Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain
Abstract
:1. Introduction
2. Materials and Methods
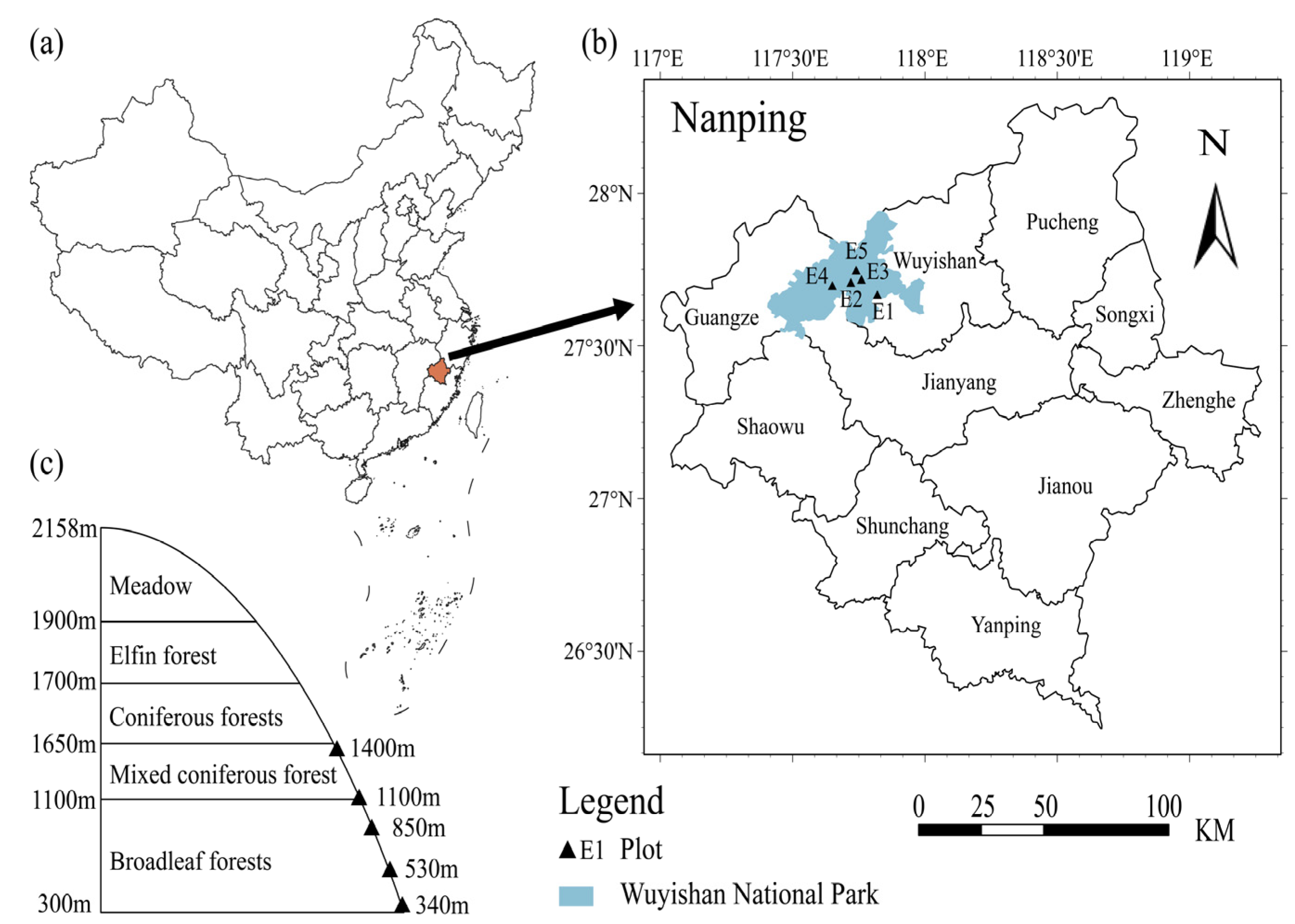
2.1. Study Area
2.2. Sample Setting and Soil Sampling
2.3. Soil Physical and Chemical Properties Determination
2.4. DNA Extraction and High-Throughput Sequencing
2.5. Statistical Analysis
3. Results
3.1. Structure of Soil Microbial Communities in Moso Bamboo Forests at Different Altitudes
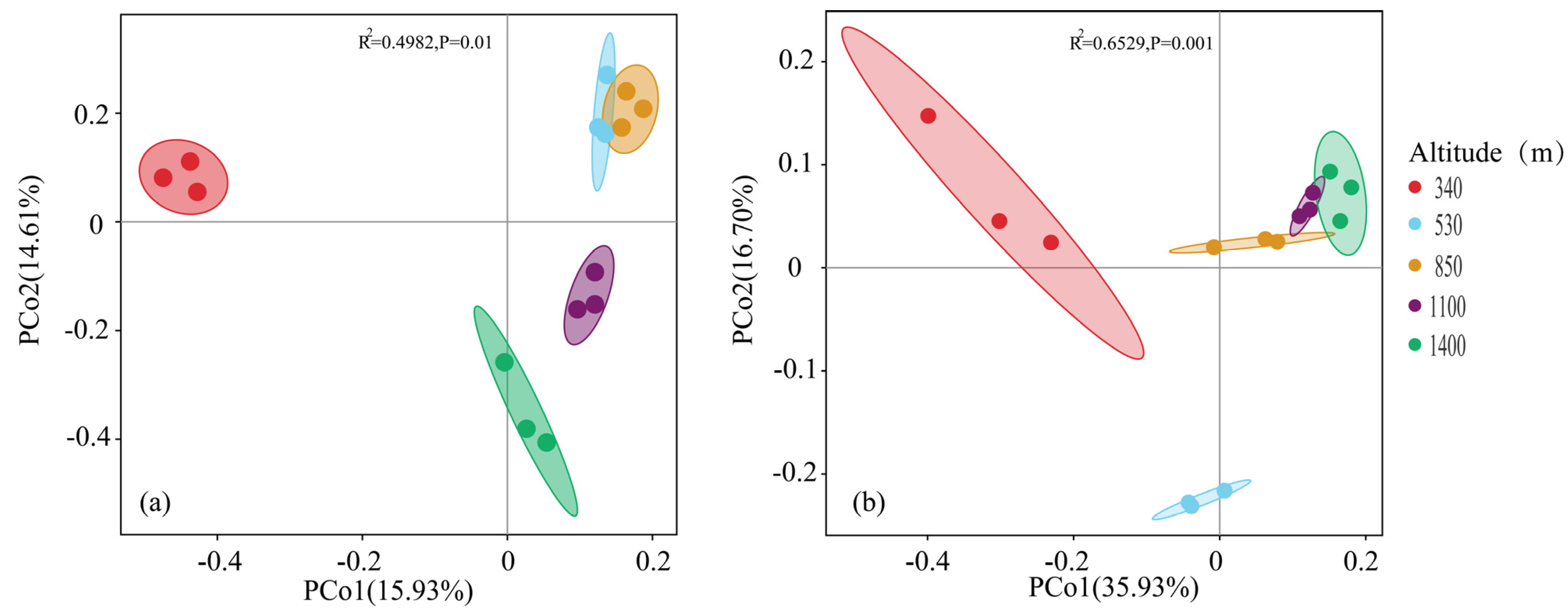
3.1.1. OTU Cluster Analysis
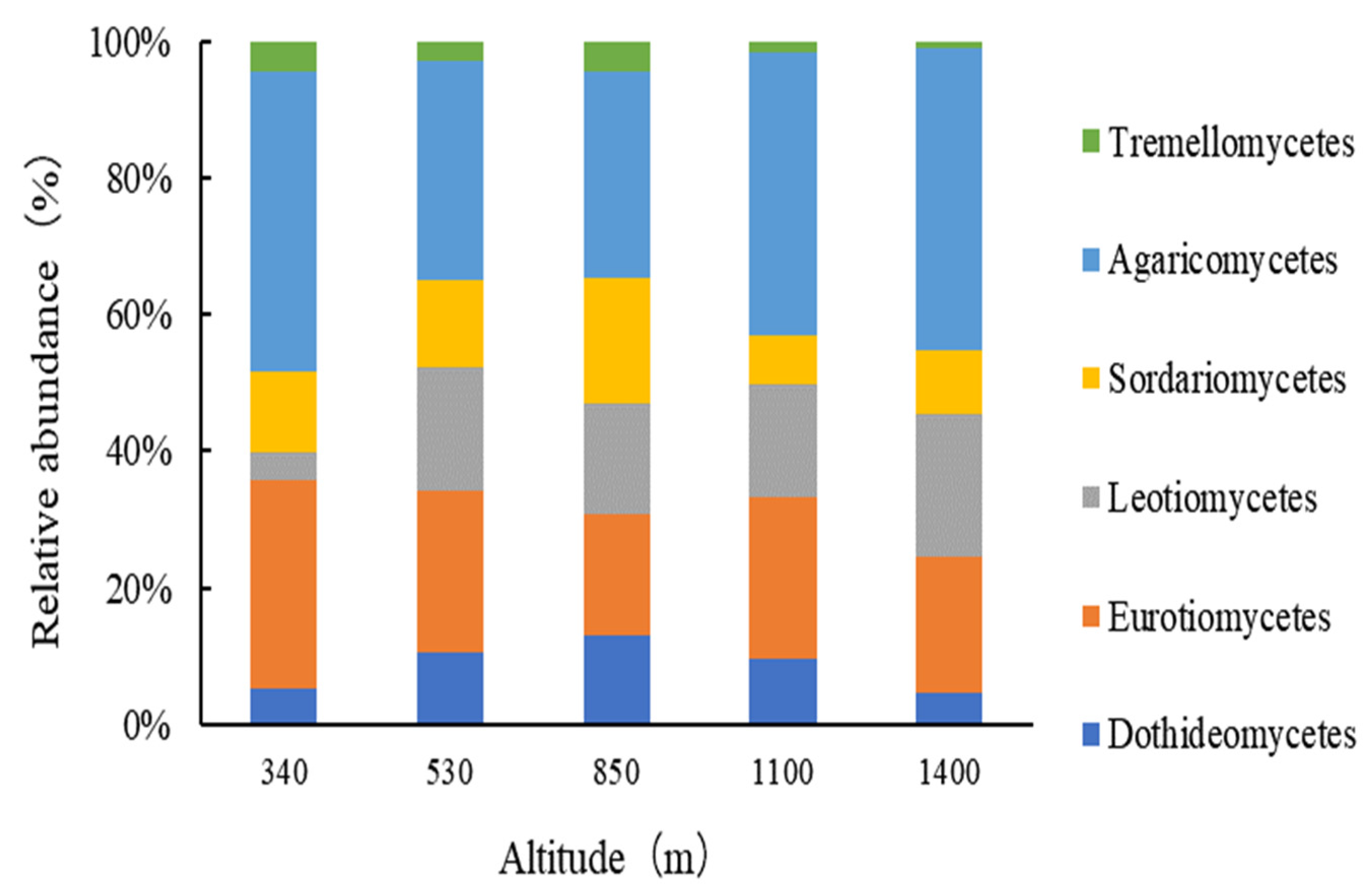
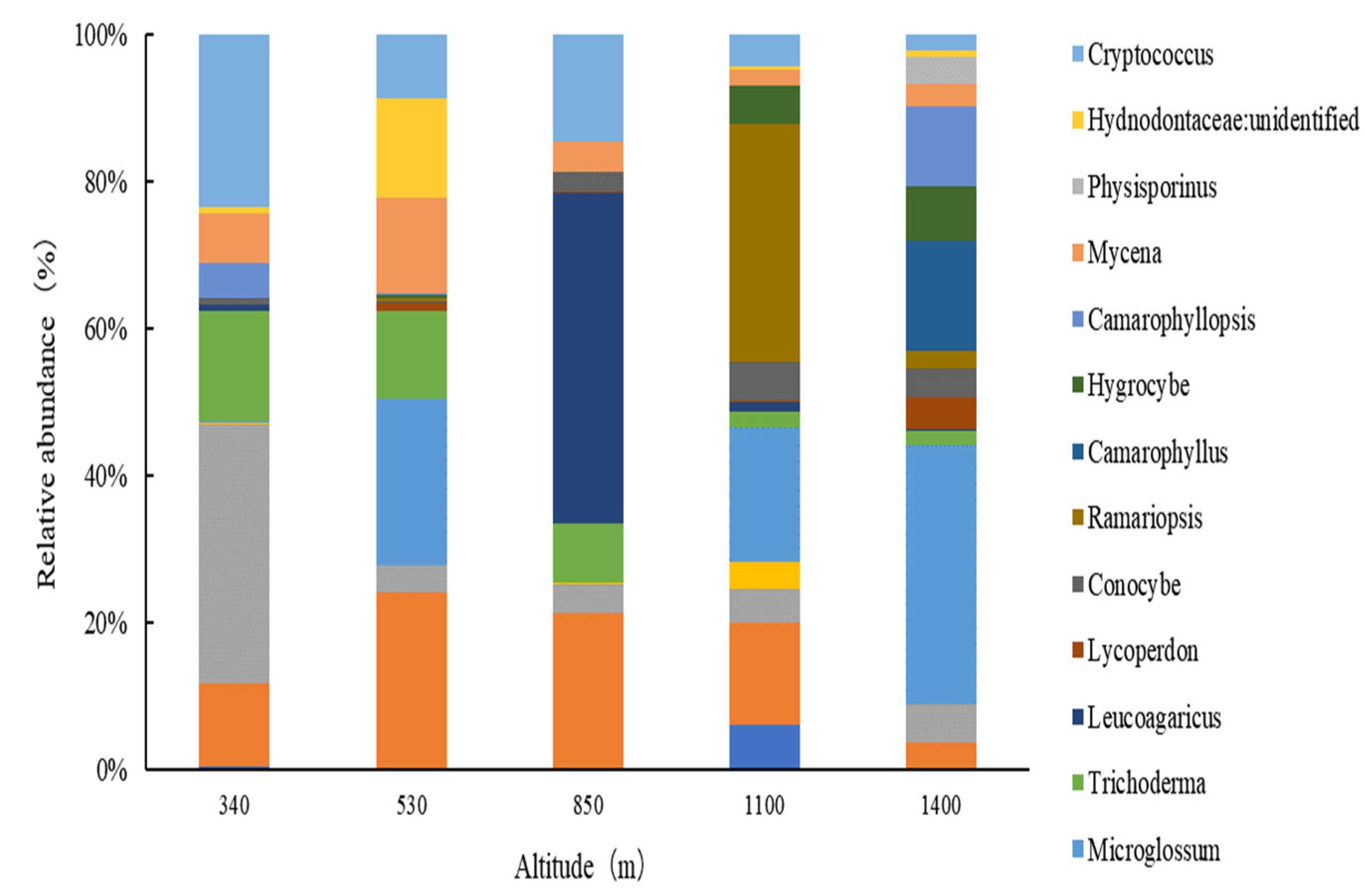
3.1.2. Fungal Community Structure
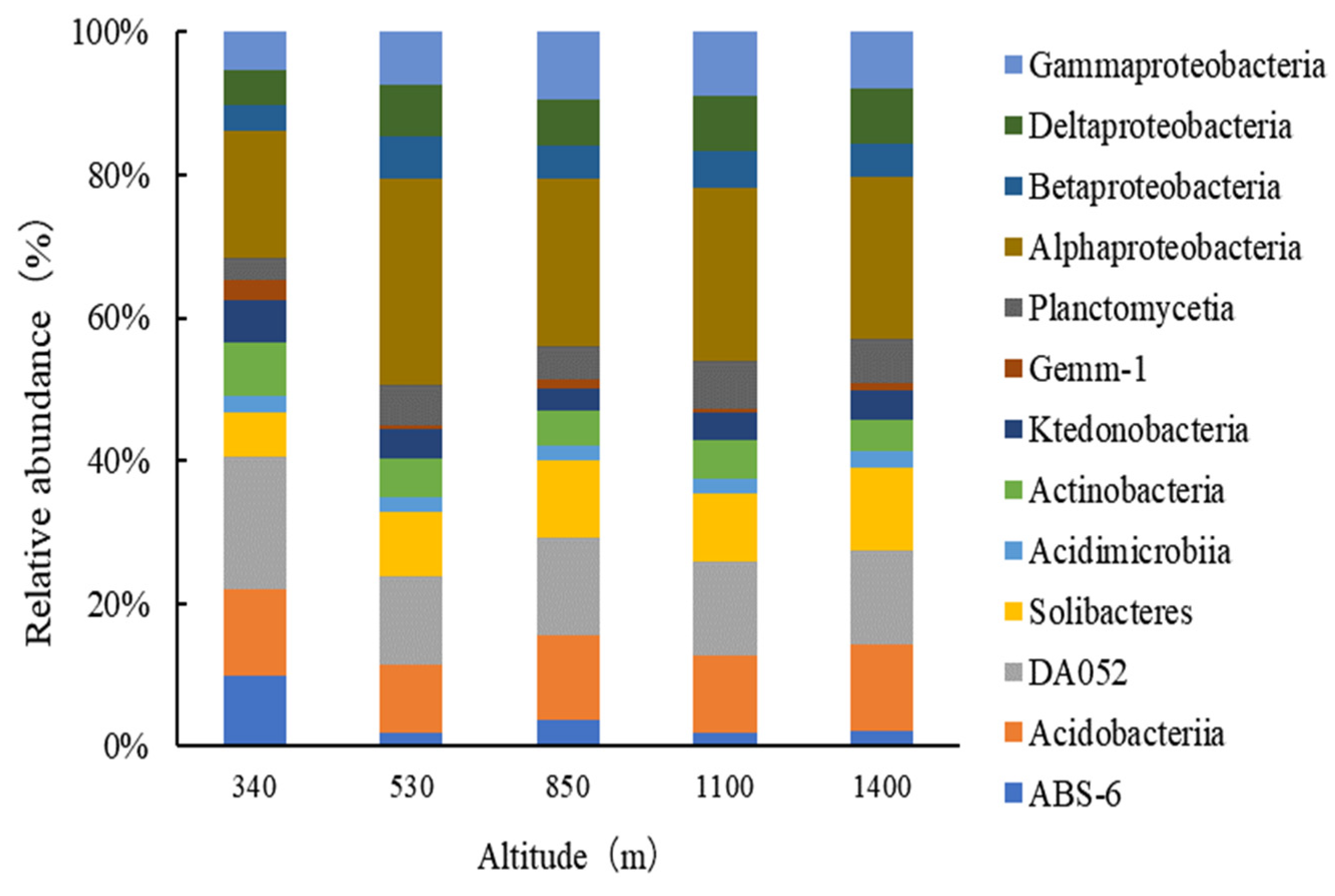
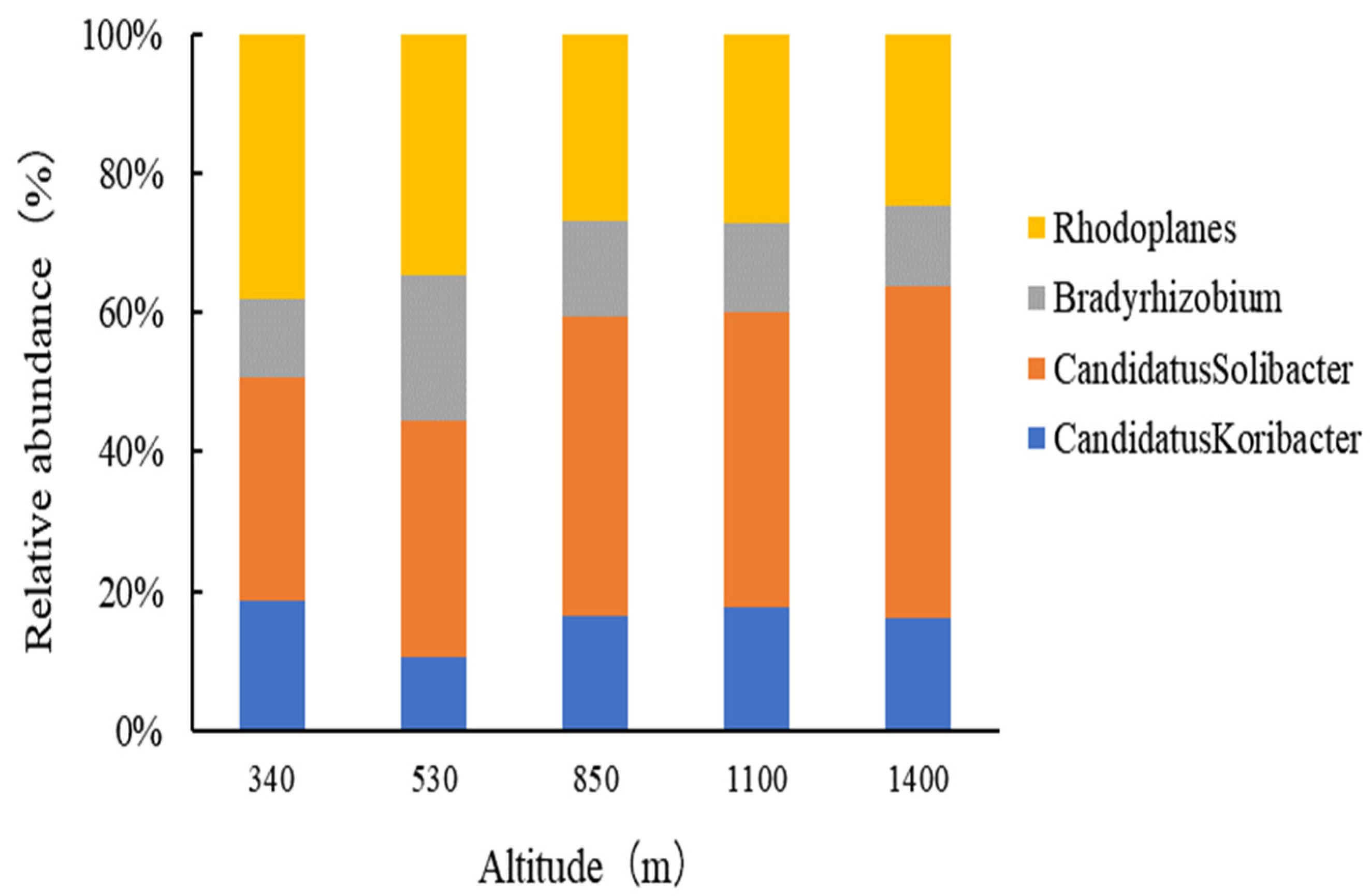
3.1.3. Bacterial Community Structure
3.1.4. Diversity Indices
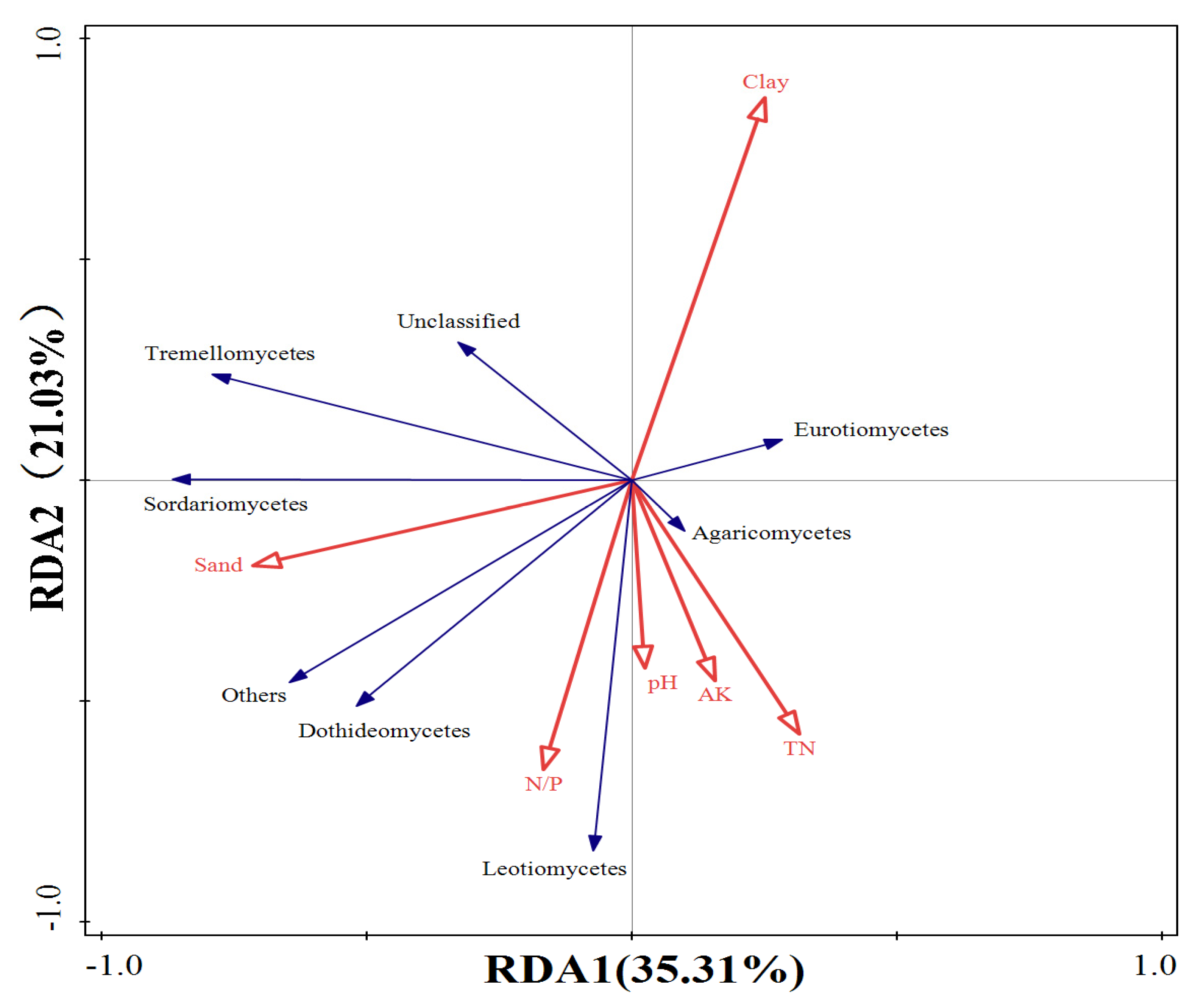
3.2. Relationship between Soil Microbial Community Structure and Environmental Factors
4. Discussion
4.1. Characterization of Soil Microbial Community Structure in Moso Bamboo Forests
4.2. Factors Affecting Soil Microbial Communities in Moso Bamboo Forests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Zhang, X.; Ni, H.; Gai, X.; Huang, Z.; Du, X.; Zhong, Z. Soil Carbon and Associated Bacterial Community Shifts Driven by Fine Root Traits along a Chronosequence of Moso Bamboo (Phyllostachys edulis) Plantations in Subtropical China. Sci. Total Environ. 2021, 752, 142333. [Google Scholar] [CrossRef]
- Li, Z.K.; Zhang, Y.R.; Deng, Z.W.; Liu, J.Y.; Rong, J.D.; Chen, L.G.; He, T.Y.; Zheng, Y.S. Effects of enclosure term on fine root functional traits of Moso bamboo (Phyllostachys edulis) in the Wuyi Mountains. Acta Ecol. Sin. 2024, 1–13. [Google Scholar]
- Ye, L.Q.; Ku, W.P.; Liu, J.; Xu, m.Y.; Meng, F.R.; Fu, W.J.; Liu, J.; Jin, J.; Wu, J.S. Effects of enclosure term on community structure and undergrowth diversity of (Phyllostachys edulis). Acta Ecol. Sin. 2020, 40, 921–930. [Google Scholar]
- Peng, H.; Chen, H.W.; Li, Q.J.; Shen, Q.H.; Zhou, H.M. The effects of Phyllostachys edulis invasion in cunninghamia lanceolata forest on the diversity of fungal community. Soil Fertil. Sci. China 2024, 2, 105–110. [Google Scholar]
- Wang, X.; Wang, Y.X.; Ji, H.X.; Shi, M.; Wang, H.L.; Song, X.Z.; Li, Q. Response of phosphorus fractions in rhizosphere soil of Moso bamboo to nitrogen and biochar additions. Chin. J. Ecol. 2024, 1–11. [Google Scholar]
- FAO. Global Forest Resources Assessment 2010: Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- SFAPRC. Forest Resources in China—The 8th National Forest Inventory; Statse Forestry Administration, P.R. China: Beijing, China, 2015.
- Xiang, J.; Gu, J.; Wang, G.; Bol, R.; Yao, L.; Fang, Y.; Zhang, H. Soil pH Controls the Structure and Diversity of Bacterial Communities along Elevational Gradients on Huangshan, China. Eur. J. Soil Biol. 2024, 120, 103586. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Zhang, H.; Chen, Y.; Shao, Y.; Yuan, Z. Effect of Altitude Gradients on the Spatial Distribution Mechanism of Soil Bacteria in Temperate Deciduous Broad-Leaved Forests. Microorganisms 2024, 12, 1034. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Zhang, W.; Zhu, J.; Chen, B.; Jiang, L.; Xu, D.; Li, W.; Liu, J.; He, Z. Soil Environments Regulate Dominant Soil Fungal Communities along an Elevational Gradient in Subtropical Forests. Forests 2024, 15, 643. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.G.; Liang, C. Aboveground-belowground biodiversity linkages differ in early and late successional temperate forests. Sci. Rep. 2015, 5, 12234. [Google Scholar] [CrossRef]
- Song, X.C.; Guo, L.M.; Tian, H.D.; Deng, X.j.; Zhao, L.S.; Cao, J.Z. Variation of soil microbial community diversity along an elevational gradient in the Mao’er Mountain forest. Acta Ecol. Sin. 2017, 37, 5428–5435. [Google Scholar]
- Li, X.; Lyu, M.; Zhang, Q.; Feng, J.; Liu, X.; Zhu, B.; Wang, X.; Yang, Y.; Xie, J. Warming Reduces Priming Effect of Soil Organic Carbon Decomposition Along a Subtropical Elevation Gradient. Glob. Biogeochem. Cycles 2024, 38, e2024GB008113. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.X.; Zhao, F.Z.; Guo, Y.X.; Wang, L.; Ren, C.J. Structure characteristics of soil microbial community in Quercus aliena var. acuteserrata Forests at different altitudes in Qinling Mountains. Sci. Silvae Sin. 2021, 57, 22–31. [Google Scholar]
- Liu, B.R. Recent Advances in altitudinal distribution patterns of biodiversity. Ecol. Environ. Sci. 2021, 30, 438–444. [Google Scholar]
- Xiong, X.L.; Ren, Y.B.; Lyu, M.K.; Li, X.J.; Nie, Y.Y.; Xie, J.S. Distribution characteristics of soil organic carbon and total nitrogen storage in typical forest soils at different altitudes in Wuyishan Mountain. Res. Soil Water Conserv. 2022, 29, 83–88. [Google Scholar]
- Ma, J.P.; Pang, D.B.; Chen, L.; Wan, H.Y.; Chen, G.L.; Li, X.B. Characteristics of soil microbial community structure under vegetation at different altitudes in Helan Mountains. Acta Ecol. Sin. 2022, 42, 667–676. [Google Scholar]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Green, L.J. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Singh, D.; Takahashi, K.; Kim, M.; Chun, J.; Adams, J.M. A Hump-Backed Trend in Bacterial Diversity with Elevation on Mount Fuji, Japan. Microb. Ecol. 2012, 63, 429–437. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Sun, Y.M.; Lin, C.; Cai, L.P.; Zhang, H.X. Ecological stoichiometric characteristics of soil carbon, nitrogen and phosphorus in Moso bamboo forests at different altitudes in Wuyi Mountain. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2022, 51, 367–373. [Google Scholar]
- Sun, Y.; Chen, X.; Zhong, A.; Guo, S.; Zhang, H. Variations in Microbial Residue and Its Contribution to SOC between Organic and Mineral Soil Layers along an Altitude Gradient in the Wuyi Mountains. Forests 2023, 14, 1678. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lin, C.; Cheng, H.; Jin, C.S.; Xu, Z.k.; Wei, Z.C.; Ma, X.Q. Variation of soil organic carbon content of Moso Bamboo forest along altitudinal gradient in Wuyi Mountain in China. Soils 2019, 51, 821–828. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus Subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Qian, D.; Qiu, Z.; Hou, Y.; Li, Q.; Shen, W. Land-Use Intensification Exerts a Greater Influence on Soil Microbial Communities than Seasonal Variations in the Taihu Lake Region, China. Sci. Total Environ. 2024, 943, 173630. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wei, H.; Ming, A.; Shu, W.; Shen, W. Tree Species Mixing Begets Admixture of Soil Microbial Communities: Variations along Bulk Soil, Rhizosphere Soil and Root Tissue. Geoderma 2023, 438, 116638. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, X.; Zhao, Y.P.; Wang, X.Y.; Zhang, Y.T.; Liang, Y.B.; Wen, Y.X.; Chen, Y.H. Effects of Mine Remediation Mode on Microbial Community Structure and Function in the Rhizosphere Soil of Robinia Pseudoacacia. Environ. Sci. 2024, 1–17. [Google Scholar]
- Li, Y.; Yang, H.V.; Yu, Q.L.; Guo, D.G.; Zhang, Q.X. Soil Microbial Diversity and Its Response to Heavy Metals in Yuncheng Salt Lake Wetland. Environ. Sci. 2024, 1–14. [Google Scholar]
- Rao, D.A.; Liu, P.Y.; Zou, L.Y.; Teng, Y.; Yu, H.Y.; Yin, G.T. Effects of long-term continuous cropping and reductive soil disinfestation on fungal community in flue-cured tobacco rhizosphere. Soil Fertil. Sci. China 2022, 4, 47–56. [Google Scholar]
- Zhao, Q.Q.; Xie, J.K.; Gao, Y.C.; Zhang, W.; Wang, J.N.; Chen, G.H. The distribution pattern of soil fungal community in coastal wetlands with different hydrologic conditions in the Yellow River Estuary. Acta Sci. Circumstantiae 2022, 42, 95–103. [Google Scholar]
- Li, Y.Y.; Xu, T.T.; Ai, Z.; Ma, F. Fungal community diversity and driving factors in rhizosphere soil of Caragana species across semi-arid regions. Chin. J. Appl. Ecol. 2021, 32, 4289–4297. [Google Scholar]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil Multifunctionality Is Affected by the Soil Environment and by Microbial Community Composition and Diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Yang, X.J.; Meng, Z.Y.; Li, Y.; Yang, Y.Z.; Li, Y.G.; Su, Y.; Li, M. Analysis of fungal community structure in rhizosphere soil of dominant tree species in Helan Mountain. For. Eng. 2024, 1–11. [Google Scholar]
- Zhang, X.F.; Zang, C.P.; Dong, Q.M.; Huo, L.A.; Tong, Y.S.; Zhang, X.; Yang, Z.Z.; Yu, Y.; Cao, Q. Soil fungal community structure in perennial cultivated grassland around Qinghai Lake. Acta Agrestia Sin. 2024, 1–16. [Google Scholar]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M. A few Ascomycota taxa dominate soil fungal communities world wide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Wijayawardene, N.; Hyde, K.D.; Dai, D.Q. Outline of Ascomycota. Fungal Divers. 2018, 89, 246–254. [Google Scholar]
- Zhang, E.H.; Liu, P.P.; He, P.; Jian, Y.; Xu, Y.T.; Chen, C.X.; Lu, Y.Z.; Lan, X.Z.; Suo, L.S.M. Physiochemical properties and microbial community structure in rhizosphere soil of dracocephalum tanguticum. J. Agric. Sci. Technol. 2024, 26, 201–213. [Google Scholar]
- Huang, F.; Wang, W.R.; Rao, X.; He, R.; Wang, J.; Jian, S.G.; Shen, W.j.; Ren, H. Soil improvements and microbial community development following establishment of plant communities in a tropical coral island. Acta Ecol. Sin. 2019, 39, 6227–6237. [Google Scholar]
- Zhao, X.; Liu, H.L.; Yang, P.; Qu, Y.P.; Wang, S.M.; Zhang, X. Effects of drip irrigation on bacterial diversity and community structure in rhizosphere soil of alfalfa. Microbiol. China 2019, 46, 2579–2590. [Google Scholar]
- Choudhary, M.; Sharma, P.C.; Jat, H.S.; Dash, A.; Rajashekar, B.; McDonald, A.J.; Jat, M.L. Soil Bacterial Diversity under Conservation Agriculture-Based Cereal Systems in Indo-Gangetic Plains. Biotech 2018, 8, 304. [Google Scholar] [CrossRef]
- An, X.R.; Jiang, S.T.; Xie, X.Y.; Xu, Y.C.; Dong, C.X.; Shen, Q.R. Effects of reducing chemical fertilizers combined with organic fertilizers on soil microbial community in litchi orchards. Chin. J. Appl. Ecol. 2022, 33, 1099–1108. [Google Scholar]
- Zhou, Y.I.; Jia, X.; Zhao, Y.H.; Wang, Q.; Ye, X.; An, Y.R. Review on soil microbial patterns along the elevation gradient based on the knowledge mapping analysis. J. Ecol. Rural Environ. 2021, 37, 1281–1291. [Google Scholar]
- Klimek, B.; Jaźwa, M.; Choczyński, M.; Stolarczyk, M.; Niklińska, M. The Drivers of Soil Microbial Communities Structure on Forest Stands along the Altitudinal Gradient in Western Carpathians. Acta Oecologica 2020, 108, 103643. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Jia, X.; Zhao, Y.H.; Chen, N.N.; Yan, J.; Tang, J.Q.; Wang, Q.; Liu, L. Altitude distribution of fungal community in Huoditang in Qinling Mountains, Northwest China. Chin. J. Appl. Ecol. 2021, 32, 2589–2596. [Google Scholar]
- Peay, K.G.; Baraloto, C. Fine Paul VA. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.H.; Chen, L.; Wang, J.T.; Zhang, L.M. Soil pH determines fungal diversity along an elevation gradient in southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Man, B.Y.; Xiang, X.; Luo, Y.; Mao, X.T.; Zhang, C.; Sun, B.H.; Wang, X. Characteristics and influencing factors of soil fungal community of typical vegetation types in Mount Huangshan, East China. Mycosystema 2021, 40, 2735–2751. [Google Scholar]
- Si, G.C.; Yuan, Y.L.; Wang, J.; Xia, Y.Q.; Lei, T.Z.; Zhang, G.X. Microbial community and soil enzyme activities along an altitudinal gradient in Sejila mountains. Microbiol. China 2014, 41, 2001–2011. [Google Scholar]
- Yu, Y.Y.; Li, M.S.; Liu, X.L.; Yin, W.P.; Li, G.F.; Mu, L.Q.; Cui, X.Y.; Cheng, Z.C. Soil bacterial community composition and diversity of typical permafrost in Greater Khingan Mountains. Microbiol. China 2020, 47, 2759–2770. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ju, Z.J.; Chen, H.; Fu, Y.; Zhao, B.; Zhang, J.Y.; Lu, M.Q.; Cui, J.S.; Zhang, L.L. Spatial distribution of Quinolone Antibiotics and its correlation relationship with microbial community in soil of shijiazhuang city. Environ. Sci. 2022, 43, 4684–4696. [Google Scholar]
- Wang, S.K.; Zhao, X.Y.; Jia, K.F.; Gao, B.L.; Qu, H.; Mo, W.; Lian, J.; Chen, M.; Zhu, Y.C. Soil bacterial diversity and its vertical distribution in Stipa klemenzii Community of Urad Desert Steppe. J. Desert Res. 2016, 36, 1564–1570. [Google Scholar]
- Liu, B.R.; Niu, S.F.; Zhang, W.W. Effects of sol particle size on enzyme activities and the amount of soil microorganism in rhizosphere of Caragana korshinskii in desert steppe. Acta Ecol. Sin. 2019, 39, 9171–9178. [Google Scholar]
- Wang, X.J.; Zhang, Z.C.; Yu, Z.Q.; Shen, G.F.; Cheng, H.F.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Zhang, X.P.; Jia, S.X. The effect of soil physical and chemical properties on soil microbial community in agro-ecosystem. Soils Crops 2013, 2, 138–144. [Google Scholar]


| ID | Altitude (m) | Longitude (°) | Latitude (°) | SOC (g/kg) | TN (g/kg) | TP (g/kg) | AP (mg/kg) | AK (mg/kg) | C/N | C/P | N/P | BD (g/cm3) | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | 340 | 117.82 | 27.67 | 25.69 b | 2.65 b | 0.35 b | 3.75 c | 49.72 b | 17.11 a | 126.04 c | 7.43 d | 0.98 a | 4.47 a |
| E2 | 530 | 117.72 | 27.71 | 34.41 b | 2.86 b | 0.24 b | 2.45 c | 63.89 b | 22.47 a | 256.41 ab | 12.41 bc | 1.07 a | 4.96 c |
| E3 | 850 | 117.76 | 27.72 | 54.87 a | 5.42 a | 0.34 b | 7.09 b | 75.28 b | 18.15 a | 291.48 ab | 16.06 ab | 0.93 a | 4.21 d |
| E4 | 1100 | 117.65 | 27.70 | 59.20 a | 6.12 a | 0.34 b | 7.69 b | 106.11 a | 16.92 a | 305.36 a | 18.22 a | 0.63 b | 4.80 bc |
| E5 | 1400 | 117.74 | 27.75 | 70.68 a | 6.10 a | 0.71 a | 11.72 a | 117.22 a | 20.04 a | 173.53 bc | 8.61 cd | 0.70 b | 4.42 b |
| Altitude (m) | CHAO | ACE | Shannon | |
|---|---|---|---|---|
| 340 | 2273.91 ± 315.38 a | 2126.98 ± 205.77 a | 7.09 ± 0.19 ab | |
| 530 | 3316.08 ± 237.74 b | 3134.75 ± 193.43 b | 7.86 ± 0.35 a | |
| Fungi | 850 | 2627.20 ± 393.18 ab | 2575.24 ± 278.29 ab | 7.96 ± 0.31 a |
| 1100 | 3278.24 ± 438.06 b | 3046.39 ± 401.11 b | 7.50 ± 0.52 ab | |
| 1400 | 2010.11 ± 804.18 a | 1907.70 ± 711.76 a | 6.86 ± 0.91 b | |
| 340 | 14,174.60 ± 1794.40 a | 14,561.00 ± 1914.33 a | 9.83 ± 0.41 a | |
| 530 | 19,464.10 ± 2433.39 b | 19,934.36 ± 2825.05 b | 10.48 ± 0.18 b | |
| Bacteria | 850 | 20,622.13 ± 2561.67 b | 20,987.01 ± 2412.87 b | 10.35 ± 0.10 b |
| 1100 | 20,167.14 ± 1693.96 b | 20,838.70 ± 1589.23 b | 10.65 ± 0.07 b | |
| 1400 | 18,738.03 ± 2259.18 b | 18,915.81 ± 2072.57 b | 10.52 ± 0.43 b |
| Environmental Factor | Explanatory Volume of Environmental Factors (%) | Pseudo-F | p |
|---|---|---|---|
| Sand | 19.600 | 3.200 | 0.028 |
| Clay | 17.300 | 3.300 | 0.012 |
| AK | 15.900 | 3.700 | 0.016 |
| pH | 4.700 | 1.100 | 0.394 |
| N/P | 2.300 | 0.500 | 0.746 |
| TN | 1.700 | 0.400 | 0.860 |
| Environmental Factor | Explanatory Volume of Environmental Factors (%) | Pseudo-F | p |
|---|---|---|---|
| Clay | 37.300 | 7.700 | 0.008 |
| pH | 17.100 | 4.500 | 0.008 |
| AK | 10.300 | 3.200 | 0.010 |
| N/P | 6.400 | 2.500 | 0.052 |
| TN | 4.800 | 1.600 | 0.184 |
| C/P | 3.900 | 1.300 | 0.238 |
| Sand | 2.800 | 1.100 | 0.342 |
| C/N | 1.300 | 0.500 | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, X.; Cai, J.; Li, Y.; Zhou, Y.; Zhang, H.; Zheng, K. Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain. Plants 2024, 13, 2471. https://doi.org/10.3390/plants13172471
Sun Y, Chen X, Cai J, Li Y, Zhou Y, Zhang H, Zheng K. Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain. Plants. 2024; 13(17):2471. https://doi.org/10.3390/plants13172471
Chicago/Turabian StyleSun, Yiming, Xunlong Chen, Jianwei Cai, Yangzhuo Li, Yuhan Zhou, Houxi Zhang, and Kehui Zheng. 2024. "Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain" Plants 13, no. 17: 2471. https://doi.org/10.3390/plants13172471






