Soil Microbial Diversity, Composition, and Markers
3.4. Function Classification of Rhizosphere Microbial Communities
3.5. Co-Occurrence Associations between Microorganism and Plant Biomass and Plant Nutrients Concentration
4. Discussion
4.1. Soil Chemistry Properties under Different Soil Cu Levels
4.2. Different Cu-Tolerant Biomarkers and Functional Groups Identified under Different Soil Cu Levels
4.3. The Interaction between Microorganisms and Soil Chemistry Properties and Plant Biomass and Nutrient Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamma, D.; Christakopoulos, P. Biotransformation of citrus by-products into value added products. Waste Biomass Valori. 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Zema, D.; Calabrò, P.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Yao, T.S.; Zhou, Y.; Zhou, C.Y. Advances in Copper Resistant Mechanisms and Control Methods of Citrus Canker. Acta Hortic. Sin. 2016, 43, 1711–1718. (In Chinese) [Google Scholar]
- Girotto, E.; Ceretta, C.A.; Brunetto, G.; Miotto, A.; Tiecher, T.L.; De Conti, L.; Lourenzi, C.R.; Lorensini, F.; Gubiani, P.I.; Da Silva, L.S. Copper availability assessment of Cu contaminated vineyard soils using black oat cultivation and chemical extractants. Environ. Monit. Assess. 2014, 186, 9051–9063. [Google Scholar] [CrossRef]
- Fan, J.; He, Z.; Ma, L.Q.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soils Sediments 2011, 11, 639–648. [Google Scholar] [CrossRef]
- Ying, J.G.; Liu, X.H.; Li, J.B.; Wu, Q.; Peng, S.A.; Jiang, C.C. Analysis of Cu and Mn contents in the soil and leaves of citrus orchards in Nanfeng and Quzhou. South China Fruits 2016, 45, 15–18. (In Chinese) [Google Scholar]
- Zhao, Y.B.; Han, J.; Yang, G.B.; Long, L.Z.; Tan, Z.H.; Li, X.X.; Zhou, W.J.; Deng, Z.N.; Ma, X.F. Analysis of soil and leaf and fruit mineral elements in the main sweet orange producing areas in Hunan Province. South China Fruits 2020, 49, 27–33. (In Chinese) [Google Scholar]
- Jiang, Y.N.; Fu, H.M.; Liu, B.H.; Liu, S.Q.; Tang, X.S.; Zhai, J.; Deng, C.L. Research on soil and leaf nutrient status in red soil navel orange orchard. South Horticult. 2020, 31, 6–11. (In Chinese) [Google Scholar]
- Mo, X.; Chen, C.; Riaz, M.; Moussa, M.G.; Chen, X.; Wu, S.; Tan, Q.; Sun, X.; Zhao, X.; Shi, L. Fruit Characteristics of Citrus Trees Grown under Different Soil Cu Levels. Plants 2022, 11, 2943. [Google Scholar] [CrossRef]
- Hippler, F.W.; Cipriano, D.O.; Boaretto, R.M.; Quaggio, J.A.; Gaziola, S.A.; Azevedo, R.A.; Mattos-Jr, D. Citrus rootstocks regulate the nutritional status and antioxidant system of trees under copper stress. Environ. Exp. Bot. 2016, 130, 42–52. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Tang, X.; Yan, L.; El-Desouki, Z.; Li, Y.; Wang, X.; Jiang, C. Insight into mechanisms of biochar-fertilizer induced of microbial community and microbiology of nitrogen cycle in acidic soil. J. Environ. Manag. 2023, 336, 117602. [Google Scholar] [CrossRef]
- Han, G.Q.; Wang, B.; Chen, G.Q.; Wang, H.X.; Zhang, H.B.; Zhang, X.J.; Xiong, Z.T. Effects of heavy metal combined pollution on soil microbial indicators and soil enzymatic activity. J. Soil Water Conserv. 2010, 24, 238–242. (In Chinese) [Google Scholar]
- Dai, J.; Becquer, T.; Rouiller, J.H.; Reversat, G.; Bernhard-Reversat, F.; Lavelle, P. Influence of heavy metals on C and N mineralisation and microbial biomass in Zn-, Pb-, Cu-, and Cd-contaminated soils. Appl. Soil Ecol. 2004, 25, 99–109. [Google Scholar] [CrossRef]
- Cui, J.L.; Zhao, Y.P.; Chan, T.S.; Zhang, L.L.; Tsang, D.C.; Li, X.D. Spatial distribution and molecular speciation of copper in indigenous plants from contaminated mine sites: Implication for phytostabilization. J. Hazard Mater. 2020, 381, 121208. [Google Scholar] [CrossRef]
- Chung, H.; Kim, M.J.; Ko, K.; Kim, J.H.; Kwon, H.A.; Hong, I.; Park, N.; Lee, S.W.; Kim, W. Effects of graphene oxides on soil enzyme activity and microbial biomass. Sci. Total Environ. 2015, 514, 307–313. [Google Scholar] [CrossRef]
- Deforest, J.L.; Moorhead, D.L. Effects of elevated pH and phosphorus fertilizer on soil C, N and P enzyme stoichiometry in an acidic mixed mesophytic deciduous forest. Soil Biol. Biochem. 2020, 150, 107996. [Google Scholar] [CrossRef]
- Wu, J.X.; Li, S.Y.; Han, G.D. Study on soil carbon, nitrogen, phosphorus acquisition enzyme activity, and microbial entropy in Stipa breviflora desert grassland of Inner Mongolia. Pratacultural Sci. 2024, 42, 1–11. (In Chinese) [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil. Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B. Urease inhibitors: A review. Indian J. Biotechnol. 2012, 11, 381–388. [Google Scholar]
- Wang, J.; Zhao, Y.; Dai, T.; Fan, X.; Liu, Y.; Tang, B. Influence of Cu and Cd Pollution on Activeness of Urease in Soil. Environ. Sci. Manag. 2014, 39, 45–48. (In Chinese) [Google Scholar]
- Mounissamy, V.C.; Kundu, S.; Selladurai, R.; Saha, J.K.; Biswas, A.K.; Adhikari, T.; Patra, A.K. Effect of soil amendments on microbial resilience capacity of acid soil under copper stress. Bull. Environ. Contam. Toxcol. 2017, 99, 625–632. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamic. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, L.C.; Deng, L.; Guo, X.B.; Han, F.; Ju, W.L.; Wang, X.; Chen, H.S.; Tan, W.F.; Zhang, X.C. Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci. Total Environ. 2019, 658, 1440–1451. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–790. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, Q.; Chao, Y. Structural development and assembly patterns of the root-associated microbiomes during phytoremediation. Sci. Total Environ. 2018, 644, 1591–1601. [Google Scholar] [CrossRef]
- Li Destri Nicosia, M.G.; Mosca, S.; Mercurio, R.; Schena, L. Dieback of pinus nigra seedlings caused by a strain of trichoderma viride. Plant Dis. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Zhou, X.; He, Z.; Liang, Z.; Stoffella, P.J.; Fan, J.; Yang, Y.; Powell, C.A. Long-term use of copper-containing fungicide affects microbial properties of citrus grove soils. Soil Sci. Soc. Am. J. 2011, 75, 898–906. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph: Saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, C.; Li, M.; Tan, Q.; Sun, X.; Pan, Z.; Deng, X.; Hu, C. Effects of potassium on fruit soluble sugar and citrate accumulations in Cara Cara navel orange (Citrus sinensis L. Osbeck). Sci. Hortic. 2021, 283, 110057. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agriculture Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Harrison, S.; Lepp, N.; Phipps, D. Uptake of Copper by excised roots II. Copper desorption from the free space. Z Pflanzenphysiol. 1979, 94, 27–34. [Google Scholar] [CrossRef]
- Moussa, M.G.; Sun, X.; Ismael, M.A.; Elyamine, A.M.; Rana, M.S.; Syaifudin, M.; Hu, C. Molybdenum-induced effects on grain yield, macro–micro-nutrient uptake, and allocation in mo-inefficient winter wheat. J. Plant Growth Regul. 2021, 41, 1516–1531. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Zhang, J.; Dai, Y.; Zhang, X.; Xie, S. Operational performance, biomass and microbial community structure: Impacts of backwashing on drinking water biofilter. Environ. Sci. Pollut. Res. 2015, 22, 546–554. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Riaz, M.; Liu, B.; Xia, H.; El-desouki, Z.; Jiang, C.C. Two-year study of bio-char: Achieving excellent capability of potassium supply via alter clay mineral composition and potassium-dissolving bacteria activity. Sci. Total Environ. 2020, 717, 137286. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y. FASTP: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microb. 2009, 75, 7537. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.Y.; Wang, Y.; Chen, W.Y. Review on the mechanism of plant rhizosphere soil enzyme response to heavy metal pollution. Asian J. Ecotox. 2020, 15, 11–20. (In Chinese) [Google Scholar]
- Zhang, M.K.; Wang, L.P. Impact of heavy metals pollution on soil organic matter accumulation. Chin. J. Appl. Ecol. 2007, 18, 1479–1483. (In Chinese) [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of cadmium, copper and zinc on plants, soil microorganisms and soil enzymes. J. Elementol. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Ali, N.; Dashti, N.; Al-Mailem, D.; Eliyas, M.; Radwan, S. Indigenous soil bacteria with the combined potential for hydrocarbon consumption and heavy metal resistance. Environ. Sci. Pollut. Res. 2012, 19, 812–820. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Stone, L.F. Physical, chemical, and biological changes in the rhizosphere and nutrient availability. J. Plant Nutr. 2006, 29, 1327–1356. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Azarbad, H.; Niklińska, M.; Van Gestel, C.A.; Van Straalen, N.M.; Röling, W.F.; Laskowski, R. Microbial community structure and functioning along metal pollution gradients. Environ. Toxicol. Chem. 2013, 32, 1992–2002. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, M.M.; Xu, S.H.; Xu, Y. Effects of Copper Pollution on Microbial Communities in Wheat Root System. Environ. Sci. 2021, 42, 996–1003. [Google Scholar]
- Krumova, E.; Andreyinski, N.; Abrashev, R.; Stoyancheva, G.; Kostadinova, N.; Miteva-Staleva, J.; Dishlijska, V.; Spasova, B.; Angelova, M. Comparison of the oxidative stress response of two aspergillus fumigatus strains isolated from polluted soils against combined heavy metal toxicity. Geomicrobiol. J. 2021, 38, 515–523. [Google Scholar] [CrossRef]
- Fernandez-Calvio, D.; Bååth, E. Interaction between pH and Cu toxicity on fungal and bacterial performance in soil. Soil Biol. Biochem. 2016, 96, 20–29. [Google Scholar] [CrossRef]
- Wu, S.; Wu, K.; Shi, L.; Sun, X.; Tan, Q.; Hu, C. Recruitment of specific microbes through exudates affects cadmium activation and accumulation in Brassica napus. J. Hazard. Mater. 2023, 442, 130066. [Google Scholar] [CrossRef]
- Lv, Z.; Rønn, R.; Liao, H.; Rensing, C.; Chen, W.; Huang, Q.; Hao, X. Soil aggregates affect the legacy effect of copper pollution on the microbial communities. Soil Biol.Biochem. 2023, 182, 109048. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; He, L. Genetic diversity and characterization of heavy metal-resistant endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour. Technol. 2010, 101, 501–509. [Google Scholar] [CrossRef]
- Guo, D.C.; Fan, Z.Z.; Lu, S.Y.; Ma, Y.J.; Nie, X.H.; Tong, F.P.; Peng, X.W. Changes in rhizosphere bacterial communities during remediation of heavy metal accumulating plants around the Xikuangshan mine in southern China. Sci. Rep. 2019, 9, 1947. [Google Scholar] [CrossRef]
- Camacho-Chab, J.C.; Castañeda-Chávez, M.D.R.; Chan-Bacab, M.J. Biosorption of cadmium by non-toxic extracellular polymeric substances (EPS) synthesized by bacteria from marine intertidal biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Pan, F.S.; Meng, Q.; Wang, Q.; Luo, S.; Chen, B.; Khan, K.Y.; Yang, X.E.; Feng, Y. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 2016, 154, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Granja-Travez, R.S.; Bugg, T.D.H. Characterization of multicopper oxidase CopA from Pseudomonas putida KT2440 and Pseudomonas fluorescens Pf-5: Involvement in bacterial lignin oxidation. Arch. Biochem. Biophys. 2018, 660, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, J.; Wang, Z. Responses of bacterial communities and resistance genes on microplastics to antibiotics and heavy metals in sewage environment. J. Hazard. Mater. 2021, 402, 123550. [Google Scholar] [CrossRef]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; Abu Al-Soud, W.; Sorensen, S.J.; He, Z.; White, D.; Sinclair, A.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.A.; Lima, A.I.G.; Figueira, E.M.D.A.P. Screening possible mechanisms mediating cadmium resistance in Rhizobium leguminosarum bv. viciae isolated from contaminated Portuguese soils. Microb. Ecol. 2006, 52, 176–186. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Gowthami, S.; Thirumarimurugan, M.; Sivakumar, V.M.; Sukanya, K. Impending heavy metal tolerance of fungus isolated from dye industrial sludge. Int. J. Mater. Prod. Technol. 2017, 55, 308–318. [Google Scholar] [CrossRef]
- Cai, Z.; Du, W.; Zhang, Z.; Guan, L.; Zeng, Q.; Chai, Y.; Dai, C.; Lu, L. The aspergillus fumigatus transcription factor AceA is involved not only in Cu but also in Zn detoxification through regulating transporters CrpA and ZrcA. Cell. Microbiol. 2018, 20, e12873. [Google Scholar] [CrossRef]
- Lacerda, E.C.M.; dos Passos Galluzzi Baltazar, M.; dos Reis, T.A.; do Nascimento, C.A.O.; Côrrea, B.; Gimenes, L.J. Copper biosorption from an aqueous solution by the dead biomass of penicillium ochrochloron. Environ. Monit. Assess. 2019, 191, 247. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, G.; Xing, S.; Fu, W.; Liu, X.; Wu, H.; Chen, B. Structure and diversity of fungal communities in long-term copper-contaminated agricultural soil. Sci. Total Environ. 2022, 806, 151302. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Singh, M.; Soni, S.K.; Singh, R.; Kalra, A. Biodiversity acts as insurance of productivity of bacterial communities under abiotic perturbations. ISME J. 2014, 8, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Shen, X.; Yang, F.; Wang, F.; Qin, C.Y.; Zou, D.Y.; Hu, Q.Y.; Lin, J.X.; Wang, J.H. Research progress on the mechanism of Arbuscular Mycorrhizal Fungi (AMF) Mediated Mineral Elements Uptake by Plants. Acta Agrestia Sin. 2023, 31, 1609–1621. (In Chinese) [Google Scholar]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of apple replant problems based on different soil disinfection treatments-links to soil microbial community structure? Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Emmett, B.; Nelson, E.B.; Kessler, A.; Bauerle, T.L. Fine-root system development and susceptibility to pathogen colonization. Planta 2014, 239, 325–340. [Google Scholar] [CrossRef]
- Weiβ, S.; Bartsch, M.; Winkelmann, T. Transcriptomic analysis of molecular responses in Malus domestica ‘M26’ roots affected by apple replant disease. Plant Mol. Biol. 2017, 94, 303–318. [Google Scholar]
- Daud, M.; Ali, S.; Variath, M.; Zhu, S. Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 2013, 93, 2593–2602. [Google Scholar] [CrossRef]
- Zeng, Q.; Ling, Q.; Wu, J.; Yang, Z.; Liu, R.; Qi, Y. Excess copper-induced changes in antioxidative enzyme activity, mineral nutrient uptake and translocation in sugarcane seedlings. Bull. Environ. Contam. Toxcol. 2019, 103, 834–840. [Google Scholar] [CrossRef]
- Miotto, A.; Ceretta, C.A.; Brunetto, G.; Nicoloso, F.T.; Girotto, E.; Farias, J.G.; Tiecher, T.L.; De Conti, L.; Trentin, G. Copper uptake, accumulation and physiological changes in adult grapevines in response to excess copper in soil. Plant Soil 2014, 374, 593–610. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Shi, J.C.; He, Y.; Chen, Y.X.; Wang, Y.J.; Yang, X.L.; Wang, T.Y.; Wu, L.S.; Xu, J.M. Enhanced soil function and health by soybean root microbial communities during in situ remediation of Cd-contaminated soil with the application of soil amendments. mSystems 2023, 8, e01049-22. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Petr, K.; Reinhard, W.N.; Marek, K.; Elnaz, A.; Moudrý, J., Jr.; Ladislav, M. Preliminary Findings on Cadmium Bioaccumulation and Photosynthesis in Rice (Oryza sativa L.) and Maize (Zea mays L.) Using Biochar Made from C3- and C4-Originated Straw. Plants 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, C.S.; van der Meer, J.R. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal-plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, 101, e02978. [Google Scholar] [CrossRef]
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Koh, R.H.; Song, H.G. Effects of application of Rhodopseudomonas sp. on seed germination and growth of tomato under axenic conditions. J. Microbiol. Biotechnol. 2007, 17, 1805–1810. [Google Scholar] [PubMed]
- Lee, K.H.; Koh, R.H.; Song, H.G. Enhancement of growth and yield of tomato by Rhodopseudomonas sp. under greenhouse conditions. J. Microbiol. 2008, 46, 641–646. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, D.H.; Tan, W.B.; Yu, H.; Xi, B.D.; Jiang, Y.H.; Dang, Q.L. Different Responses of Soil Microbial Community Structure to Irrigation with Treated Wastewater from Domestic and Industrial Sources. Environ. Sci. 2020, 41, 4253–4261. [Google Scholar]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Kitto, S.L.; Caplan, J.L. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 2012, 160, 1642–1661. [Google Scholar] [CrossRef]
- Xu, Y.X.; Wang, G.H.; Jin, J. Bacterial communities in soybean rhizosphere in response to soil type, soybean genotype, and their growth stage. Soil Biol. Biochem. 2009, 41, 919–925. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jinbo, X.; Fei, P.; Huaibo, S. Erratum to: Divergent responses of soil fungi functional groups to short-term warming. Microb. Ecol. 2014, 68, 708–715. [Google Scholar]
- Junsheng, H.; Bin, H.; Kaibin, Q. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar]
- Chen, Y.Y.; Xia, W.Y.; Zhao, H.; Zeng, M. Effects of deep vertical rotary tillage on soil enzyme activity, microbial community structure, and functional diversity of cultivated land. Acta Ecol. Sin. 2022, 42, 5009–5021. (In Chinese) [Google Scholar]
- Yao, Q.; Li, X.L.; Ai, W.D.; Christie, P. Bi-directional transfer of phosphorus between red clover and perennial ryegrass via arbuscular mycorrhizal hyphal links. Eur. J. Soil Biol. 2003, 39, 47–54. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z.; et al. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef]





| Treatments | TCu (mg kg−1) | ACu (mg kg−1) | SOM (g kg−1) | pH | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Cu 38 | 33.18 ± 0.39 e | 0.47 ± 0.04 e | 16.77 ± 0.95 d | 6.68 ± 0.10 a | 70.74 ± 7.62 a | 39.12 ± 3.70 a | 493.13 ± 18.73 b |
| Cu 108 | 69.85 ± 2.70 d | 25.40 ± 1.30 d | 17.91 ± 0.25 cd | 6.58 ± 0.02 b | 64.89 ± 4.40 ab | 36.66 ± 5.67 a | 550.38 ± 26.68 a |
| Cu 178 | 103.30 ± 4.59 c | 42.16 ± 3.11 c | 19.69 ± 0.37 ab | 6.57 ± 0.03 b | 72.55 ± 1.32 a | 33.94 ± 2.47 a | 532.82 ± 3.25 a |
| Cu 318 | 167.12 ± 5.81 b | 87.79 ± 2.69 b | 20.48 ± 0.92 a | 6.57 ± 0.05 b | 59.87 ± 1.38 b | 14.45 ± 0.74 b | 566.00 ± 4.55 a |
| Cu 388 | 201.27 ± 13.76 a | 115.65 ± 10.52 a | 18.63 ± 0.71 bc | 6.51 ± 0.03 b | 49.96 ± 2.54 c | 11.99 ± 0.74 b | 550.06 ± 20.50 a |
| Treatments | FRO /U g−1 | αG /nmol (g h)−1 | βG /nmol (g h)−1 | CBH /nmol (g h)−1 | βX /nmol (g h)−1 | NAG /nmo (g h)−1 | ACP /nmol (g h)−1 |
|---|---|---|---|---|---|---|---|
| Cu 38 | 1.85 ± 0.08 e | 6.37 ± 0.14 a | 5.20 ± 0.06 a | 1.16 ± 0.04 b | 1.05 ± 0.04 a | 2.06 ± 0.12 d | 11.31 ± 0.26 a |
| Cu 108 | 4.84 ± 0.09 c | 5.04 ± 0.02 b | 4.09 ± 0.06 b | 1.05 ± 0.05 cd | 1.08 ± 0.04 a | 3.97 ± 0.11 b | 11.38 ± 0.23 a |
| Cu 178 | 5.85 ± 0.06 b | 3.63 ± 0.04 c | 4.69 ± 0.12 a | 1.06 ± 0.01 c | 1.06 ± 0.02 a | 4.19 ± 0.11 a | 10.80 ± 0.26 a |
| Cu 318 | 6.21 ± 0.13 a | 1.11 ± 0.09 d | 5.11 ± 0.08 a | 1.57 ± 0.06 a | 0.81 ± 0.02 c | 3.2 ± 0.04 c | 5.49 ± 0.13 c |
| Cu 388 | 3.44 ± 0.17 d | 1.10 ± 0.07 d | 3.96 ± 0.60 b | 0.98 ± 0.04 d | 0.88 ± 0.01 b | 2.24 ± 0.09 d | 6.54 ± 0.55 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, X.; Huang, Q.; Chen, C.; Xia, H.; Riaz, M.; Liang, X.; Li, J.; Chen, Y.; Tan, Q.; Wu, S.; et al. Characteristics of Rhizosphere Microbiome, Soil Chemical Properties, and Plant Biomass and Nutrients in Citrus reticulata cv. Shatangju Exposed to Increasing Soil Cu Levels. Plants 2024, 13, 2344. https://doi.org/10.3390/plants13172344
Mo X, Huang Q, Chen C, Xia H, Riaz M, Liang X, Li J, Chen Y, Tan Q, Wu S, et al. Characteristics of Rhizosphere Microbiome, Soil Chemical Properties, and Plant Biomass and Nutrients in Citrus reticulata cv. Shatangju Exposed to Increasing Soil Cu Levels. Plants. 2024; 13(17):2344. https://doi.org/10.3390/plants13172344
Chicago/Turabian StyleMo, Xiaorong, Qichun Huang, Chuanwu Chen, Hao Xia, Muhammad Riaz, Xiaomin Liang, Jinye Li, Yilin Chen, Qiling Tan, Songwei Wu, and et al. 2024. "Characteristics of Rhizosphere Microbiome, Soil Chemical Properties, and Plant Biomass and Nutrients in Citrus reticulata cv. Shatangju Exposed to Increasing Soil Cu Levels" Plants 13, no. 17: 2344. https://doi.org/10.3390/plants13172344





