From Stand to Forest: Woody Plant Recruitment in an Andean Restoration Project
Abstract
1. Introduction
2. Results
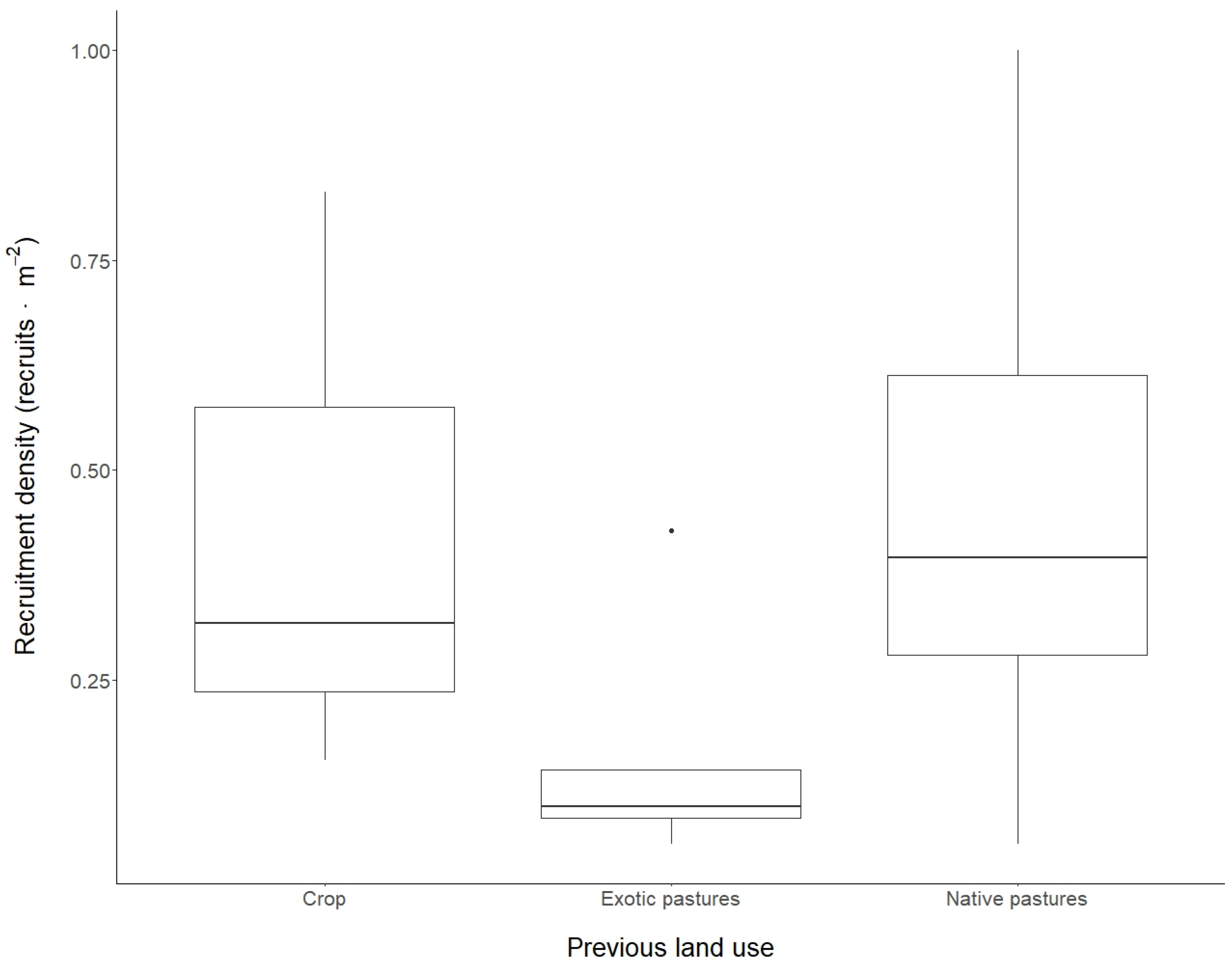
2.1. Recruitment Characteristics
2.2. Recruiment Density and Diversity and Environmental Factors
3. Discussion
3.1. Recruitment Density and Environmental Factors
3.2. Recruitment Diversity and Environmental Factors
3.3. Implications for Andean Forest Restoration
4. Conclusions
5. Materials and Methods
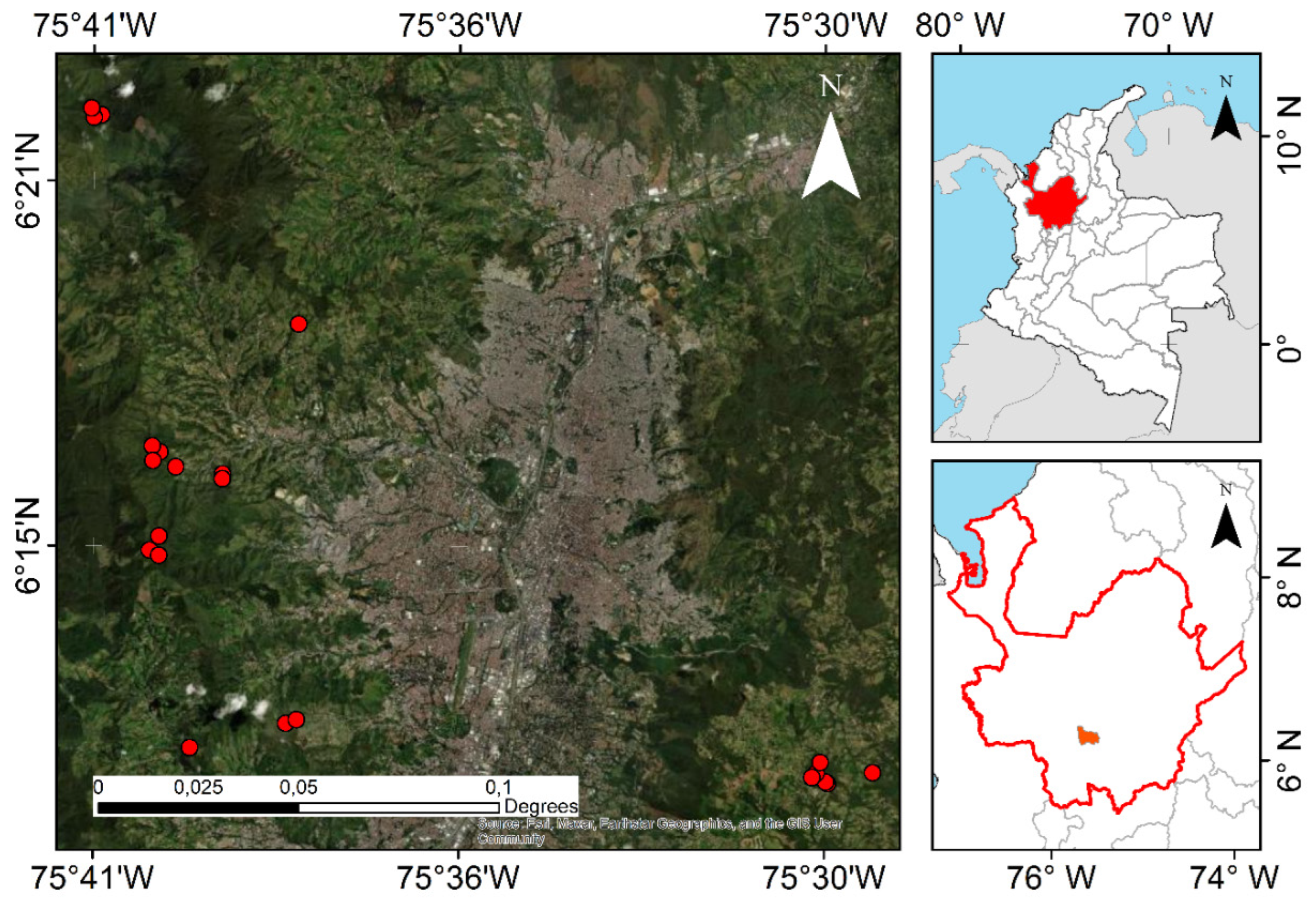
5.1. Study Area
5.2. Response Variables
5.3. Predictor Variables
5.3.1. Edaphic Conditions
5.3.2. Plantation Structure and Diversity
5.3.3. Landscape Metrics
5.4. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Family | Abundance |
|---|---|---|
| Aegiphila sp. | Lamiaceae | 2 |
| Ageratina popayanensis | Asteraceae | 1 |
| Alchornea grandiflora | Euphorbiaceae | 6 |
| Alchornea sp. | Euphorbiaceae | 1 |
| Alchornea triplinervia | Euphorbiaceae | 3 |
| Andesanthus lepidotus | Melastomataceae | 14 |
| Araliaceae | Araliaceae | 1 |
| Arecaceae sp. 1 | Arecaceae | 2 |
| Asteraceae indet. | Asteraceae | 2 |
| Asteraceae indet. 2 | Asteraceae | 3 |
| Asteraceae indet. 3 | Asteraceae | 3 |
| Austroeupatorium inulifolium | Asteraceae | 4 |
| Baccharis brachylaenoides | Asteraceae | 1 |
| Baccharis latifolia | Asteraceae | 4 |
| Baccharis nitida | Asteraceae | 17 |
| Baccharis sp. | Asteraceae | 2 |
| Bocconia frutescens | Papaveraceae | 1 |
| Cavendishia pubescens | Ericaceae | 1 |
| Cecropia sp. | Urticaceae | 1 |
| Cestrum nocturnum | Solanaceae | 1 |
| Cestrum ochraceum | Solanaceae | 3 |
| Cestrum sp. | Solanaceae | 7 |
| Cestrum tomentosum | Solanaceae | 21 |
| Chamaedorea sp. | Arecaceae | 2 |
| Citharexylum subflavescens | Verbenaceae | 3 |
| Clethra fagifolia | Clethraceae | 5 |
| Clethra revoluta | Clethraceae | 2 |
| Clethra sp. | Clethraceae | 47 |
| Clidemia ciliata | Melastomataceae | 7 |
| Clusia sp. 1 | Clusiaceae | 1 |
| Clusia sp. 2 | Clusiaceae | 1 |
| Clusia sp. 3 | Clusiaceae | 8 |
| Croton magdalenensis | Euphorbiaceae | 85 |
| Duranta erecta | Verbenaceae | 1 |
| Erythrina edulis | Fabaceae | 1 |
| Eucalyptus sp. | Myrtaceae | 3 |
| Eupatorium sp. | Myrtaceae | 1 |
| Fraxinus uhdei | Oleaceae | 70 |
| Fuchsia sp. | Onagraceae | 3 |
| Geissanthus sp. | Primulaceae | 1 |
| Hedyosmum translucidum | Chloranthaceae | 31 |
| Heliocarpus sp. | Malvaceae | 1 |
| Hyeronima antioquensis | Phyllanthaceae | 1 |
| Ladenbergia macrocarpa | Rubiaceae | 7 |
| Lantana camara | Verbenaceae | 1 |
| Lepechinia bullata | Lamiaceae | 10 |
| Lycianthes radiata | Solanaceae | 1 |
| Melastomataceae indet. 1 | Melastomataceae | 1 |
| Meriania nobilis | Melastomataceae | 16 |
| Miconia caudata | Melastomataceae | 28 |
| Miconia domociliata | Melastomataceae | 1 |
| Miconia jahnii | Melastomataceae | 1 |
| Miconia lehmannii | Melastomataceae | 6 |
| Miconia sp. | Melastomataceae | 1 |
| Miconia stenostachya | Melastomataceae | 5 |
| Miconia theaezans | Melastomataceae | 305 |
| Monochaetum multiflorum | Melastomataceae | 1 |
| Montanoa quadrangularis | Asteraceae | 92 |
| Morella pubescens | Myricaceae | 13 |
| Myrsine coriacea | Primulaceae | 186 |
| Myrsine latifolia | Primulaceae | 2 |
| Nectandra acutifolia | Lauraceae | 2 |
| Ocotea leucoxylon | Lauraceae | 8 |
| Oreopanax sp. | Araliaceae | 10 |
| Palicourea acetosoides | Rubiaceae | 109 |
| Palicourea angustifolia | Rubiaceae | 31 |
| Palicourea apicata | Rubiaceae | 2 |
| Palicourea garciae | Rubiaceae | 2 |
| Palicourea sp. | Rubiaceae | 5 |
| Palicourea sp. 2 | Rubiaceae | 2 |
| Palicourea thyrsiflora | Rubiaceae | 65 |
| Paratrophis insignis | Moraceae | 1 |
| Persea caerulea | Lauraceae | 8 |
| Piper sp. 1 | Piperaceae | 2 |
| Piper sp. 2 | Piperaceae | 1 |
| Prunus integrifolia | Rosaceae | 1 |
| Psidium guajava | Myrtaceae | 18 |
| Rhamnus sphaerosperma | Rhamnaceae | 1 |
| Rubiaceae | Rubiaceae | 1 |
| Saurauia sp. | Actinidiaceae | 8 |
| Senna pistaciifolia | Fabaceae | 1 |
| Senna sp. | Fabaceae | 1 |
| Siparuna grandiflora | Siparunaceae | 1 |
| Solanaceae indet. 1 | Solanaceae | 1 |
| Solanaceae indet. 2 | Solanaceae | 1 |
| Solanum dolosum | Solanaceae | 11 |
| Solanum nutans | Solanaceae | 3 |
| Solanum sp. | Solanaceae | 1 |
| Solanum stellatiglandulosum | Solanaceae | 1 |
| Solanum sycophanta | Solanaceae | 2 |
| Toxicodendron striatum | Anacardiaceae | 9 |
| Urera sp. | Urticaceae | 15 |
| Verbesina helianthoides | Asteraceae | 121 |
| Verbesina nudipes | Asteraceae | 4 |
| Verbesina sp. | Asteraceae | 5 |
| Viburnum undulatum | Viburnaceae | 49 |
| Vismia baccifera | Hypericaceae | 18 |
| Weinmannia balbisiana | Cunoniaceae | 2 |
| Weinmannia pubescens | Cunoniaceae | 49 |
| Plot | Total Recruits | Number of Species | Sample Coverage | Maximum Sample Coverage |
|---|---|---|---|---|
| PM01 | 86 | 7 | 95.39% | 97.64% |
| PM02 | 101 | 19 | 92.12% | 95.23% |
| PM03 | 11 | 6 | 65.37% | 79.75% |
| PM04 | 17 | 7 | 77.18% | 86.47% |
| PM05 | 11 | 5 | 65.91% | 83.23% |
| PM07 | 31 | 9 | 87.31% | 92.39% |
| PM08 | 34 | 9 | 79.59% | 84.77% |
| PM09 | 41 | 8 | 95.35% | 99.37% |
| PM10 | 118 | 27 | 83.94% | 88.31% |
| PM12 | 68 | 11 | 94.20% | 97.88% |
| PM14 | 20 | 4 | 90.95% | 98.77% |
| PM16 | 167 | 25 | 93.42% | 94.51% |
| PM19 | 57 | 9 | 94.86% | 98.65% |
| PM20 | 61 | 16 | 82.02% | 85.05% |
| PM21 | 192 | 21 | 95.86% | 98.81% |
| PM22 | 64 | 11 | 95.36% | 97.63% |
| PM23 | 91 | 15 | 94.55% | 97.56% |
| PM24 | 56 | 13 | 83.99% | 87.22% |
| PM25 | 101 | 19 | 99.02% | 99.95% |
| PM26 | 175 | - | - | - |
| PM27 | 125 | 15 | 96.84% | 99.29% |
| PM28 | 176 | 23 | 93.76% | 95.67% |

References
- Balboni, C.; Berman, A.; Burgess, R.; Olken, B.A. The Economics of Tropical Deforestation. Annu. Rev. Econom. 2023, 15, 723–754. [Google Scholar] [CrossRef]
- Alroy, J. Effects of Habitat Disturbance on Tropical Forest Biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 6056–6061. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The Exceptional Value of Intact Forest Ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Mitchard, E.T.A. The Tropical Forest Carbon Cycle and Climate Change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating Extinction Risk from Climate Change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The Last Frontiers of Wilderness: Tracking Loss of Intact Forest Landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef]
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global Land Change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple Successional Pathways in Human-Modified Tropical Landscapes: New Insights from Forest Succession, Forest Fragmentation and Landscape Ecology Research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Rodríguez, V.; Rito, K.F.; Farfán, M.; Navía, I.C.; Mora, F.; Arreola-Villa, F.; Balvanera, P.; Bongers, F.; Castellanos-Castro, C.; Catharino, E.L.M.; et al. Landscape-Scale Forest Cover Drives the Predictability of Forest Regeneration across the Neotropics. Proc. R. Soc. B Biol. Sci. 2023, 290, 20222203. [Google Scholar] [CrossRef]
- Senior, R.A.; Hill, J.K.; Edwards, D.P. Global Loss of Climate Connectivity in Tropical Forests. Nat. Clim. Chang. 2019, 9, 623–626. [Google Scholar] [CrossRef]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; The University of Chicago Press: Chicago, IL, USA; London, UK, 2014; ISBN 9780226117911. [Google Scholar]
- Society for Ecological Restoration (SER). What Is Ecological Restoration? Available online: https://www.ser-rrc.org/what-is-ecological-restoration/ (accessed on 25 August 2024).
- Poorter, L.; Amissah, L.; Bongers, F.; Hordijk, I.; Kok, J.; Laurance, S.G.W.; Lohbeck, M.; Martínez-Ramos, M.; Matsuo, T.; Meave, J.A.; et al. Successional Theories. Biol. Rev. 2023, 98, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Werden, L.K.; Calderón-Morales, E.; Alvarado, J.P.; Gutiérrez, L.M.; Nedveck, D.A.; Powers, J.S. Using Large-Scale Tropical Dry Forest Restoration to Test Successional Theory. Ecol. Appl. 2020, 30, e02116. [Google Scholar] [CrossRef] [PubMed]
- König, L.A.; Medina-Vega, J.A.; Longo, R.M.; Zuidema, P.A.; Jakovac, C.C. Restoration Success in Former Amazonian Mines Is Driven by Soil Amendment and Forest Proximity. Philos. Trans. R. Soc. B Biol. Sci. 2022, 378, 20210086. [Google Scholar] [CrossRef] [PubMed]
- Caughlin, T.; de la Peña-Domene, M.; Martínez-Garza, C. Demographic Costs and Benefits of Natural Regeneration during Tropical Forest Restoration. Ecol. Lett. 2019, 22, 34–44. [Google Scholar] [CrossRef]
- Burnett, K.M.; Ticktin, T.; Bremer, L.L.; Quazi, S.A.; Geslani, C.; Wada, C.A.; Kurashima, N.; Mandle, L.; Pascua, P.; Depraetere, T.; et al. Restoring to the Future: Environmental, Cultural, and Management Trade-Offs in Historical versus Hybrid Restoration of a Highly Modified Ecosystem. Conserv. Lett. 2019, 12, e12606. [Google Scholar] [CrossRef]
- Catterall, C.P. Fauna as Passengers and Drivers in Vegetation Restoration: A Synthesis of Processes and Evidence. Ecol. Manag. Restor. 2018, 19, 54–62. [Google Scholar] [CrossRef]
- Lozano-Baez, S.E.; Domínguez-Haydar, Y.; Meli, P.; van Meerveld, I.; Vásquez Vásquez, K.; Castellini, M. Key Gaps in Soil Monitoring during Forest Restoration in Colombia. Restor. Ecol. 2021, 29, e13391. [Google Scholar] [CrossRef]
- Clerici, N.; Armenteras, D.; Kareiva, P.; Botero, R.; Ramírez-Delgado, J.P.; Forero-Medina, G.; Ochoa, J.; Pedraza, C.; Schneider, L.; Lora, C.; et al. Deforestation in Colombian Protected Areas Increased during Post-Conflict Periods. Sci. Rep. 2020, 10, 4971. [Google Scholar] [CrossRef]
- Etter, A.; McAlpine, C.; Wilson, K.; Phinn, S.; Possingham, H. Regional Patterns of Agricultural Land Use and Deforestation in Colombia. Agric. Ecosyst. Environ. 2006, 114, 369–386. [Google Scholar] [CrossRef]
- Murcia, C.; Guariguata, M. La Restauración Ecológica En Colombia: Tendencias, Necesidades y Oportunidades; Documentos Ocasionales 107; CIFOR: Bogor, Indonesia, 2014; ISBN 9786021504352. [Google Scholar]
- Murcia, C.; Guariguata, M.R.; Andrade, Á.; Andrade, G.I.; Aronson, J.; Escobar, E.M.; Etter, A.; Moreno, F.H.; Ramírez, W.; Montes, E. Challenges and Prospects for Scaling-up Ecological Restoration to Meet International Commitments: Colombia as a Case Study. Conserv. Lett. 2016, 9, 213–220. [Google Scholar] [CrossRef]
- Dent, D.H.; Estrada-Villegas, S. Uniting Niche Differentiation and Dispersal Limitation Predicts Tropical Forest Succession. Trends Ecol. Evol. 2021, 36, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Catterall, C.P. Roles of Non-Native Species in Large-Scale Regeneration of Moist Tropical Forests on Anthropogenic Grassland. Biotropica 2016, 48, 809–824. [Google Scholar] [CrossRef]
- Catterall, C.P. Values of Weedy Regrowth for Rainforest Restoration. Ecol. Manag. Restor. 2020, 21, 9–13. [Google Scholar] [CrossRef]
- Bohlman, S.A. Species Diversity of Canopy Versus Understory Trees in a Neotropical Forest: Implications for Forest Structure, Function and Monitoring. Ecosystems 2015, 18, 658–670. [Google Scholar] [CrossRef]
- Rissanen, K.; Martin-Guay, M.O.; Riopel-Bouvier, A.S.; Paquette, A. Light Interception in Experimental Forests Affected by Tree Diversity and Structural Complexity of Dominant Canopy. Agric. For. Meteorol. 2019, 278, 107655. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Junqueira, A.B.; Crouzeilles, R.; Peña-Claros, M.; Mesquita, R.C.G.; Bongers, F. The Role of Land-Use History in Driving Successional Pathways and Its Implications for the Restoration of Tropical Forests. Biol. Rev. 2021, 96, 1114–1134. [Google Scholar] [CrossRef]
- Hordijk, I.; Poorter, L.; Martínez-Ramos, M.; Bongers, F.; Mendoza, R.D.L.; Romero, P.J.; van der Sande, M.; Muñoz, R.; Jansma, R.; Fujisawa, N.; et al. Land Use Legacies Affect Early Tropical Forest Succession in Mexico. Appl. Veg. Sci. 2024, 27, e12784. [Google Scholar] [CrossRef]
- Borges, M.; Melo, C. Frugivory and Seed Dispersal of Miconia Theaezans (Bonpl.) Cogniaux (Melastomataceae) by Birds in a Transition Palm Swamp: Gallery Forest in Central Brazil. Braz. J. Biol. 2012, 72, 25–31. [Google Scholar] [CrossRef]
- Begnini, R.M.; Castellani, T.T. Seed Rain under the Canopies of Female and Male Myrsine Coriacea, a Pioneer Tree from the Brazilian Atlantic Forest. J. Trop. Ecol. 2013, 29, 391–399. [Google Scholar] [CrossRef]
- Pérez-Rojo, A.F. Fisiología de La Semilla y Germinación de Montanoa Quadrangularis. Bachelor’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2006. [Google Scholar]
- Mopán-Chilito, A.M.; Montilla, S.O.; Buitrago-Torres, D.L.; Saldaña-Vidal, A.L.; Aristizabal, J.F. Using a Phylogenetic Framework to Assess the Role of Fruit Size in Food Selection by the Andean Night Monkey (Aotus Lemurinus). Int. J. Primatol. 2022, 43, 273–290. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, M.A.; Lee, C.; Clement, W.L.; Donoghue, M.J. Fruit Syndromes in Viburnum: Correlated Evolution of Color, Nutritional Content, and Morphology in Bird-Dispersed Fleshy Fruits. BMC Evol. Biol. 2020, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Buitrón-Jurado, G.; Ramírez, N. Dispersal Spectra, Diaspore Size and the Importance of Endozoochory in the Equatorial Andean Montane Forests. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 299–311. [Google Scholar] [CrossRef]
- Castaño, G.J.; Arias, A. Evaluación de La Avifauna de La Microcuenca de La Quebrada Santa Helena, Zona Centro Oriental de Medellín; Instituto para el Manejo Integral de la Cuenca del Río Medellín (Mi Río)–Sociedad Antioqueña de Ornitología: Medellín, Colombia, 1999. [Google Scholar]
- Chazdon, R.L.; Norden, N.; Colwell, R.K.; Chao, A. Monitoring Recovery of Tree Diversity during Tropical Forest Restoration: Lessons from Long-Term Trajectories of Natural Regeneration. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20210069. [Google Scholar] [CrossRef]
- Ledo, A.; Cayuela, L.; Manso, R.; Condés, S. Recruitment Patterns and Potential Mechanisms of Community Assembly in an Andean Cloud Forest. J. Veg. Sci. 2015, 26, 876–888. [Google Scholar] [CrossRef]
- Freeman, A.N.D.; Freebody, K.; Montenero, M.; Moran, C.; Shoo, L.P.; Catterall, C.P. Enhancing Bird-Mediated Seed Dispersal to Increase Rainforest Regeneration in Disused Pasture—A Restoration Experiment. For. Ecol. Manag. 2021, 479, 118536. [Google Scholar] [CrossRef]
- Kelm, D.H.; Wiesner, K.R.; Helversen, O.V.O.N. Effects of Artificial Roosts for Frugivorous Bats on Seed Dispersal in a Neotropical Forest Pasture Mosaic. Conserv. Biol. 2008, 22, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Armenteras, D.; Rodríguez, N.; Retana, J.; Morales, M. Understanding Deforestation in Montane and Lowland Forests of the Colombian Andes. Reg. Environ. Chang. 2011, 11, 693–705. [Google Scholar] [CrossRef]
- Armenteras, D.; Cabrera, E.; Rodríguez, N.; Retana, J. National and Regional Determinants of Tropical Deforestation in Colombia. Reg. Environ. Chang. 2013, 13, 1181–1193. [Google Scholar] [CrossRef]
- Hoyos-Estrada, C. Evaluación de La Regeneración de Especies Del Bosque Natural Bajo Dosel de Coníferas y En Bosque Secundario En La Cuenca de La Quebrada Piedras Blancas. Master’s Thesis, Universidad Nacional de Colombia, Medellín, Colombia, 2003. [Google Scholar]
- Vallejo-Mayo, L.Y.; Rivera-Díaz, O. Floristic Inventory in Andean Forest Areas of the Central Cordillera of Colombia (El Peñol, Antioquia). Caldasia 2022, 44, 8–18. [Google Scholar] [CrossRef]
- López González, W.; Montoya, Á.D. Bosques Montanos Del Neotrópico Beta Diversity in Neotropical Mountain Forests. Caldasia 2010, 32, 175–189. [Google Scholar]
- Restrepo Correa, Z. Dominancia de Árboles Andinos En Colombia: Una Relación de Pocas Especies. In Descubrimientos Recientes Contados por Investigadores Locales; Calderón-caro, J., Benavides, A.M., Cepeda, D., Eds.; Observatorio de Bosques de Antioquia: Medellin, Colombia, 2021; p. 5. [Google Scholar]
- Kopittke, P.M.; Moore, K.L.; Lombi, E.; Gianoncelli, A.; Ferguson, B.J.; Blamey, F.P.C.; Menzies, N.W.; Nicholson, T.M.; McKenna, B.A.; Wang, P.; et al. Identification of the Primary Lesion of Toxic Aluminum in Plant Roots. Plant Physiol. 2015, 167, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Merchan, D. Restauración Ecológica: Evaluación de Modelos y Factores de Sitio En Zonas Degradadas Por Uso Agropecuario En Medellín—Antioquia. Master’s Thesis, Universidad Nacional de Colombia, Medellín, Colombia, 2019. [Google Scholar]
- McDaniel, P.; Lowe, D.; Arnalds, O.; Ping, C. Andisols. In Handbook of Soil Sciences; Huang, P., Li, Y., Summer, M., Eds.; Taylor & Francis: Abingdon, UK, 2012; pp. 29–33. [Google Scholar]
- Cano-Arboleda, L.V.; Villegas, J.C.; Restrepo, A.C.; Quintero-Vallejo, E. Complementary Effects of Tree Species on Canopy Rainfall Partitioning: New Insights for Ecological Restoration in Andean Ecosystems. For. Ecol. Manag. 2022, 507, 119969. [Google Scholar] [CrossRef]
- Holl, K.D.; Reid, J.L.; Cole, R.J.; Oviedo-Brenes, F.; Rosales, J.A.; Zahawi, R.A. Applied Nucleation Facilitates Tropical Forest Recovery: Lessons Learned from a 15-Year Study. J. Appl. Ecol. 2020, 57, 2316–2328. [Google Scholar] [CrossRef]
- Holdridge, L. Life Zone Ecology; Tropical Science Center: San José, Costa Rica, 1967. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Kohyama, T.S.; Kohyama, T.I.; Sheil, D. Definition and Estimation of Vital Rates from Repeated Censuses: Choices, Comparisons and Bias Corrections Focusing on Trees. Methods Ecol. Evol. 2018, 9, 809–821. [Google Scholar] [CrossRef]
- Rich, P.M.; Wood, J.; Vieglais, D.A.; Burek, K.; Webb, N. Hemiview User Manual; Delta-T Devices, Ltd.: Cambridge, UK, 1999. [Google Scholar]
- Tiede, D. Vector-Based Landscape Analysis Tools Extension; Z_GIS from University of Salzburg: Salzburg, Austria, 2012. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780387874579. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Pinheiro, J.; Bates, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. 2023. Available online: https://cran.r-project.org/web/packages/nlme/ (accessed on 25 August 2024).
- Spiess, A. QpcR: Modelling and Analysis of Real-Time PCR Data. 2018. Available online: https://www.rdocumentation.org/packages/qpcR/versions/1.4-1 (accessed on 25 August 2024).


| Species | Number of Individuals | Presence in Plots | Dispersal Mode |
|---|---|---|---|
| Miconia theaezans | 305 | 71.43% | Endozoochory [31] |
| Myrsine coriacea * | 186 | 80.95% | Endozoochory [32] |
| Verbesina helianthoides | 121 | 57.14% | Anemochory (achene) |
| Palicourea acetosoides | 109 | 4.76% | Endozoochory [32] |
| Montanoa quadrangularis * | 92 | 38.10% | Anemochory [33] |
| Croton magdalenensis * | 85 | 57.14% | Endozoocoria [34] |
| Fraxinus uhdei | 70 | 4.76% | Anemochory (samara) |
| Palicourea thyrsiflora | 65 | 14.28% | Endozoochory [32] |
| Viburnum undulatum | 49 | 42.86% | Endozoochory [35] |
| Weinmannia pubescens * | 49 | 23.80% | Anemochory [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piquer-Doblas, M.; Correa-Londoño, G.A.; Osorio-Vélez, L.F. From Stand to Forest: Woody Plant Recruitment in an Andean Restoration Project. Plants 2024, 13, 2474. https://doi.org/10.3390/plants13172474
Piquer-Doblas M, Correa-Londoño GA, Osorio-Vélez LF. From Stand to Forest: Woody Plant Recruitment in an Andean Restoration Project. Plants. 2024; 13(17):2474. https://doi.org/10.3390/plants13172474
Chicago/Turabian StylePiquer-Doblas, Marina, Guillermo A. Correa-Londoño, and Luis F. Osorio-Vélez. 2024. "From Stand to Forest: Woody Plant Recruitment in an Andean Restoration Project" Plants 13, no. 17: 2474. https://doi.org/10.3390/plants13172474
APA StylePiquer-Doblas, M., Correa-Londoño, G. A., & Osorio-Vélez, L. F. (2024). From Stand to Forest: Woody Plant Recruitment in an Andean Restoration Project. Plants, 13(17), 2474. https://doi.org/10.3390/plants13172474






