Molecular Insights into the Role of Sterols in Microtuber Development of Potato Solanum tuberosum L.
Abstract
:1. Introduction
2. Results
2.1. Microtuber Development
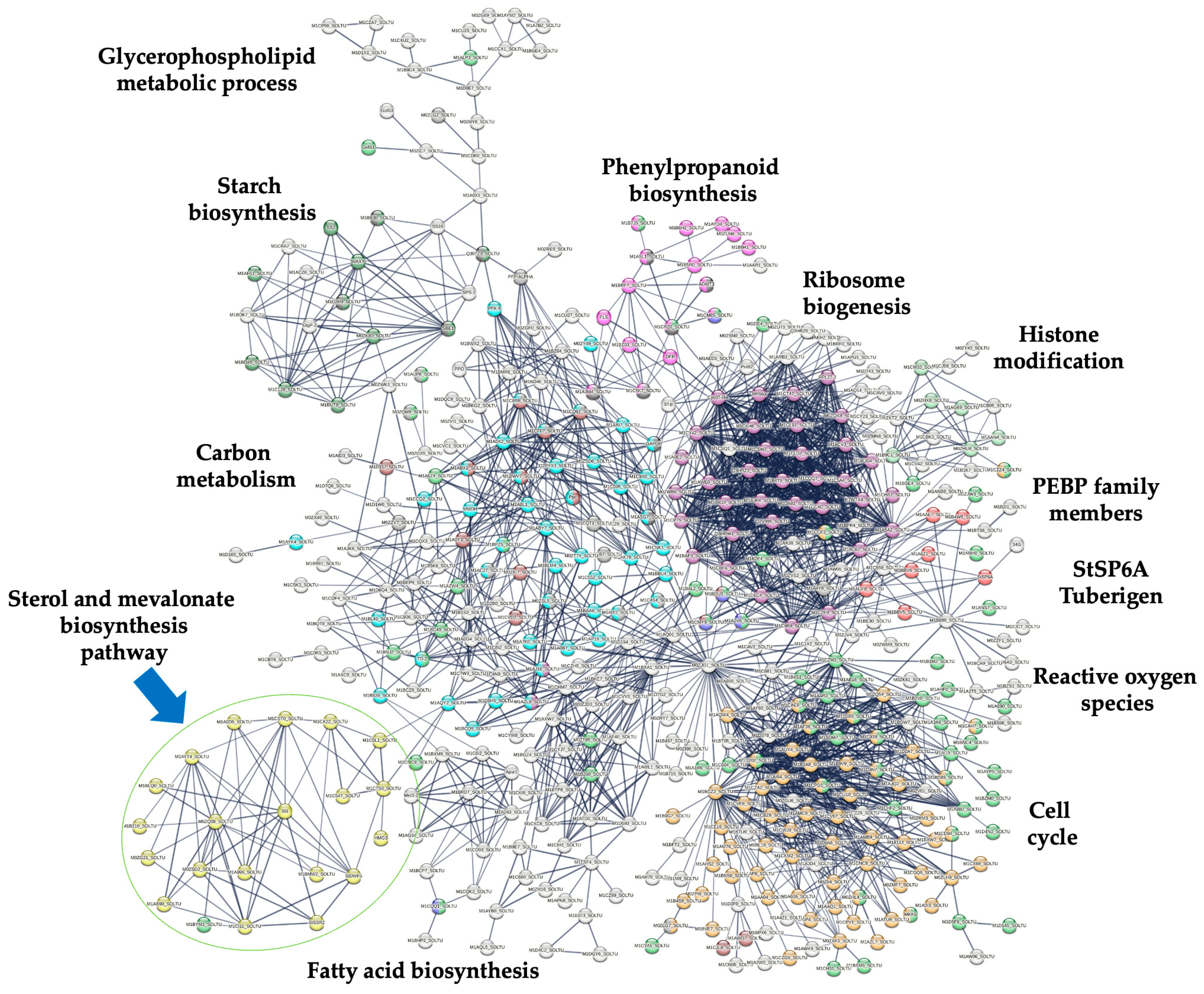
2.2. Identification of Up-Regulated Transcripts Involved in PS Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Material and MTs Induction
4.2. Isolation of RNA, qPCR, and Transcriptome Sequencing
4.3. Analysis of DEG and Interaction Analysis of PS Enzymes
4.4. Transcriptional Analysis through qPCR of Genes Involved in PS Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diener, A.C.; Li, H.; Zhou, W.; Whoriskey, W.J.; Nes, W.D.; Fink, G.R. STEROL METHYLTRANSFERASE 1 Controls the Level of Cholesterol in Plants. Plant Cell 2000, 12, 853–870. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant Sterols: Biosynthesis, Biological Function and Their Importance to Human Nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Hartmann, M. Plant Sterols and the Membrane Environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Souter, M.; Topping, J.; Pullen, M.; Friml, J.; Palme, K.; Hackett, R.; Grierson, D.; Lindsey, K. Hydra Mutants of Arabidopsis Are Defective in Sterol Profiles and Auxin and Ethylene Signaling. Plant Cell 2002, 14, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Kawagoe, Y.; Hogan, P.; Delmer, D. Sitosterol-β-Glucoside as Primer for Cellulose Synthesis in Plants. Science 2002, 295, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; DeBolt, S.; Bulone, V. Deciphering the Molecular Functions of Sterols in Cellulose Biosynthesis. Front. Plant Sci. 2012, 3, 25882. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; Fujioka, S.; Takatsuto, S.; Stierhof, Y.; Stransky, H.; Yoshida, S.; Jürgens, G. A Link between Sterol Biosynthesis, the Cell Wall, and Cellulose in Arabidopsis. Plant J. 2004, 38, 227–243. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, F.; Li, J. Promotive Effect of Brassinosteroids on Cell Division Involves a Distinct CycD3 -induction Pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Carland, F.; Fujioka, S.; Nelson, T. The Sterol Methyltransferases SMT1, SMT2, and SMT3 Influence Arabidopsis Development through Nonbrassinosteroid Products. Plant Physiol. 2010, 153, 741–756. [Google Scholar] [CrossRef]
- Mayer, U.; Ruiz, R.A.T.; Berleth, T.; Miséra, S.; Jürgens, G. Mutations Affecting Body Organization in the Arabidopsis Embryo. Nature 1991, 353, 402–407. [Google Scholar] [CrossRef]
- Schrick, K.; Mayer, U.; Horrichs, A.; Kuhnt, C.; Bellini, C.; Dangl, J.; Schmidt, J.; Jürgens, G. FACKEL Is a Sterol C-14 Reductase Required for Organized Cell Division and Expansion in Arabidopsis Embryogenesis. Genes Dev. 2000, 14, 1471–1484. [Google Scholar] [CrossRef]
- Jang, J.-C.; Fujioka, S.; Tasaka, M.; Seto, H.; Takatsuto, S.; Ishii, A.; Aida, M.; Yoshida, S.; Sheen, J. A Critical Role of Sterols in Embryonic Patterning and Meristem Programming Revealed by the Fackel Mutants of Arabidopsis thaliana. Genes Dev. 2000, 14, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Pullen, M.; Clark, N.; Zarinkamar, F.; Topping, J.; Lindsey, K. Analysis of Vascular Development in the Hydra Sterol Biosynthetic Mutants of Arabidopsis. PLoS ONE 2010, 5, e12227. [Google Scholar] [CrossRef]
- Douglas, T.J.; Paleg, L.G. Inhibition of Sterol Biosynthesis and Stem Elongation of Tobacco Seedlings Induced by Some Hypocholesterolemic Agents. J. Exp. Bot. 1981, 32, 59–68. [Google Scholar] [CrossRef]
- Babiychuk, E.; Bouvier-Navé, P.; Compagnon, V.; Suzuki, M.; Muranaka, T.; Van Montagu, M.; Kushnir, S.; Schaller, H. Allelic Mutant Series Reveal Distinct Functions for Arabidopsis Cycloartenol Synthase 1 in Cell Viability and Plastid Biogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3163–3168. [Google Scholar] [CrossRef]
- Hong, Z.; Ueguchi-Tanaka, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. The Rice Brassinosteroid-Deficient Dwarf2 Mutant, Defective in the Rice Homolog of Arabidopsis DIMINUTO/DWARF1, Is Rescued by the Endogenously Accumulated Alternative Bioactive Brassinosteroid, Dolichosterone. Plant Cell 2005, 17, 2243–2254. [Google Scholar] [CrossRef]
- Moehninsi; Lange, I.; Lange, B.M.; Navarre, D.A. Altering Potato Isoprenoid Metabolism Increases Biomass and Induces Early Flowering. J. Exp. Bot. 2020, 71, 4109–4124. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zheng, C.; Zhao, Y.; Li, Q.; Liu, J.; Deng, R.; Lei, T.; Wang, S.; Wang, X. RNA Interference Knockdown of the Brassinosteroid Receptor BRI1 in Potato (Solanum tuberosum L.) Reveals Novel Functions for Brassinosteroid Signaling in Controlling Tuberization. Sci. Hortic. 2021, 290, 110516. [Google Scholar] [CrossRef]
- Schuler, I.; Milon, A.; Nakatani, Y.; Ourisson, G.; Albrecht, A.M.; Benveniste, P.; Hartman, M.A. Differential Effects of Plant Sterols on Water Permeability and on Acyl Chain Ordering of Soybean Phosphatidylcholine Bilayers. Proc. Natl. Acad. Sci. USA 1991, 88, 6926–6930. [Google Scholar] [CrossRef]
- Grandmougin-Ferjani, A.; Schuler-Muller, I.; Hartmann, M.A. Sterol Modulation of the Plasma Membrane H+-ATPase Activity from Corn Roots Reconstituted into Soybean Lipids. Plant Physiol. 1997, 113, 163–174. [Google Scholar] [CrossRef]
- Schrick, K.; Mayer, U.; Martin, G.; Bellini, C.; Kuhnt, C.; Schmidt, J.; Jürgens, G. Interactions between Sterol Biosynthesis Genes in Embryonic Development of Arabidopsis. Plant J. 2002, 31, 61–73. [Google Scholar] [CrossRef]
- Lindsey, K.; Pullen, M.L.; Topping, J.F. Importance of Plant Sterols in Pattern Formation and Hormone Signalling. Trends Plant Sci. 2003, 8, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Closa, M.; Vranová, E.; Bortolotti, C.; Bigler, L.; Arró, M.; Ferrer, A.; Gruissem, W. The Arabidopsis thaliana FPP Synthase Isozymes Have Overlapping and Specific Functions in Isoprenoid Biosynthesis, and Complete Loss of FPP Synthase Activity Causes Early Developmental Arrest: Arabidopsis thaliana FPP Synthase Mutants. Plant J. 2010, 63, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Chen, F.; Li, P.P.; Chen, Y.M.; Chen, M.; Yang, Q. Identification and Characterization of Brassinosteroid Biosynthesis and Signaling Pathway Genes in Solanum Tuberosum. Russ. J. Plant Physiol. 2019, 66, 628–636. [Google Scholar] [CrossRef]
- Arnqvist, L.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Reduction of Cholesterol and Glycoalkaloid Levels in Transgenic Potato Plants by Overexpression of a Type 1 Sterol Methyltransferase cDNA. Plant Physiol. 2003, 131, 1792–1799. [Google Scholar] [CrossRef]
- Kärenlampi, S.O.; White, P.J. Potato proteins, lipids, and minerals. In Advances in Potato Chemistry and Technology; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2009; pp. 99–125. [Google Scholar] [CrossRef]
- Nahar, N.; Westerberg, E.; Arif, U.; Huchelmann, A.; Olarte Guasca, A.; Beste, L.; Dalman, K.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Transcript Profiling of Two Potato Cultivars during Glycoalkaloid-Inducing Treatments Shows Differential Expression of Genes in Sterol and Glycoalkaloid Metabolism. Sci. Rep. 2017, 7, 43268. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Rohmer, M.; Schwender, J. Two Independent Biochemical Pathways for Isopentenyl Diphosphate and Isoprenoid Biosynthesis in Higher Plants. Physiol. Plant. 1997, 101, 643–652. [Google Scholar] [CrossRef]
- Krits, P.; Fogelman, E.; Ginzberg, I. Potato Steroidal Glycoalkaloid Levels and the Expression of Key Isoprenoid Metabolic Genes. Planta 2007, 227, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-K.; Zhao, Y.; Chen, S.-Y.; Kennelly, E.J. Solanum Steroidal Glycoalkaloids: Structural Diversity, Biological Activities, and Biosynthesis. Nat. Prod. Rep. 2021, 38, 1423–1444. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Herrera-Isidrón, L.; Flores-López, J.A.; Recoder-Meléndez, O.S.; Barraza, A.; Cabrera-Ponce, J.L. Solanum Tuberosum Microtuber Development under Darkness Unveiled through RNAseq Transcriptomic Analysis. Int. J. Mol. Sci. 2022, 23, 13835. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C. Control of Isoprenoid Biosynthesis in Higher Plants. Adv. Bot. Res. 1988, 14, 25–91. [Google Scholar] [CrossRef]
- Bach, T.J.; Boronat, A.; Campos, N.; Ferrer, A.; Vollack, K.-U. Mevalonate Biosynthesis in Plants. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 107–122. [Google Scholar] [CrossRef]
- Yang, Z.; Park, H.; Lacy, G.H.; Cramer, C.L. Differential Activation of Potato 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Genes by Wounding and Pathogen Challenge. Plant Cell 1991, 3, 397–405. [Google Scholar] [CrossRef]
- Learned, R.M. Light Suppresses 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Gene Expression in Arabidopsis thaliana. Plant Physiol. 1996, 110, 645–655. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, O.R.; Oh, J.Y.; Jang, M.-G.; Yang, D.-C. Functional Analysis of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Encoding Genes in Triterpene Saponin-Producing Ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Lozano, E.; Herrera-Isidrón, L.; Flores-López, J.A.; Recoder-Meléndez, O.S.; Uribe-López, B.; Barraza, A.; Cabrera-Ponce, J.L. Exploring the Potential Role of Ribosomal Proteins to Enhance Potato Resilience in the Face of Changing Climatic Conditions. Genes 2023, 14, 1463. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Ward, B.L.; Bostock, R.M. Differential Induction and Suppression of Potato 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Genes in Response to Phytophthora Infestans and to Its Elicitor Arachidonic Acid. Plant Cell 1992, 4, 1333–1344. [Google Scholar] [CrossRef]
- Korth, K.L.; Stermer, B.A.; Bhattacharyya, M.K.; Dixon, R.A. HMG-CoA Reductase Gene Families That Differentially Accumulate Transcripts in Potato Tubers Are Developmentally Expressed in Floral Tissues. Plant Mol. Biol. 1997, 33, 545–551. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kato-Emori, S.; Tomita, K.; Ezura, H. Detection of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Protein Cm-HMGR during Fruit Development in Melon (Cucumis melo L.). Theor. Appl. Genet. 2002, 104, 779–785. [Google Scholar] [CrossRef]
- Cordier, H.; Karst, F.; Bergès, T. Heterologous Expression in Saccharomyces Cerevisiae of an Arabidopsis thaliana cDNA Encoding Mevalonate Diphosphate Decarboxylase. Plant Mol. Biol. 1999, 39, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Cunillera, N.; Arró, M.; Delourme, D.; Karst, F.; Boronat, A.; Ferrer, A. Arabidopsis thaliana Contains Two Differentially Expressed Farnesyl-Diphosphate Synthase Genes. J. Biol. Chem. 1996, 271, 7774–7780. [Google Scholar] [CrossRef]
- Corey, E.J.; Matsuda, S.P.; Bartel, B. Isolation of an Arabidopsis thaliana Gene Encoding Cycloartenol Synthase by Functional Expression in a Yeast Mutant Lacking Lanosterol Synthase by the Use of a Chromatographic Screen. Proc. Natl. Acad. Sci. USA 1993, 90, 11628–11632. [Google Scholar] [CrossRef] [PubMed]
- Rondet, S.; Taton, M.; Rahier, A. Identification, Characterization, and Partial Purification of 4α-Carboxysterol–C3-Dehydrogenase/ C4-Decarboxylase from Zea mays. Arch. Biochem. Biophys. 1999, 366, 249–260. [Google Scholar] [CrossRef]
- Song, J.; Sun, S.; Ren, H.; Grison, M.; Boutté, Y.; Bai, W.; Men, S. The SMO1 Family of Sterol 4α-Methyl Oxidases Is Essential for Auxin- and Cytokinin-Regulated Embryogenesis. Plant Physiol. 2019, 181, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Lovato, M.A.; Hart, E.A.; Segura, M.J.R.; Giner, J.-L.; Matsuda, S.P.T. Functional Cloning of an Arabidopsis thaliana cDNA Encoding Cycloeucalenol Cycloisomerase. J. Biol. Chem. 2000, 275, 13394–13397. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Han, B.; Forestier, E.; Hu, Z.; Gao, N.; Lu, W.; Schaller, H.; Li, J.; Hou, S. Sterols Are Required for Cell-fate Commitment and Maintenance of the Stomatal Lineage in A. rabidopsis. Plant J. 2013, 74, 1029–1044. [Google Scholar] [CrossRef]
- Men, S.; Boutté, Y.; Ikeda, Y.; Li, X.; Palme, K.; Stierhof, Y.D.; Hartmann, M.A.; Moritz, T.; Grebe, M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 2008, 10, 237–244. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Kelly, S.L. Molecular Diversity of Sterol 14α-demethylase Substrates in Plants, Fungi and Humans. FEBS Letters 1998, 425, 263–265. [Google Scholar] [CrossRef]
- Kushiro, M.; Nakano, T.; Sato, K.; Yamagishi, K.; Asami, T.; Nakano, A.; Takatsuto, S.; Fujioka, S.; Ebizuka, Y.; Yoshida, S. Obtusifoliol 14α-Demethylase (CYP51) Antisense Arabidopsis Shows Slow Growth and Long Life. Biochem. Biophys, Res. Commun. 2001, 285, 98–104. [Google Scholar] [CrossRef]
- Kim, H.B.; Schaller, H.; Goh, C.-H.; Kwon, M.; Choe, S.; An, C.S.; Durst, F.; Feldmann, K.A.; Feyereisen, R. Arabidopsis Cyp51 Mutant Shows Postembryonic Seedling Lethality Associated with Lack of Membrane Integrity. Plant Physiol. 2005, 138, 2033–2047. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Yin, L.; Zhang, Q.; Xu, W.; Jia, Y.; Xia, K.; Zhang, M. The Putative Obtusifoliol 14α-demethylase OSCYP51H3 Affects Multiple Aspects of Rice Growth and Development. Physiol. Plant. 2022, 174, e13764. [Google Scholar] [CrossRef] [PubMed]
- He, J.-X.; Fujioka, S.; Li, T.-C.; Kang, S.G.; Seto, H.; Takatsuto, S.; Yoshida, S.; Jang, J.-C. Sterols Regulate Development and Gene Expression in Arabidopsis. Plant Physiol. 2003, 131, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Topping, J.F.; May, V.J.; Muskett, P.R.; Lindsey, K. Mutations in the HYDRA1 Gene of Arabidopsis Perturb Cell Shape and Disrupt Embryonic and Seedling Morphogenesis. Development 1997, 124, 4415–4424. [Google Scholar] [CrossRef]
- Bouvier-Navé, P.; Husselstein, T.; Benveniste, P. Two Families of Sterol Methyltransferases Are Involved in the First and the Second Methylation Steps of Plant Sterol Biosynthesis. Eur. J. Biochem. 1998, 256, 88–96. [Google Scholar] [CrossRef]
- Schaeffer, A.; Bronner, R.; Benveniste, P.; Schaller, H. The Ratio of Campesterol to Sitosterol That Modulates Growth in Arabidopsis Is Controlled by STEROL METHYLTRANSFERASE 2;1. Plant J. 2001, 25, 605–615. [Google Scholar] [CrossRef]
- Schaller, H. The Role of Sterols in Plant Growth and Development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Neelakandan, A.K.; Song, Z.; Wang, J.; Richards, M.H.; Wu, X.; Valliyodan, B.; Nguyen, H.T.; Nes, W.D. Cloning, Functional Expression and Phylogenetic Analysis of Plant Sterol 24C-Methyltransferases Involved in Sitosterol Biosynthesis. Phytochemistry 2009, 70, 1982–1998. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Nie, X.; Boutté, Y.; Grison, M.; Li, P.; Kuang, S.; Men, S. Sterol Methyl Oxidases Affect Embryo Development via Auxin-Associated Mechanisms. Plant Physiol. 2016, 171, 468–482. [Google Scholar] [CrossRef]
- Gachotte, D.; Husselstein, T.; Bard, M.; Lacroute, F.; Benveniste, P. Isolation and Characterization of an Arabidopsis thaliana cDNA Encoding a Δ 7 -sterol-C-5-desaturase by Functional Complementation of a Defective Yeast Mutant. Plant J. 1996, 9, 391–398. [Google Scholar] [CrossRef]
- Choe, S.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tissier, C.P.; Gregory, B.D.; Ross, A.S.; Tanaka, A.; Yoshida, S.; Tax, F.E.; et al. The Arabidopsis Dwf7/Ste1 Mutant Is Defective in the Δ 7 Sterol C-5 Desaturation Step Leading to Brassinosteroid Biosynthesis. Plant Cell 1999, 11, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Ohyama, K.; Yasumoto, S.; Seki, H.; Sakuma, T.; Yamamoto, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Aoki, T.; et al. Sterol Side Chain Reductase 2 Is a Key Enzyme in the Biosynthesis of Cholesterol, the Common Precursor of Toxic Steroidal Glycoalkaloids in Potato. Plant Cell 2014, 26, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Klahre, U.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Yokota, T.; Nomura, T.; Yoshida, S.; Chua, N.-H. The Arabidopsis DIMINUTO/DWARF1 Gene Encodes a Protein Involved in Steroid Synthesis. Plant Cell 1998, 10, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Kauschmann, A.; Jessop, A.; Koncz, C.; Szekeres, M.; Willmitzer, L.; Altmann, T. Genetic Evidence for an Essential Role of Brassinosteroids in Plant Development. Plant J. 1996, 9, 701–713. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein–Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Sherry, S.T. dbSNP: The NCBI Database of Genetic Variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


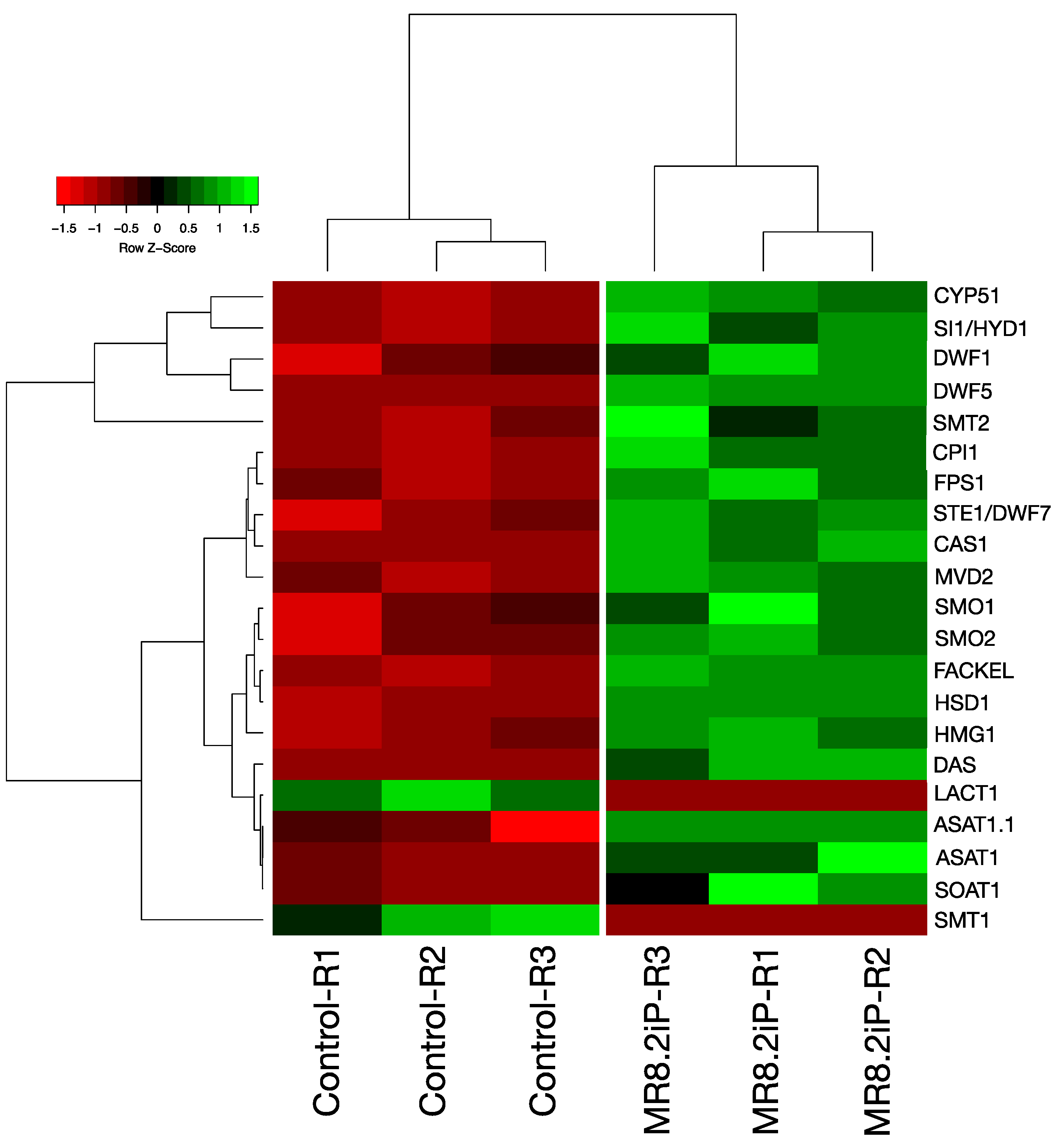



| No | ID String v.12 | Arabidopsis | Annotation |
|---|---|---|---|
| 1 | HMG3 | HMGR3 | Hydroxy-3-methylglutaryl-coenzyme A reductase 1-like |
| 2 | M1C7S0_SOLTU | MVD2 | Diphosphomevalonate decarboxylase-like |
| 3 | M1CX22_SOLTU | FPS1 | Farnesyl pyrophosphate synthase 1-like |
| 4 | M1CST0_SOLTU | CAS1 | Cycloartenol synthase |
| 5 | M1AYT4_SOLTU | SMO1-1 | Fatty acid hydroxylase domain-containing protein |
| 6 | M1AB90_SOLTU | HSD1 | 3βHSD domain-containing protein |
| 7 | M1A0D5_SOLTU | CPI1 | Cycloeucalenol cycloisomerase |
| 8 | M1BCS3_SOLTU | CYP51 | Sterol 14-demethylase |
| 9 | M0ZSD2_SOLTU | FACKEL | Delta14-sterol reductase |
| 10 | SI1 | HYD1 | Cholestenol Delta-isomerase |
| 11 | M1BLQ0_SOLTU | SMT2 | 24-methylenesterol C-methyltransferase |
| 12 | M0ZQ08_SOLTU | SMO2 | Fatty acid hydroxylase domain-containing protein |
| 13 | M1CI11_SOLTU | STE1/DWF7 | Delta7-sterol 5-desaturase |
| 14 | StSSR1 | DWF1 | Delta (24)-sterol reductase-like |
| 15 | M1BMW2_SOLTU | DAS | Delta-amyrin synthase |
| ID String v.12 | ID | NCBI | Forward | Reverse |
|---|---|---|---|---|
| M1BCS3_SOLTU | CYP51 | XM_006348474.2 | CATACAGGCAAGGCAGAGAA | AGAGCAGCAATCAGAAGACC |
| M1C7G1_SOLTU | FD | XM_006361882.2 | GAAAGCAGGCTTACACGAATG | GAAGTAGAGCACCAGCTGAA |
| M0ZSD2_SOLTU | FACKEL | XM_006341108.2 | AAGACGTGTGGGCTGAATAC | TACGGTACGGTGAGGCTAAT |
| M1CX22_SOLTU | FPS1 | XM_006344841.2 | GGAGGTGTACTCTGTGCTTAAA | GATAGTCCTCGATTCAGCTTCC |
| SI1 | SI1 | NM_001288433.1 | CACATGGTCCTTGAGGGATATT | CTCGGCCTACGTATCTTGAATC |
| M1A996_SOLTU | SMT1 | XM_015310447.1 | CTAAACCGTATTGGCGGAATTG | GGGCATGACATGTAGCTTCTA |
| M1BLQ0_SOLTU | SMT2 | XM_006364446.2 | CCCAATGGATTCTCTCACTCTC | GCCCGTTTACCCTTAACCT |
| StSP6A | StSP6A | XM_006365395.2 | GGGAAACCTTTGGCAATGAAG | CCCAATTGCTGGAACAACAC |
| M1A0D5_SOLTU | CPI1 | XM_015309779.1 | GCAACACCTAGTCTGTGGTTAG | CACAACACCAAGGCACAAAG |
| HMG3 | HMG1 | XP_015159610.1 | GCGACCTGTTAAGCCTCTATAC | CAACCCAGTGGTTAGGTACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Isidron, L.; Valencia-Lozano, E.; Uribe-Lopez, B.; Délano-Frier, J.P.; Barraza, A.; Cabrera-Ponce, J.L. Molecular Insights into the Role of Sterols in Microtuber Development of Potato Solanum tuberosum L. Plants 2024, 13, 2391. https://doi.org/10.3390/plants13172391
Herrera-Isidron L, Valencia-Lozano E, Uribe-Lopez B, Délano-Frier JP, Barraza A, Cabrera-Ponce JL. Molecular Insights into the Role of Sterols in Microtuber Development of Potato Solanum tuberosum L. Plants. 2024; 13(17):2391. https://doi.org/10.3390/plants13172391
Chicago/Turabian StyleHerrera-Isidron, Lisset, Eliana Valencia-Lozano, Braulio Uribe-Lopez, John Paul Délano-Frier, Aarón Barraza, and José Luis Cabrera-Ponce. 2024. "Molecular Insights into the Role of Sterols in Microtuber Development of Potato Solanum tuberosum L." Plants 13, no. 17: 2391. https://doi.org/10.3390/plants13172391





