Integrated Analysis of Metabolomics, Flavoromics, and Transcriptomics for Evaluating New Varieties of Amomum villosum Lour.
Abstract
1. Introduction
2. Results
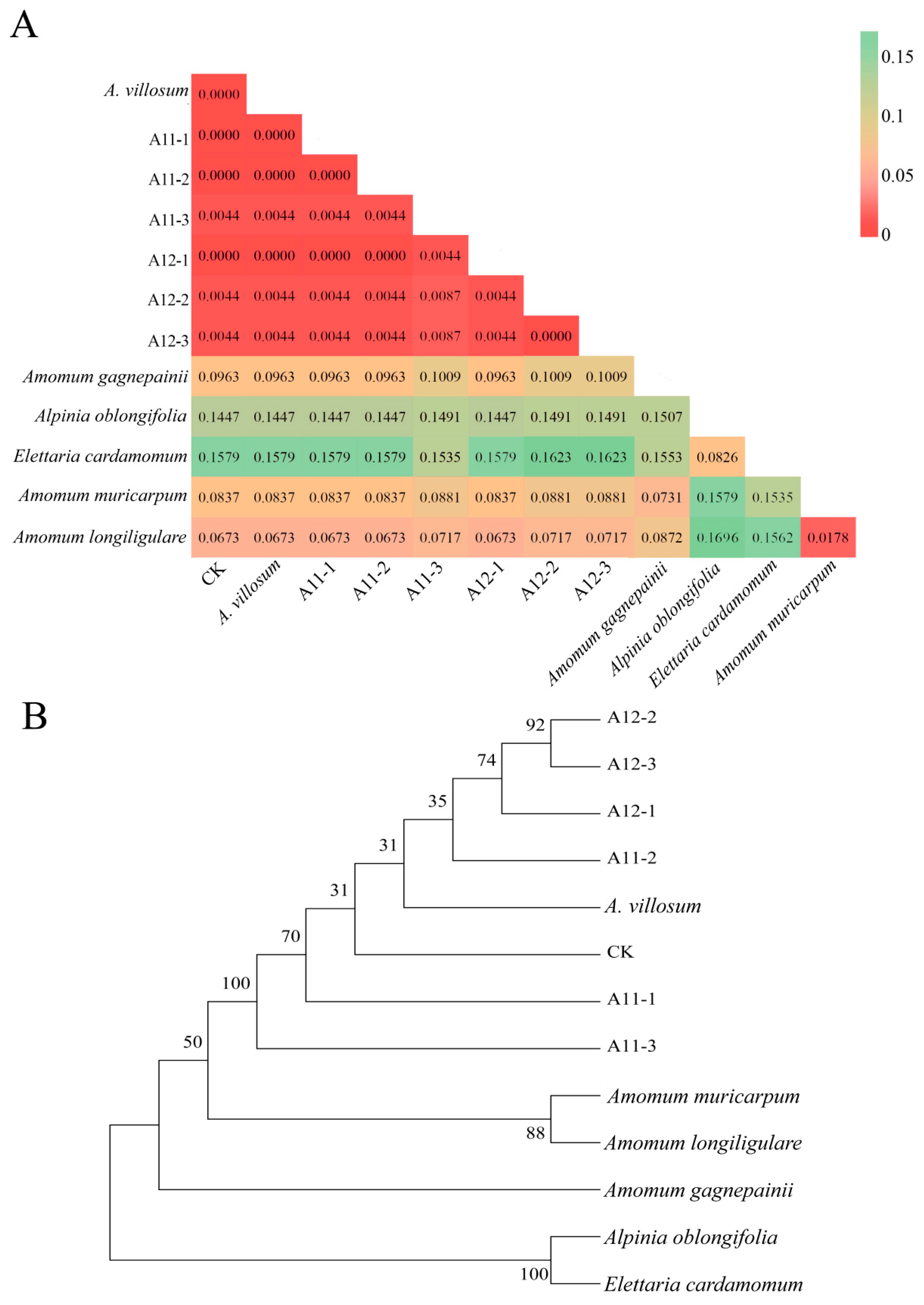
2.1. DNA Barcode Analysis
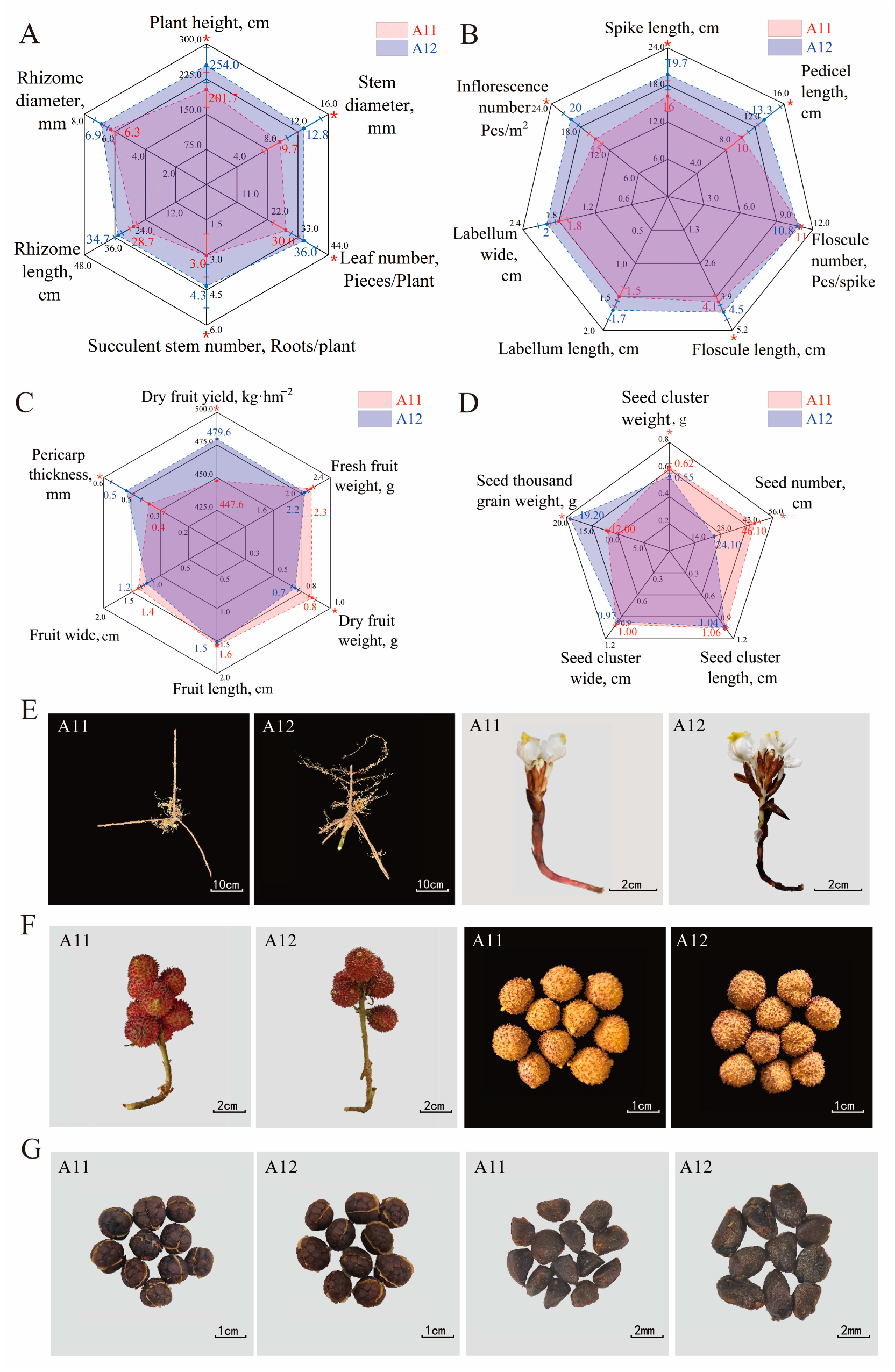
2.2. Comparison of Plant Traits and Agronomic Characteristics
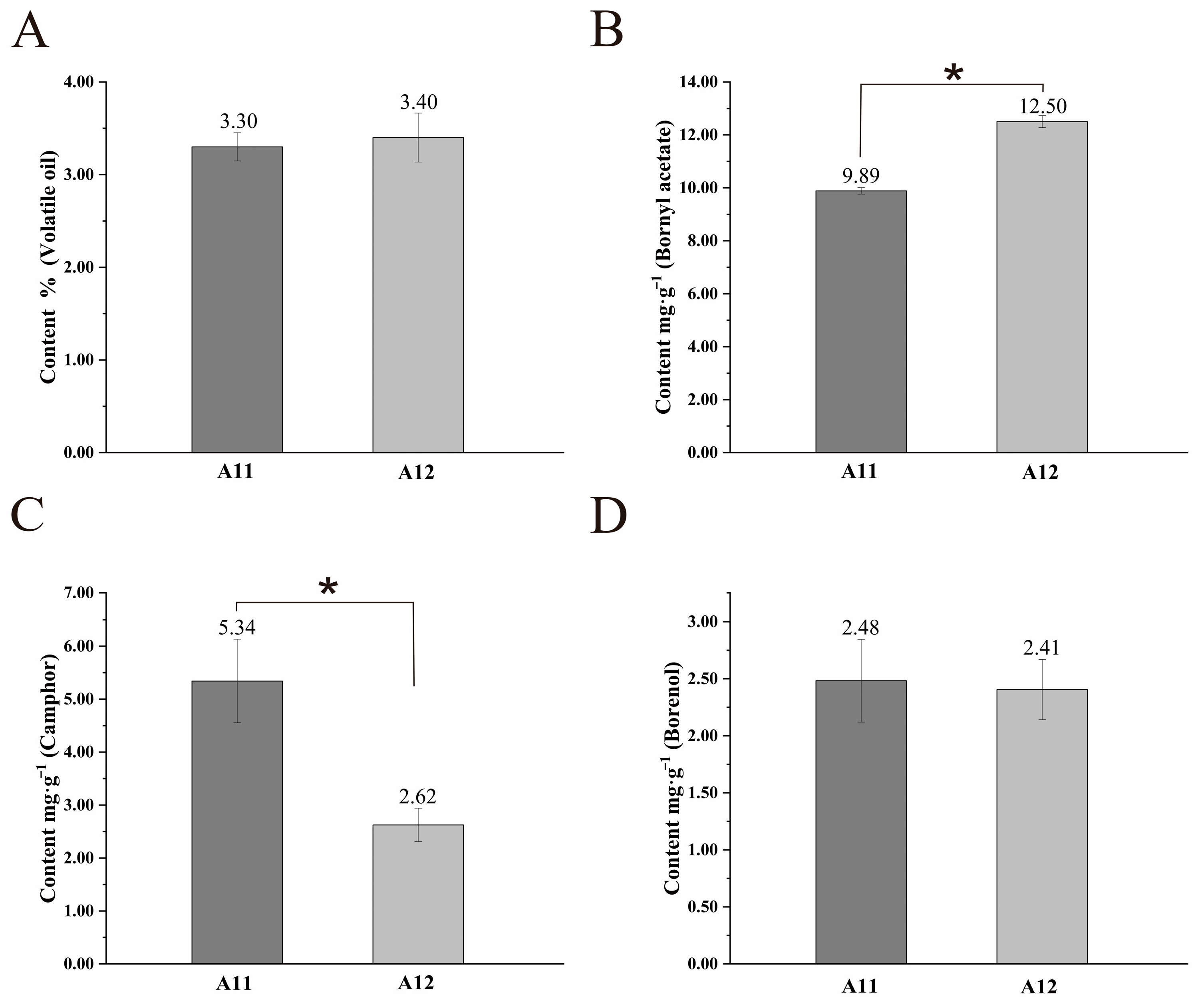
2.3. Differences in the Content of Major Active Ingredients
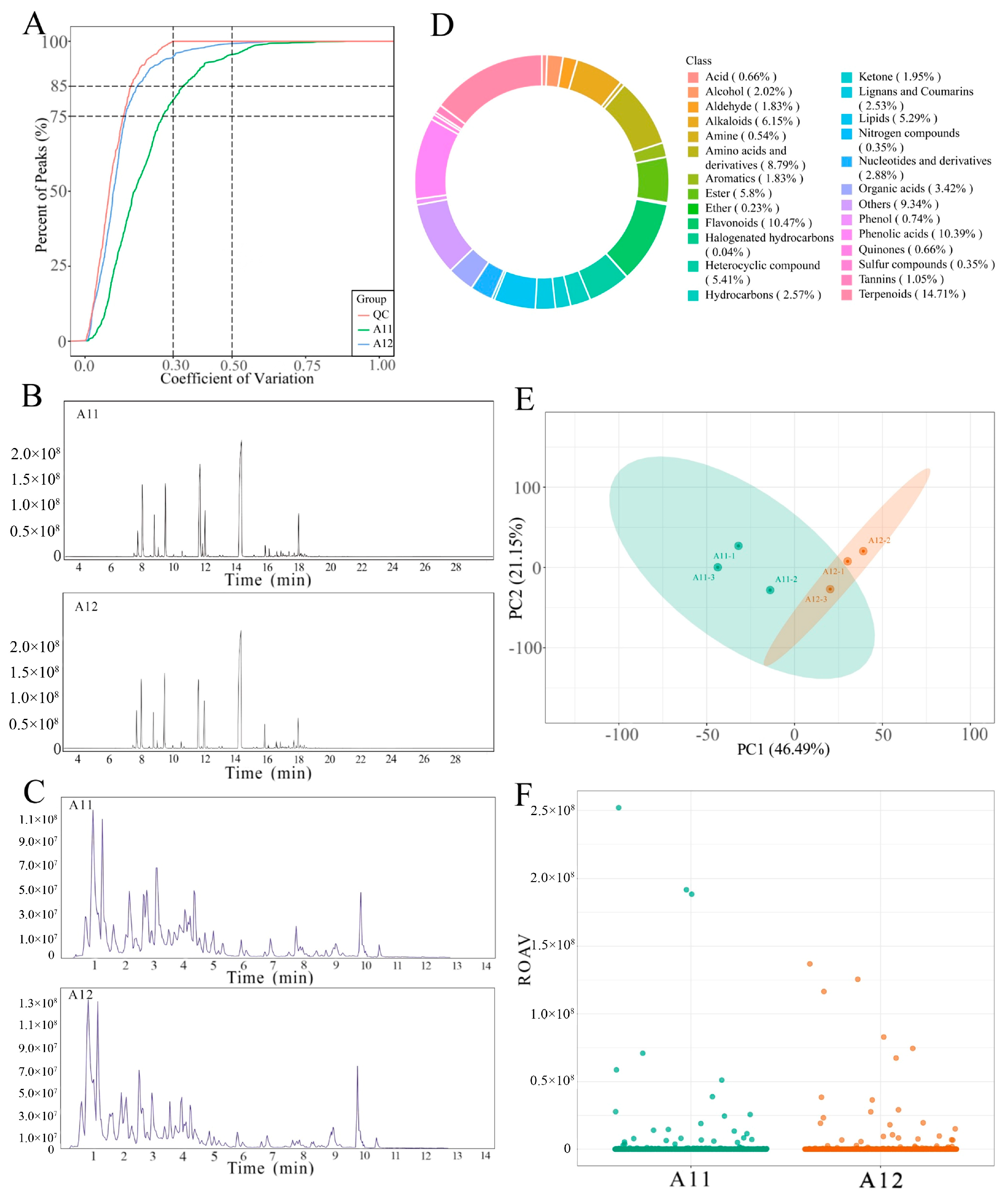
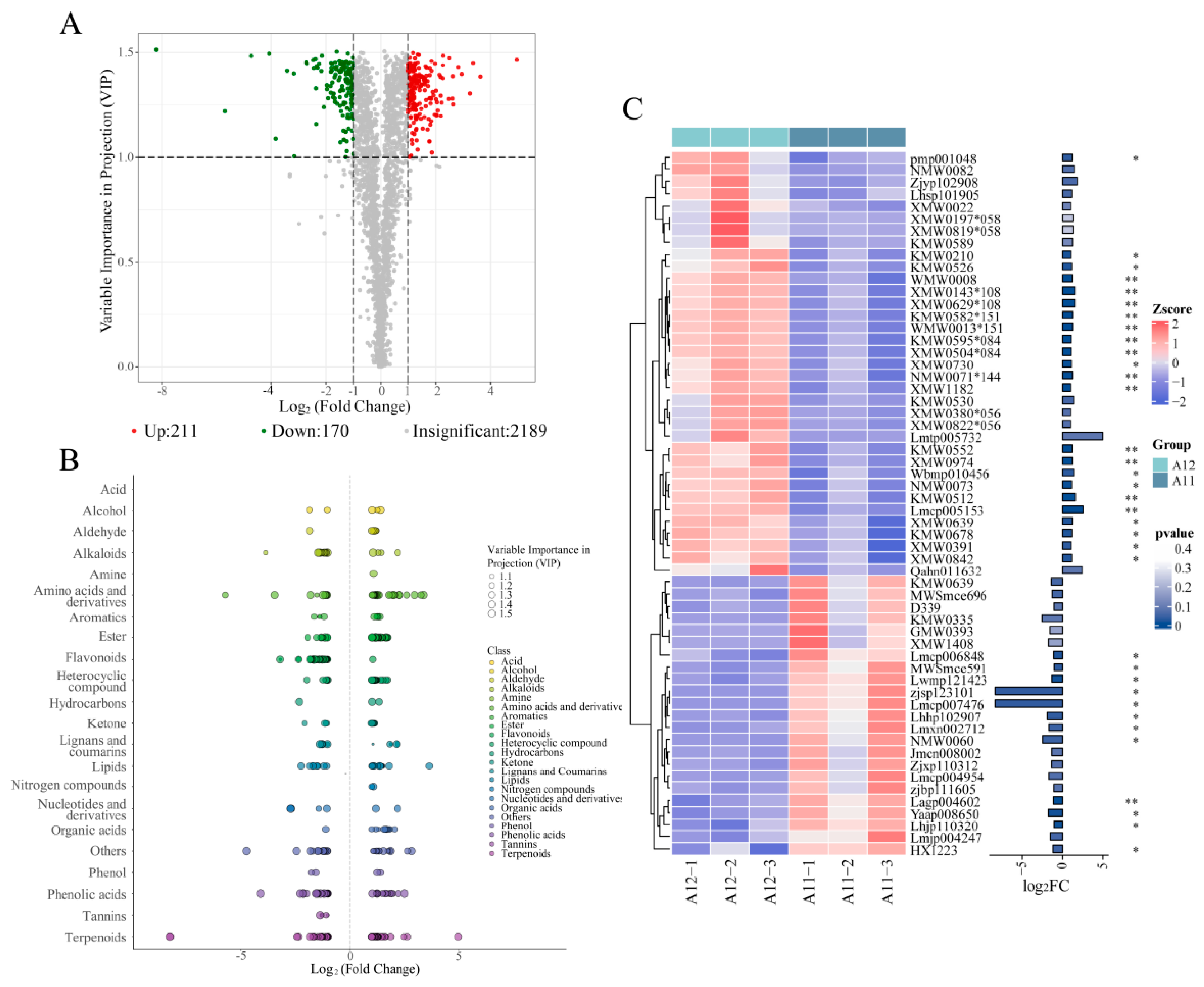
2.4. Metabolomics Analysis Results
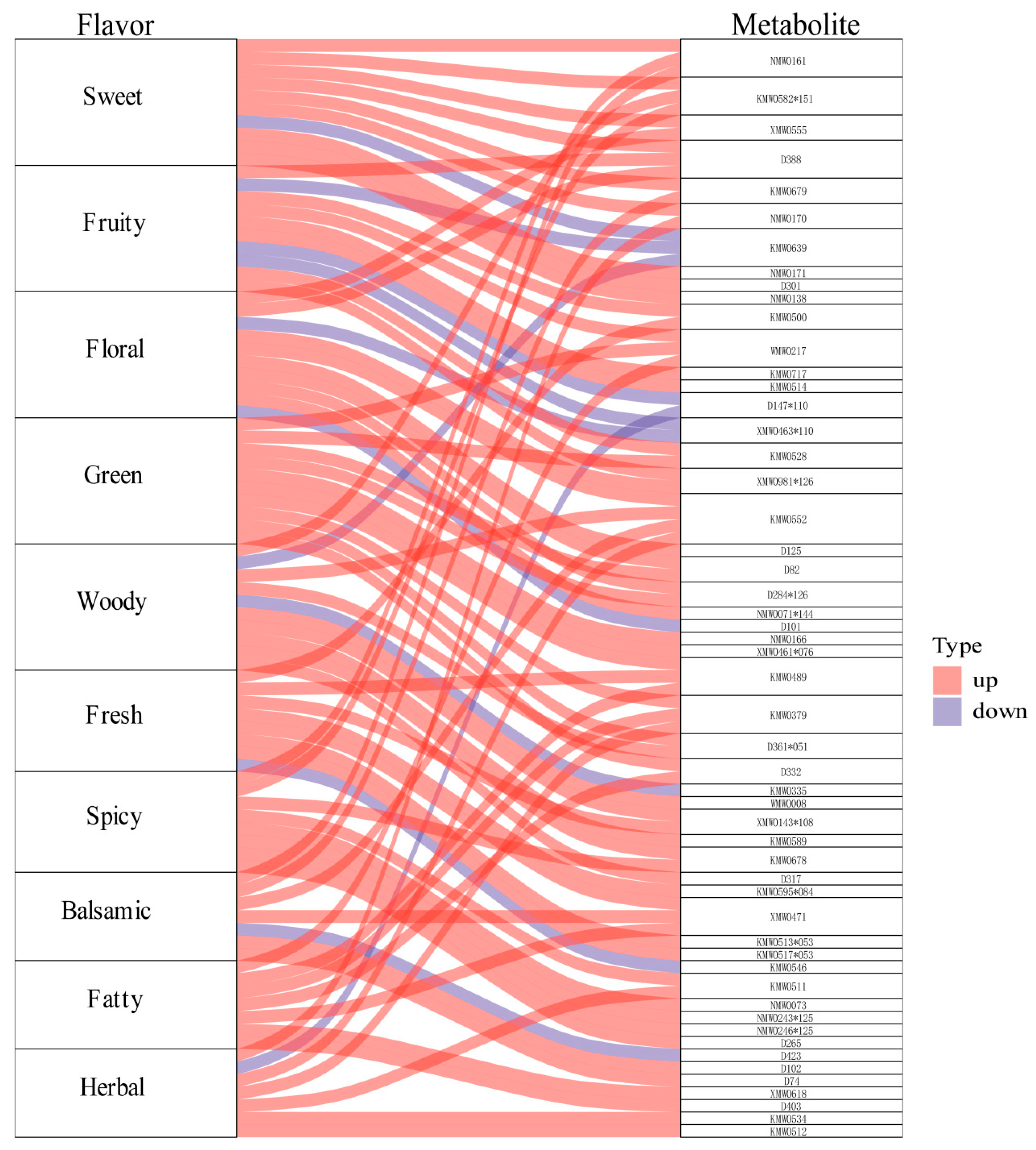
2.5. Differential Metabolite Flavoromics Analysis Results
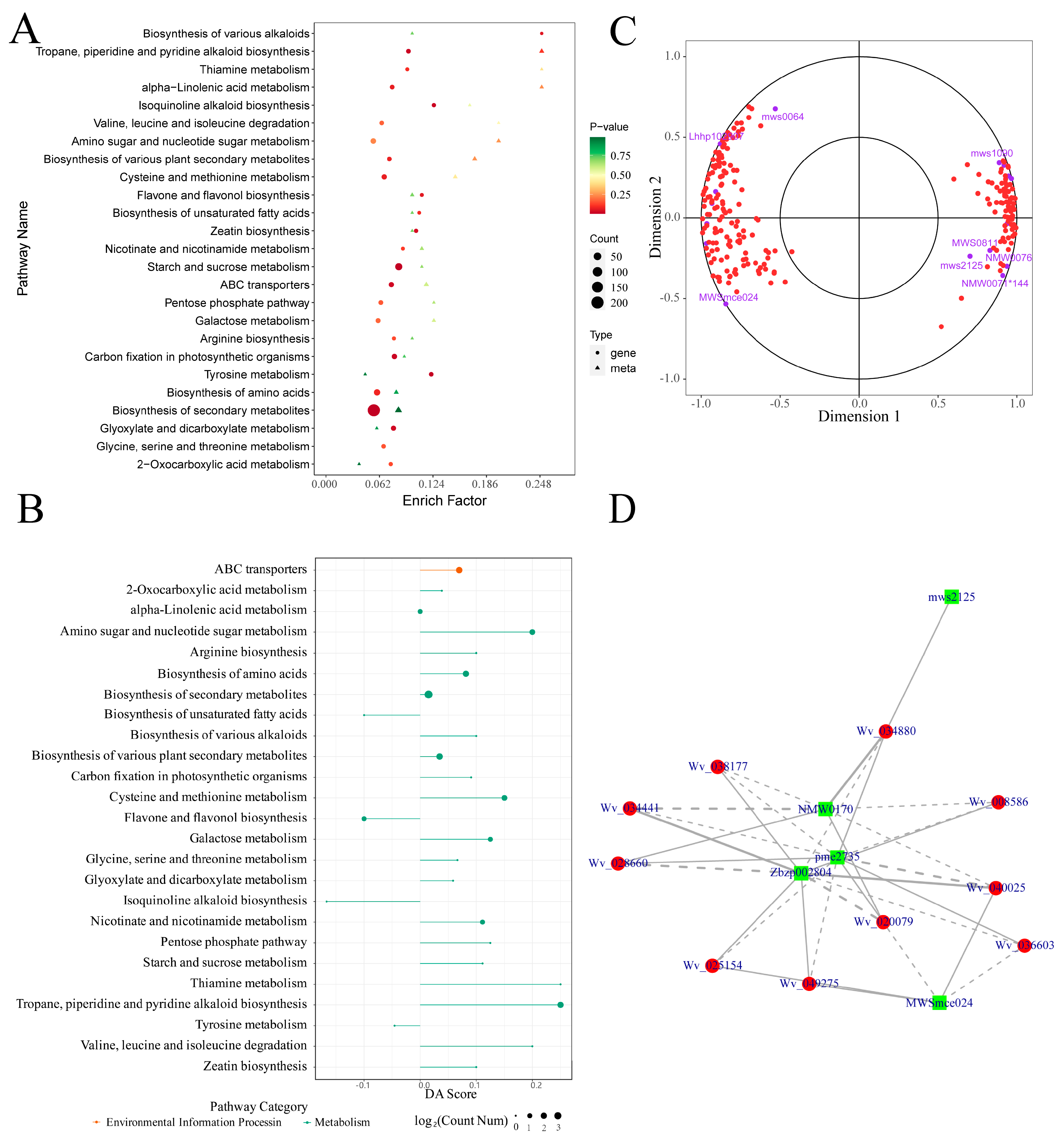
2.6. Transcriptomic Analysis Results
2.7. Metabolome and Transcriptome Correlation Analysis Results
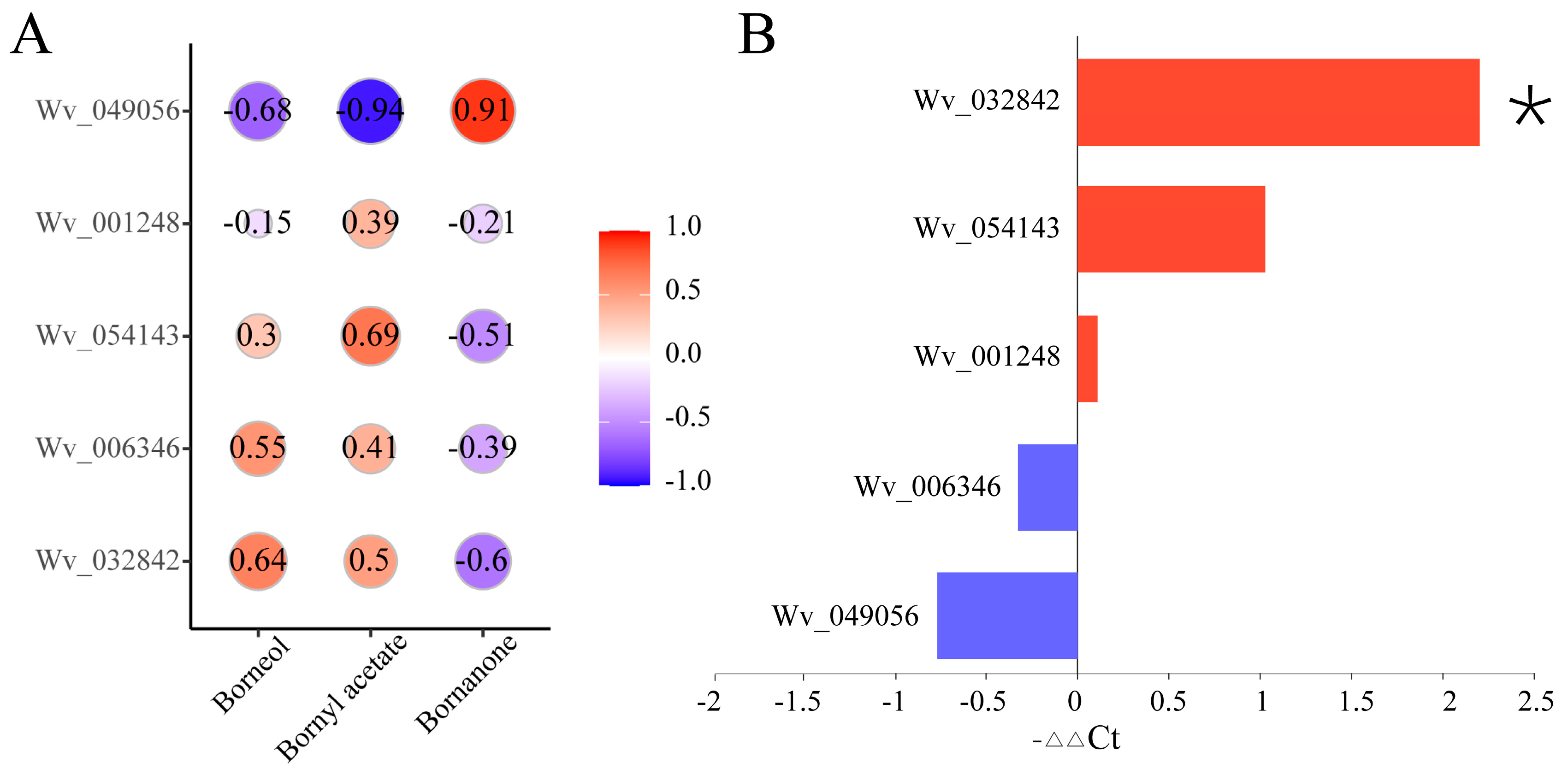
2.8. Analysis of the Correlation between the Main Active Ingredients and Key Genes, and qPCR Validation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Barcode Molecular Identification
4.3. Investigation of Plant Morphology and Medicinal Characteristics
4.4. Determination of Active Ingredient Content
4.5. Metabolomics Testing
4.5.1. Detection of Metabolites Based on the LC-MS Platform
4.5.2. Detection of Metabolites Based on the GC-MS Platform
4.6. Flavoromics Analysis
4.7. Transcriptomic Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Ge, L.; Fong Leong, W.; Huang, L.; Li, S. Advanced development in phytochemicals analysis of medicine and food dual purposes plants used in China (2011–2014). J. Chromatogr. 2016, 1428, A39–A54. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Zhang, Y.; Jiang, C.; Liu, C.; Huang, L. Research progress and strategic considerations for the regeneration of Chinese medicine resources. Sci. Sin. 2023, 53, 1274–1286. [Google Scholar] [CrossRef]
- Rao, K.S.; Haran, R.H.; Rajpoot, V.S. Value addition: A novel strategy for quality enhancement of medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100415. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, N.; Davoud, T.; Mohsen, H. Editorial: Advances in biotechnology-based breeding of medicinal plants. Front. Plant Sci. 2023, 14, 1228951. [Google Scholar] [CrossRef]
- Liang, H.; Lin, X.; Yang, P.; Sun, Y.; Wu, Q.; Alimujiang, S.; Zhao, H.; Ma, D.; Zhan, R.; Yang, J. Genome-Wide Identification of BAHD Superfamily and Functional Characterization of Bornyl Acetyltransferases Involved in the Bornyl Acetate Biosynthesis in Wurfbainia villosa. Front. Plant Sci. 2022, 13, 860152. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zeng, J.; Guan, J.; Xu, Y.; Jia, R.B.; Chen, J.; Jiang, G.; Zhou, C. Dietary supplement of Amomum villosum Lour. polysaccharide attenuates ulcerative colitis in BALB/c mice. Foods 2022, 11, 3737. [Google Scholar] [CrossRef] [PubMed]
- China Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; pp. 264–265.
- Wan, J.; Pang, Y.; Yang, Q.; Wang, D.; Zhang, W. Development and Utilization of Aromatic Plant Resources in Hainan. Mod. Chin. Med. 2015, 17, 276–284. [Google Scholar]
- Yang, D.; Zhang, Y.; Shu, Y.; Cao, H. Research Progress on Chemical Constituents and Pharmacological Effects of Amomum villosum. Guangdong Chem. Ind. 2022, 49, 111–114. [Google Scholar]
- Wu, M.; Li, Q.; Hu, Z.; Li, X.; Chen, S. The complete Amomum kravanh chloroplast genome sequence and phylogenetic analysis of the commelinids. Molecules 2017, 22, 1875. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L.; Zheng, Z.; Feng, Z. The impact of Amomum villosum cultivation on seasonal rainforest in Xishuangbanna, Southwest China. Biodivers. Conserv. 2006, 15, 2971–2985. [Google Scholar] [CrossRef]
- Feng, Y.L.; Li, X. The combined effects of soil moisture and irradiance on growth, biomass allocation, morphology and photosynthesis in Amomum villosum. Agrofor. Syst. 2007, 71, 89–98. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Wang, Y.; Yang, D.; He, G.; Peng, J.; Zhang, L. Research Progress on Cultivation of Amomum villosum Lour.: Original Plant of the Southern Famous Medicinal Fructus Amomi Villosi. World Chin. Med. 2022, 17, 1163–1170. [Google Scholar]
- Li, A.; Li, A.; Luo, C.; Liu, B. Geographical specificity of metabolomic traits among Amomum villosum Lour. Fruits from different areas in China. LWT-Food Sci. Technol. 2024, 191, 115619. [Google Scholar] [CrossRef]
- Ding, P.; Fang, Q.; Zhang, D. Comparative studies on bioactivities of Yunnan introduced Amomum villosum and Amomum villosum. Chin. Pharm. J. 2004, 39, 342–344. [Google Scholar]
- Wang, B.; Chen, H.; Qu, P.; Lin, R.; He, S.; Li, W.; Zhang, C.; Shi, X.; Liu, Y.; Du, H.; et al. Effect of Different Cultivation Patterns on Amomum villosum Yield and Quality Parameters, Rhizosphere Soil Properties, and Rhizosphere Soil Microbes. Horticulturae 2023, 9, 306. [Google Scholar] [CrossRef]
- Gao, R.; Hu, Y.; Dan, Y.; Hao, L.; Liu, X.; Song, J. Chinese herbal medicine resources: Where we stand. Chin. Herb. Med. 2020, 12, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, D.A.; Olaitan, E.O. Mutagenesis in crop improvement: Methods and applications. J. Crop Improv. 2024, 38, 156–178. [Google Scholar]
- Yang, J.; Liu, F.; Peng, Y.; Liu, Y.; Zhou, W.; Zhan, R.; Xu, H.; Chen, W. Radiation Mutagenesis of Amomum villosum Lour. and its Mutation Analysis Based on ISSR. J. Plant Genet. Resour. 2012, 13, 748–753. [Google Scholar] [CrossRef]
- He, G.; Yang, S.; Wang, Y. An integrated chemical characterization based on FT-NIR, and GC–MS for the comparative metabolite profiling of 3 species of the genus Amomum. Anal. Chim. Acta 2023, 1280, 341869. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, H.Y.; Wei, J.S.; Zhao, Y.Y.; Lin, X.J.; Su, J.; Li, F.; Li, M.; Ma, D.; Tan, X.; et al. Chromosome-level genome assembly and functional characterization of terpene synthases provide insights into the volatile terpenoid biosynthesis of Wurfbainia villosa. Plant J. 2022, 112, 630–645. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, K.; Li, W.; Peng, Y.; Yi, Y.; Qiao, M.; Fu, Y. Characterization of flavor and taste profile of different radish (Raphanus Sativus L.) varieties by headspace-gas chromatography-ion mobility spectrometry (GC/IMS) and E-nose/tongue. Food Chem. X 2024, 22, 101419. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Ma, L.; Pei, D.; Di, D.; Liu, J. Polysaccharides from different cultivars of wolfberry: Physicochemical characteristics and protection against oxidative stress. Ind. Crops Prod. 2023, 197, 116548. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.; Yan, W. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWT-Food Sci. Technol. 2023, 187, 115322. [Google Scholar] [CrossRef]
- Ouyang, H.; Fan, Y.; Wei, S.; Chang, Y.; He, J. Study on the Chemical Profile of Chrysanthemum (Chrysanthemum morifolium) and the Evaluation of the Similarities and Differences between Different Cultivars. Chem. Biodivers. 2022, 9, e202200252. [Google Scholar] [CrossRef]
- Yu, L.; Wu, W.; Pan, Y.; Wang, W.; Sun, L.; Liu, Y.; Li, D. Quality evaluation of different varieties of Zanthoxylum bungeanum Maxim. peels based on phenolic profiles, bioactivity, and HPLC fingerprint. J. Food Sci. 2020, 85, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Liu, Y.; Wang, Y.; Mo, X.; Zeng, S. Aging and/or tissue-specific regulation of patchoulol and pogostone in two Pogostemon cablin (Blanco) Benth. cultivars. Physiol. Plant. 2016, 158, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zou, X.; Lan Lun, L.; Cao, C.; Jiang, B.; Zeng, Y. Discrimination of Aromi Fructus from Different Origins Using Electronic Nose Combined with Headspace-Gas Chromatography-Mass Spectrometry Coupled and Chemometrics. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 35–42. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Huang, Y.; An, W.; Liu, S.; Huang, S.; Zheng, X. Metabolism and transcriptome profiling provides insight into the genes and transcription factors involved in monoterpene biosynthesis of borneol chemotype of Cinnamomum camphora induced by mechanical damage. Peer J. 2021, 9, e11465. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.X.; Wang, T.T.; Huang, X.S.; Zhan, R.T.; Yang, J.F.; Ma, D.M. Nudix hydrolase WvNUDX24 is involved in borneol biosynthesis in Wurfbainia villosa. Plant J. 2024, 118, 1218–1231. [Google Scholar] [CrossRef]
- Afzaal, Z.; Hussain, I.; Ashraf, M.A.; Rasheed, R.; Javed, M.T.; Ansari, M.U.R.; Ansari, M.R.; Iqbal, M. Lead induced modulation in growth, chlorophyll pigment, nutrient uptake, antioxidant enzyme regulation, gene expression and fruit quality in two tomato cultivars. Int. J. Agric. Biol. 2020, 1732–1744. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Han, X.; Li, C.; Wang, F.; Nie, B.; Hou, Z.; Yang, S.; Ji, J.; Li, G.; et al. Multiomics comparison among populations of three plant sources of Amomi Fructus. Hortic. Res. 2023, 10, uhad128. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Mu, Y.; Tang, D.; Yu, J.; Deng, Z.; Liu, S.; Li, Y.; Zhang, L. Analysis of the Existing Standard and Construction of Standard System for Amomi Fructus Industry. J. Chin. Med. Mater. 2024, 47, 1–6. [Google Scholar] [CrossRef]
- Yuan, H.; Jiangfang, Y.; Liu, Z.; Su, R.; Li, Q.; Fang, C.; Huang, S.; Liu, X.; Fernie, A.R.; Luo, J. WTV2.0: A high-coverage plant volatilomics method with a comprehensive selective ion monitoring acquisition mode. Mol. Plant 2024, 17, 972–985. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Luo, X.; Yao, Y.; Wang, Y.; Dai, Z.; Cheng, T.; Huang, X.; Bai, M.; He, J.; Wu, H. Integrated Analysis of Metabolomics, Flavoromics, and Transcriptomics for Evaluating New Varieties of Amomum villosum Lour. Plants 2024, 13, 2382. https://doi.org/10.3390/plants13172382
Li Z, Luo X, Yao Y, Wang Y, Dai Z, Cheng T, Huang X, Bai M, He J, Wu H. Integrated Analysis of Metabolomics, Flavoromics, and Transcriptomics for Evaluating New Varieties of Amomum villosum Lour. Plants. 2024; 13(17):2382. https://doi.org/10.3390/plants13172382
Chicago/Turabian StyleLi, Zhenkai, Xin Luo, Yanli Yao, Yukun Wang, Zhiheng Dai, Tianle Cheng, Xinzhi Huang, Mei Bai, Junjun He, and Hong Wu. 2024. "Integrated Analysis of Metabolomics, Flavoromics, and Transcriptomics for Evaluating New Varieties of Amomum villosum Lour." Plants 13, no. 17: 2382. https://doi.org/10.3390/plants13172382
APA StyleLi, Z., Luo, X., Yao, Y., Wang, Y., Dai, Z., Cheng, T., Huang, X., Bai, M., He, J., & Wu, H. (2024). Integrated Analysis of Metabolomics, Flavoromics, and Transcriptomics for Evaluating New Varieties of Amomum villosum Lour. Plants, 13(17), 2382. https://doi.org/10.3390/plants13172382







