Soil Carbon Dioxide Emissions and Carbon Sequestration with Implementation of Alley Cropping in a Mediterranean Citrus Orchard
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characteristics of Soils
2.2. Carbon Dioxide Emission Rates
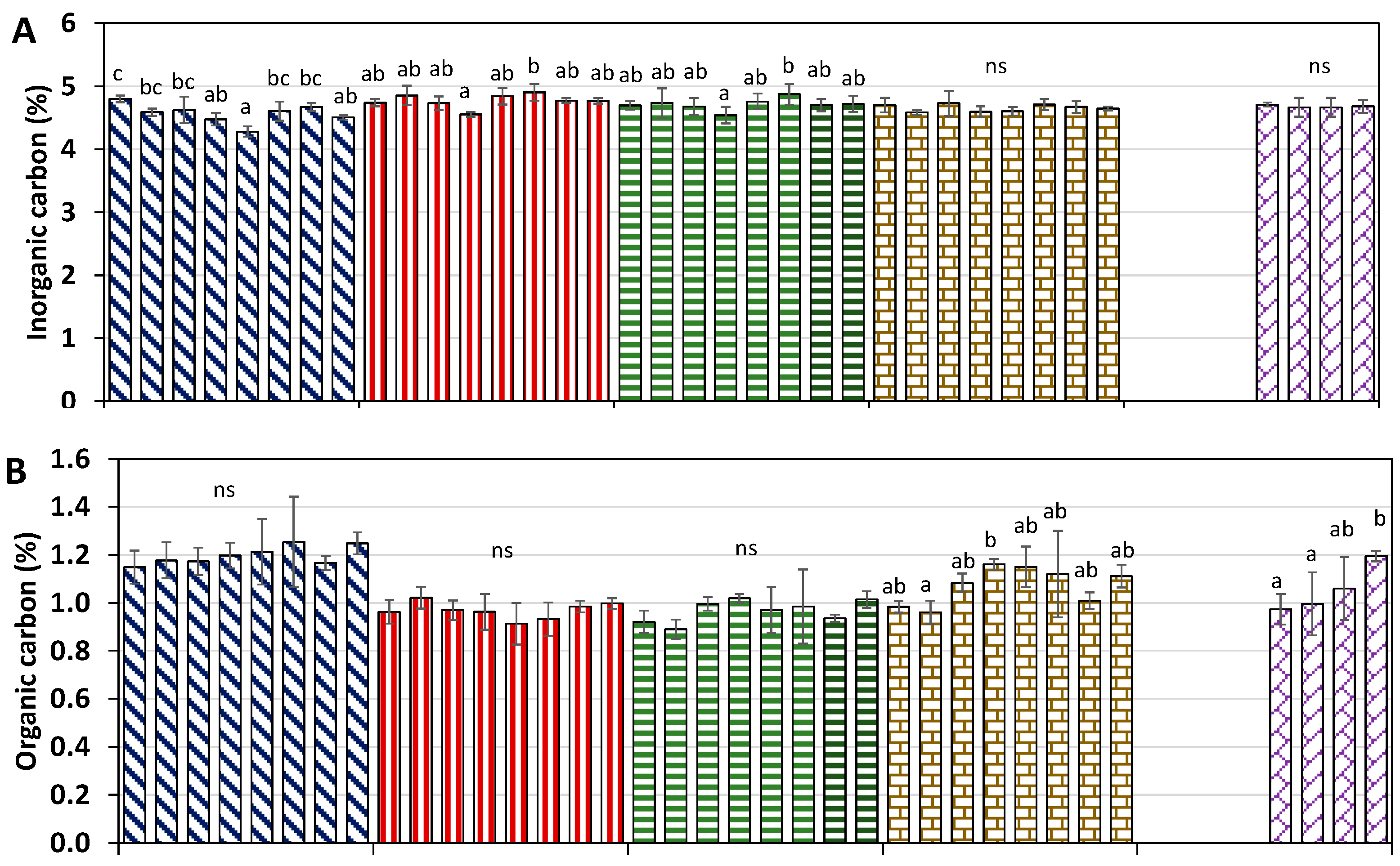
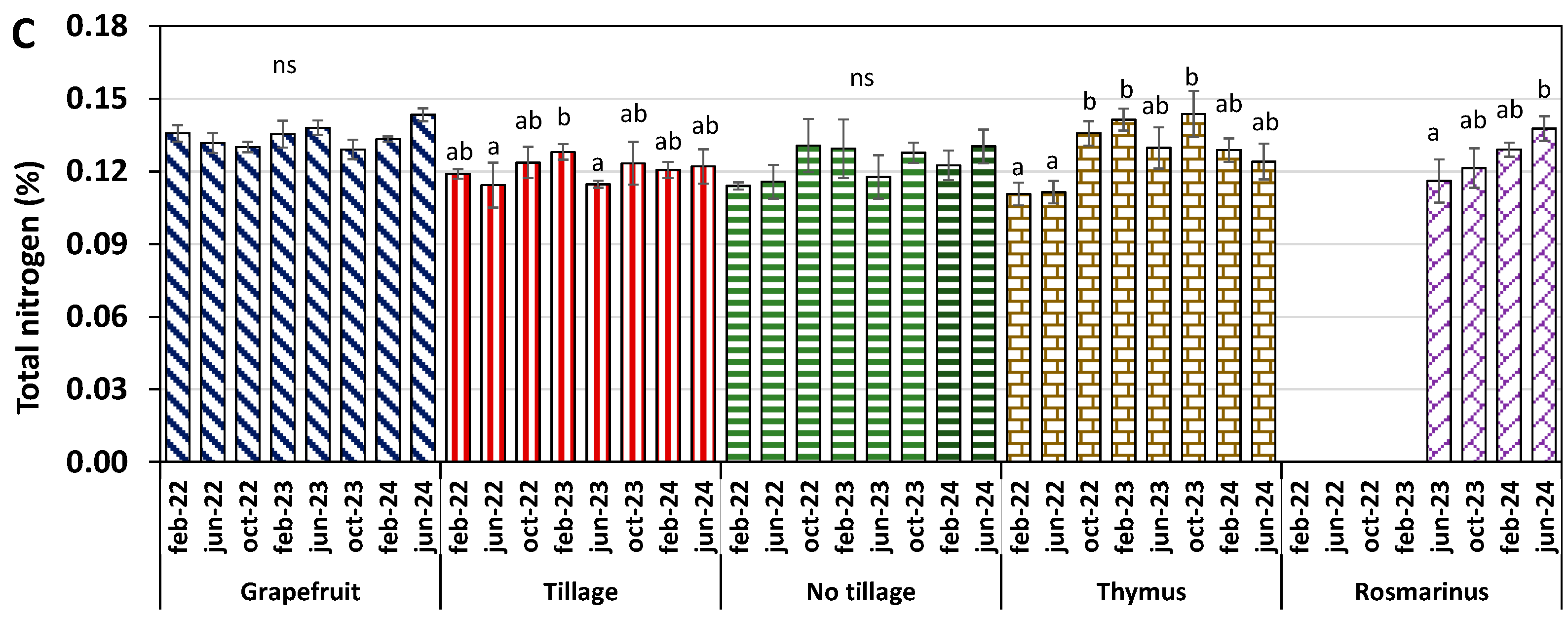
2.3. Soil Inorganic/Organic Carbon and Total Nitrogen Evolution in Soils
3. Discussion
3.1. Effect of Alley Cropping on Physicochemical Characteristics of Soils
3.2. Evolution of Carbon Dioxide Emissions
3.3. Carbon Sequestration in Soils
4. Materials and Methods
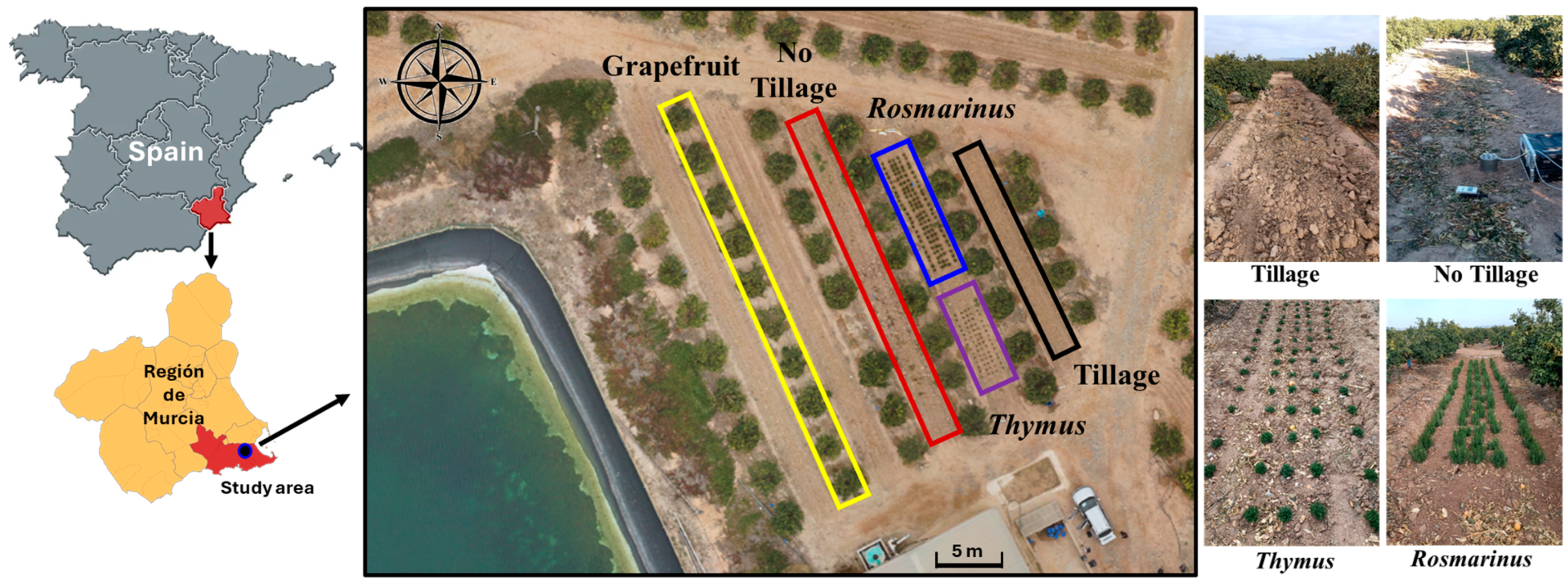
4.1. Study Area and Soil Sampling
4.2. Analytical Methods
4.3. Soil Carbon Dioxide Measurements
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallego-Schmid, A.; Chen, H.M.; Sharmina, M.; Mendoza, J.M.F. Links between circular economy and climate change mitigation in the built environment. J. Clean. Prod. 2020, 260, 121115. [Google Scholar] [CrossRef]
- Malhotra, A.; Mathur, A.; Diddi, S.; Sagar, A.D. Building institutional capacity for addressing climate and sustainable development goals: Achieving energy efficiency in India. Clim. Policy 2022, 22, 652–670. [Google Scholar] [CrossRef]
- IPCC. Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Environmental Protection Agency (EPA). 2023. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 23 July 2024).
- Xu, X.B.; Xu, Y.; Li, J.; Lu, Y.L.; Jenkins, A.; Ferrier, R.C.; Li, H.; Stenseth, N.C.; Hessen, D.O.; Zhang, L.X.; et al. Coupling of crop and livestock production can reduce the agricultural GHG emission from smallholder farms. Science 2023, 26, 106798. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration and aggregation by cover cropping. J. Soil Water Conserv. 2015, 70, 329–339. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde-Geochem. 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Wattenbach, M.; Sus, O.; Vuichard, N.; Lehuger, S.; Gottschalk, P.; Li, L.; Smith, P. The carbon balance of European croplands: A cross-site comparison of simulation models. Agric. Ecosyst. Environ. 2010, 139, 419–453. [Google Scholar] [CrossRef]
- Chabbi, A.; Lehmann, J.; Ciais, P.; Loescher, H.W.; Cotrufo, M.F.; Don, A.; SanClements, M.; Schipper, L.; Six, J.; Smith, P.; et al. Aligning agriculture and climate policy. Nat. Clim. Chang. 2017, 7, 307–309. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Koutika, L.-S.; Smith, P.; Whitehead, D.; Wollenberg, E. Put More Carbon in Soils to Meet Paris Climate Pledges; Nature Publishing Group: London, UK, 2018. [Google Scholar]
- Bellamy, P.H.; Loveland, P.J.; Bradley, R.I.; Lark, R.M.; Kirk, G.J. Carbon losses from all soils across England and Wales 1978–2003. Nature 2005, 437, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Bala, S.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, R.; DeFries, R.; Galloway, J.; Heimann, M.; Jones, C.; et al. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Lal, R. Beyond COP 21: Potential and challenges of the “4 per Thousand” initiative. J. Soil Water Conserv. 2016, 71, 20A–25A. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Review: Factors Affecting Rhizosphere Priming Effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Huo, C.; Luo, Y.; Cheng, W. Rhizosphere priming effect: A meta-analysis. Soil Biol. Biochem. 2017, 111, 78–84. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Padovan, M.P.; Panosso, A.R.; Teixeira, D.D.B.; Ferraudo, A.S.; La Scala, N. On the spatial and temporal dependence of CO2 emission on soil properties in sugarcane (Saccharum spp.) production. Soil Tillage Res. 2015, 148, 127–132. [Google Scholar] [CrossRef]
- Silva, B.O.; Moitinho, M.R.; Santos, G.A.A.; Teixeira, D.D.B.; Fernandes, C.; La Scala, N., Jr. Soil CO2 emission and short-term soil pore class distribution after tillage operations. Soil Tillage Res. 2019, 186, 224–232. [Google Scholar] [CrossRef]
- Abbas, F.; Hammad, H.M.; Fahad, S.; Ishaq, W.; Farooque, A.I.; Bakhat, H.F.; Zia, Z.; Fahad, S.; Farhad, W.; Cerdá, A. A review of soil carbon dynamics resulting from agricultural practices. Agric. Ecosyst. Environ. 2020, 268, 110319. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soiland Sustained Food Production; Food & Agriculture Organization: Rome, Italy, 2005. [Google Scholar]
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Zornoza, R.; Acosta, J.A.; Gabarrón, M.; Gómez-Garrido, M.; Sánchez-Navarro, V.; Terrero, A.; Martínez-Martínez, S.; Faz, Á.; Pérez-Pastor, A. Greenhouse gas emissions and soil organic matter dynamics in woody crop orchards with different irrigation regimes. Sci. Total Environ. 2018, 644, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Rodrigo-Comino, J.; Gimenez-Morera, A.; Keesstra, S.D. Hydrological and erosional impact and farmer’s perception on catch crops and weeds in citrus organic farming in Canyoles river watershed, Eastern Spain. Agric. Ecosyst. Environ. 2018, 258, 49–58. [Google Scholar] [CrossRef]
- Tsanis, I.K.; Seiradakis, K.D.; Sarchani, S.; Panagea, I.S.; Alexakis, D.D.; Koutroulis, A.G. The impact of soil-improving cropping practices on erosion rates: A stakeholder-oriented field experiment assessment. Land 2021, 10, 964. [Google Scholar] [CrossRef]
- Cerda, A.; Novara, A.; Moradi, E. Long-term non-sustainable soil erosion rates and soil compaction in drip-irrigated citrus plantation in Eastern Iberian Peninsula. Sci. Total Environ. 2021, 787, 147549. [Google Scholar] [CrossRef]
- Martínez-Mena, M.; Carrillo-Lopez, E.; Boix-Fayos, C.; Almagro, M.; García Franco, N.; Díaz-Pereira, E.; Montoya, I.; de Vente, J. Long-term effectiveness of sustainable land management practices to control runoff, soil erosion, and nutrient loss and the role of rainfall intensity in Mediterranean rainfed agroecosystems. Catena 2020, 187, 104352. [Google Scholar] [CrossRef]
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2019, 23, 4430–4439. [Google Scholar] [CrossRef]
- Kochiieru, M.; Versuliene, A.; Feiza, V.; Feiziene, D. Trend for soil CO2 efflux in grassland and forest land in relation with meteorological conditions and root parameters. Sustainability 2023, 15, 7193. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Pries, C.E.H.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Pe’Er, G.; Zinngrebe, Y.; Moreira, F.; Sirami, C.; Schindler, S.; Müller, R.; Bontzorlos, V.; Clough, D.; Bezák, P.; Bonn, A. A greener path for the EU Common Agricultural Policy. Science 2019, 365, 449–451. [Google Scholar] [CrossRef]
- Hillel, D.; Rosenzweig, C. The role of soils in climate change. In Handbook of Climate Change and Agroecosystems Impacts, Adaptation and Mitigation; World Scientific Publishing: Singapore, 2011; pp. 9–20. [Google Scholar]
- Hutchinson, J.J.; Campbell, C.A.; Desjardins, R.L. Some perspectives on carbon sequestration in agriculture. Agric. Meteorol. 2007, 142, 288–302. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Zahra, S.I.; Abbas, F.; Ishaq, W.; Ibrahim, M.; Hammad, H.M.; Akram, B.; Salik, M.R. Carbon sequestration potential of soils under maize production in irrigated agriculture of the Punjab province of Pakistan. J. Anim. Plant Sci. 2016, 26, 706–715. [Google Scholar]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.F.; Ferrer, A.; Peigne, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Develop. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Francaviglia, R.; Di Bene, C.; Farina, R.; Salvati, L. Soil organic carbon sequestration and tillage systems in the Mediterranean Basin: A data mining approach. Nutr. Cycl. Agroecosyst. 2017, 107, 125–137. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Li, L. Intercropping: Feed more people and build more sustainable agroecosystems. Front. Agric. Sci. Eng. 2021, 8, 373–386. [Google Scholar]
- Ebbisa, A. Mechanisms underlying cereal/legume intercropping as nature-based biofortification: A review. Food Prod. Process. Nutr. 2022, 4, 19. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Taschen, E.; Plassard, C.; Dezette, D.; Abadie, J.; Arnal, D.; Benezech, P.; Duthoit, M.; Pablo, A.-L.; Jourdan, C. Role of trees and herbaceous vegetation be neath trees in maintaining arbuscular mycorrhizal communities in temperate alley cropping systems. Plant Soil 2020, 453, 153–171. [Google Scholar] [CrossRef]
- Rosa-Schleich, J.; Loos, J.; Mußhoff, O.; Tscharntke, T. Ecological-economic trade-offs of diversified farming systems-a review. Ecol. Econ. 2019, 160, 251–263. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission. The European Green Deal; COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Regulation of the European Parliament and of the Council Establishing the Framework for Achieving Climate Neutrality and Amending Regulation (EU) 2018/1999 (European Climate Law); COM(2020) 80 Final, 2020/0036 (COD); European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Sanchez-Navarro, V.; Shahrokh, V.; Martínez-Martínez, S.; Acosta, J.A.; Almagro, M.; Martínez-Mena, M.; Boix-Fayos, C.; Díaz-Pereira, E.; Zornoza, R. Perennial alley cropping contributes to decrease soil CO2 and N2O emissions and increase soil carbon sequestration in a Mediterranean almond orchard. Sci. Total Environ. 2022, 845, 157225. [Google Scholar] [CrossRef]
- Isbell, F.; Tilman, D.; Polasky, S.; Loreau, M. The biodiversity-dependent ecosystem service debt. Ecol. Lett. 2015, 18, 119–134. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Nazzaro, F.; Mancini, E.; De Feo, V. Essential Oils from Mediterranean Aromatic Plants. The Mediterranean Diet: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2015; pp. 649–661. [Google Scholar]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Soil Survey Division. Soil Survey Manual (No. 18); United States Department of Agriculture: Washington, DC, USA, 1993.
- Yaalon, D. Soils in the Mediterranean region: What makes them different? Catena 1997, 28, 157–169. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Prentice Hall: Englewood, NJ, USA, 2001; 960p. [Google Scholar]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2021, 88, 967–988. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Debosz, K.; McCarty, G.W. Short-term effects of tillage on mineralization of nitrogen and carbon in soil. Soil Biol. Biochem. 2023, 35, 979–986. [Google Scholar] [CrossRef]
- Xue, J.F.; Pu, C.; Liu, S.L.; Chen, Z.; Chen, F.; Xiao, X.P.; Lal, R.; Zhang, H.L. Effects of tillage systems on soil organic carbon and total nitrogen in a double paddy cropping system in Southern China. Soil Tillage Res. 2015, 153, 161–168. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; Sayer, E.J. Nitrogen deposition enhances soil organic carbon and microbial residual carbon in a tropical forest. Plant Soil 2023, 484, 217–235. [Google Scholar] [CrossRef]
- Bi, X.; Chu, H.; Fu, M.; Xu, D.; Zhao, W.; Zhong, Y.; Wang, M.; Li, K.; Zhang, Y. Distribution characteristics of organic carbon (nitrogen) content, cation exchange capacity, and specific surface area in different soil particle sizes. Sci. Rep. 2023, 13, 12242. [Google Scholar] [CrossRef]
- D’Hervilly, C.; Marsden, C.; Capowiez, Y.; Beral, C.; Delapre-Cosset, L.; Bertrand, I. Trees and herbaceous vegetation strips both contribute to changes in soil fertility and soil organism communities in an agroforestry system. Plant Soil 2021, 4631, 537–553. [Google Scholar] [CrossRef]
- Wachendorf, C.; Piepho, H.P.; Beuschel, R. Determination of litter derived C and N in litterbags and soil using stable isotopes prevents overestimation of litter decomposition in alley cropping systems. Pedobiologia 2020, 81–82, 150651. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Epron, D.; Bosc, A.; Bonal, D.; Freycon, V. Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana. J. Trop. Ecol. 2006, 22, 565–574. [Google Scholar] [CrossRef]
- Concilio, A.; Chen, J.; Ma, S.; North, M. Precipitation drives interannual variation in summer soil respiration in a Mediterranean-climate, mixed-conifer forest. Clim. Chang. 2009, 92, 109–122. [Google Scholar] [CrossRef]
- Fernández-Ortega, J.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Double-cropping, tillage and nitrogen fertilization effects on soil CO2 and CH4 emissions. Agric. Ecosyst. Environ. 2024, 359, 108758. [Google Scholar] [CrossRef]
- Marwanto, S.; Agus, F. Is CO2 flux from oil palm plantations on peatland controlled by soil moisture and/or soil and air temperatures? Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 809–819. [Google Scholar] [CrossRef]
- Mccalmont, J.; Khoon, L.; Yit, K.; Teh, A.; Lewis, K.; Chocholek, M.; Rumpang, E.; Hill, T. Short and long-term carbon emissions from oil palm plantations converted from logged tropical peat swamp forest. Glob. Chang. Biol. 2021, 27, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Green, J.K.; Seneviratne, S.I.; Berg, A.M.; Findell, K.L.; Hagemann, S.; Lawrence, D.M.; Gentine, P. Large influence of soil moisture on long-term terrestrial carbon uptake. Nature 2019, 565, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.; Rezanezhad, F.; Fairbairn, L.; Slowinski, S.; Basiliko, N.; Price, J.S.; Quinton, W.L.; Roy-Leveillee, P.; Webster, K.; Van Cappellen, P. Temperature, moisture and freeze-thaw controls on CO2 production in soil incubations from northern peatlands. Sci. Rep. 2021, 11, 23219. [Google Scholar] [CrossRef]
- Moyano, F.E. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2011, 93, 930–938. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Zanatta, J.A.; Vieira, F.C.B.; Dieckow, J. Mitigating greenhouse gas emissions from a subtropical Ultisol by using long-term no-tillage in combination with legume cover crops. Soil Tillage Res. 2016, 161, 86–94. [Google Scholar] [CrossRef]
- Siles, J.A.; Vera, A.; Díaz-Lopez, M.; García, C.; van den Hoogen, J.; Crowther, T.W.; Eisenhauer, N.; Guerra, C.; Jones, A.; Orgiazzi, A.; et al. Land-use- and climate-mediated variations in soil bacterial and fungal biomass across Europe and their driving factors. Geoderma 2023, 434, 116474. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Blagodatsky, S.; Anderson, T.H.; Kuzyakov, Y. Microbial Growth and Carbon Use Efficiency in the Rhizosphere and Root-Free Soil. PLoS ONE 2014, 9, 9328. [Google Scholar] [CrossRef]
- Kuzyakov, Y.V.; Larionova, A.A. Contribution of rhizomicrobial and root respiration to the CO2 emission from soil (A review). Eurasian Soil Sc. 2006, 39, 753–764. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, X.; Smith, P.; Fan, J.; Lu, Y.; Emmett, B.; Li, R.; Dorling, S.; Chen, H.; Liu, S.; et al. Soil quality both increases crop production and improves resilience to climate change. Nat. Clim. Chang. 2022, 12, 574–580. [Google Scholar] [CrossRef]
- Raghavendra, M.; Sharma, M.P.; Ramesh, A.; Richa, A.; Billore, S.D.; Verma, R.K. Soil health indicators: Methods and applications. In Soil Analysis: Recent Trends and Applications; Rakshit, A., Ghosh, S., Chakraborty, S., Philip, V., Datta, A., Eds.; Springer: Singapore, 2020; pp. 221–253. [Google Scholar]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.R.; Cora, J.E.; Jorge, R.F.; Marcelo, A.V. Crop type influences soil aggregation and organic matter under no-tillage. Soil Tillage Res. 2009, 104, 22–29. [Google Scholar] [CrossRef]
- Mungai, N.W.; Motavalli, P.P.; Kremer, R.J. Soil organic carbon and nitrogen fractions in temperate alley cropping systems. Commun. Soil Sci. Plant Anal. 2006, 37, 977–992. [Google Scholar] [CrossRef]
- Ben Moussa-Machraoui, S.; Errouissi, F.; Ben-Hammouda, M.; Nouira, S. Comparative effects of conventional and o-tillage management on some soil properties under Mediterranean semi-Arid conditions in northwestern Tunisia. Soil Tillage Res. 2010, 106, 247–253. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Kang, B.T.; Caveness, F.E.; Tian, G.T.; Kalawole, G.O. Long-term alley cropping with four hedgerows species on an Alfisol in south western Nigeria. Effect on crop performance, soil chemical properties and nematode population. Nutr. Cycl. Agroecosyst. 1999, 54, 145–155. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Zwikel, S.; Lavee, H.; Pariente, S. Temporal evolution of salts in Mediterranean soils transect under different climatic conditions. Catena 2007, 70, 282–295. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Laboratory Methods Manual. Version No. 4.0. USDA NRCS. Soil Survey Investigations Report No. 42; U.S. Government Publishing Office: Washington, DC, USA, 2004.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]

| Before Treatments | Soil 2 Years after Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grapefruit | Alley | Grapefruit | No tillage | Tillage | Rosmarinus officinalis | Thymus hyemalis | |||
| Bulk Soil | Rhiz. Soil | Bulk Soil | Rhiz. Soil | ||||||
| pH | 7.8 ± 0.2 b | 8.5 ± 0 c | 8.3 ± 0 bc | 8.2 ± 0.1 b | 8.2 ± 0 b | 8.2 ± 0 b | 7.3 ± 0.1 a | 8.4 ± 0 bc | 7.3 ± 0.1 a |
| EC (dS/m) | 0.8 ± 0.1 c | 0.2 ± 0 a | 0.2 ± 0 a | 0.2 ± 0 a | 0.2 ± 0 a | 0.3 ± 0 b | 0.7 ± 0.1 c | 0.4 ± 0 b | 0.5 ± 0.1 b |
| Inorganic carbon (%) | 4.8 ± 0.1 b | 4.7 ± 0.1 ab | 4.5 ± 0 a | 4.7 ± 0 ab | 4.8 ± 0 b | 4.7 ± 0.1 ab | 4.9 ± 0 b | 4.6 ± 0.1 ab | 4.9 ± 0 b |
| Organic carbon (%) | 1.1 ± 0.02 b | 0.9 ± 0.05 a | 1.2 ± 0.05 c | 1 ± 0.02 a | 1 ± 0.05 a | 1.2 ± 0.02 bc | 1.4 ± 0.04 d | 1.1 ± 0.03 b | 1.2 ± 0.01 bc |
| Total Nitrogen (%) | 0.14 ± 0 abc | 0.11 ± 0 a | 0.14 ± 0.01 bc | 0.13 ± 0.01 ab | 0.12 ± 0 ab | 0.14 ± 0.01 abc | 0.24 ± 0.02 d | 0.12 ± 0.01 ab | 0.16 ± 0.01 c |
| CEC (cmol kg−1) | 11.1 ± 0.5 ab | 10.7 ± 0.4 a | 12.8 ± 0.2 b | 12.6 ± 0.4 ab | 13.8 ± 0.5 b | 14 ± 0.5 b | 18.9 ± 0.9 c | 10.8 ± 0.9 a | 18.2 ± 0.9 c |
| Clay (%) | 20.7 ± 0.9 | 21.2 ± 1.4 | 19.8 ± 0.8 | 21.9 ± 3.2 | 22.6 ± 1.1 | 22.6 ± 2.9 | 21.8 ± 3 | 22 ± 2.4 | 21 ± 1.3 |
| Silt (%) | 18.3 ± 0.7 ab | 24.6 ± 1.7 c | 16.4 ± 1.4 a | 22.9 ± 2.6 bc | 21.7 ± 2.1 bc | 21.2 ± 1.4 bc | 20.4 ± 0.9 abc | 20.2 ± 1.7 abc | 18.4 ± 1.1 ab |
| Sand (%) | 61.1 ± 0.4 bc | 54.1 ± 2.9 a | 63.8 ± 1.3 c | 55.1 ± 1.7 ab | 55.6 ± 3.1 ab | 56.2 ± 1.8 ab | 57.8 ± 3.7 abc | 57.8 ± 0.8 abc | 60.6 ± 1.6 bc |
| Available P (mg kg−1) | 96.9 ± 7.8 c | 49.6 ± 3.6 ab | 78.7 ± 3 c | 47.3 ± 6.1 ab | 39.6 ± 3.6 a | 51.3 ± 7.5 ab | 62 ± 7 b | 48.5 ± 3 ab | 57.8 ± 3.3 b |
| Available Fe (mg kg−1) | 3.82 ± 0.5 a | 7.61 ± 0.9 ab | 5.37 ± 1 a | 7.15 ± 0.7 ab | 7.41 ± 1.1 ab | 7.76 ± 1 ab | 9.41 ± 1 b | 8.7 ± 1 b | 13.98 ± 1.5 c |
| Available Mn (mg kg−1) | 8.63 ± 0.9 ab | 8.69 ± 1 ab | 8.77 ± 1 ab | 8.13 ± 1.6 ab | 10.91 ± 1.3 bc | 8.66 ± 1.1 ab | 8.87 ± 0.9 ab | 7.41 ± 1.1 a | 12.43 ± 1.5 c |
| Available Cu (mg kg−1) | 2.07 ± 0.5 | 1.87 ± 0.8 | 1.72 ± 0.8 | 2.42 ± 0.6 | 1.79 ± 0.3 | 2.86 ± 0.8 | 2.22 ± 1.3 | 2.63 ± 0.8 | 2.63 ± 0.9 |
| Available Zn (mg kg−1) | 2.76 ± 0.8 ab | 2.92 ± 0.6 ab | 1.65 ± 0.9 a | 2.63 ± 0.9 ab | 2.73 ± 0.9 ab | 2.82 ± 0.4 ab | 2.75 ± 0.8 ab | 4.38 ± 0.9 b | 2.86 ± 0.8 ab |
| Available Na (mg kg−1) | 142 ± 14 b | 40 ± 5 a | 53 ± 6 a | 46 ± 4 a | 52 ± 4 a | 136 ± 12 b | 390 ± 40 c | 153 ± 26 b | 267 ± 30 c |
| Available K (mg kg−1) | 217 ± 25 a | 247 ± 29 ab | 235 ± 27 ab | 246 ± 27 ab | 263 ± 33 ab | 296 ± 15 b | 292 ± 31 ab | 267 ± 32 ab | 249 ± 22 ab |
| Available Ca (mg kg−1) | 1650 ± 131 a | 1634 ± 107 a | 1613 ± 165 a | 1958 ± 126 ab | 2177 ± 170 b | 2046 ± 58 ab | 2534 ± 176 bc | 1846 ± 97 ab | 2812 ± 185 c |
| Available Mg (mg kg−1) | 211 ± 9 a | 211 ± 25 a | 235 ± 9 ab | 244 ± 32 ab | 243 ± 27 ab | 300 ± 22 b | 459 ± 40 c | 274 ± 15 ab | 288 ± 22 b |
| W. soluble Na (mg L−1) | 81.2 ± 5.1 c | 9.9 ± 2.2 a | 10.1 ± 2.4 a | 12.9 ± 2.6 a | 10.9 ± 1.9 a | 43.5 ± 3.3 bc | 32.1 ± 3.8 b | 37.8 ± 2.4 b | 48.1 ± 5.2 c |
| W. soluble K (mg L−1) | 20 ± 2.6 b | 12.8 ± 2.9 ab | 8.5 ± 1.6 a | 13.6 ± 2.9 ab | 9.3 ± 1.9 a | 24.6 ± 3.8 c | 11.6 ± 1.9 a | 11.7 ± 2.2 a | 14.2 ± 3.4 ab |
| W. soluble Ca (mg L−1) | 52.2 ± 3.5 c | 18 ± 2.6 ab | 13.2 ± 2.7 a | 29.1 ± 3.2 b | 20.5 ± 1.9 ab | 59.9 ± 12.7 c | 23.4 ± 2.7 ab | 21.6 ± 2.1 ab | 40.1 ± 5 bc |
| W. soluble Mg (mg L−1) | 10.4 ± 1.8 b | 3.8 ± 1 a | 3.1 ± 1 a | 5.5 ± 0.9 a | 3.4 ± 1.1 a | 16.8 ± 2.6 c | 4.2 ± 0.7 a | 4.4 ± 0.6 a | 10.9 ± 1.5 b |
| W. soluble Cl (mg L−1) | 144 ± 11.9 c | 41.9 ± 3.7 b | 11.9 ± 1.4 a | 17.4 ± 3.3 a | 12.4 ± 1.8 a | 41.5 ± 3.8 b | 34.4 ± 3.3 b | 9.8 ± 1.4 a | 33.5 ± 3 b |
| W. soluble SO4 (mg L−1) | 61.1 ± 2.5 c | 43.5 ± 3.3 c | 8.6 ± 1.8 ab | 15.7 ± 2.8 b | 10.3 ± 2 ab | 66 ± 3.4 c | 37.6 ± 5.5 bc | 6.6 ± 1.2 a | 91.8 ± 4 c |
| W. soluble NO3 (mg L−1) | 86.6 ± 6.9 c | 18.6 ± 4.6 b | 11.8 ± 1.9 ab | 55.4 ± 5.2 c | 16 ± 3.7 b | 4.1 ± 0.6 a | 15.7 ± 2 b | 7 ± 1.3 ab | 2.6 ± 0.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, J.A.; Imbernón-Mulero, A.; Gallego-Elvira, B.; Maestre-Valero, J.F.; Martínez-Martínez, S.; Martínez-Álvarez, V. Soil Carbon Dioxide Emissions and Carbon Sequestration with Implementation of Alley Cropping in a Mediterranean Citrus Orchard. Plants 2024, 13, 2399. https://doi.org/10.3390/plants13172399
Acosta JA, Imbernón-Mulero A, Gallego-Elvira B, Maestre-Valero JF, Martínez-Martínez S, Martínez-Álvarez V. Soil Carbon Dioxide Emissions and Carbon Sequestration with Implementation of Alley Cropping in a Mediterranean Citrus Orchard. Plants. 2024; 13(17):2399. https://doi.org/10.3390/plants13172399
Chicago/Turabian StyleAcosta, Jose A., Alberto Imbernón-Mulero, Belén Gallego-Elvira, Jose F. Maestre-Valero, Silvia Martínez-Martínez, and Victoriano Martínez-Álvarez. 2024. "Soil Carbon Dioxide Emissions and Carbon Sequestration with Implementation of Alley Cropping in a Mediterranean Citrus Orchard" Plants 13, no. 17: 2399. https://doi.org/10.3390/plants13172399






