Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones
Abstract
1. Introduction
2. Role of Phytohormones in Rice Resistance to BLB
2.1. Role of SA in Rice Resistance to BLB
2.2. Role of JA in Rice Resistance to BLB
| Gene | Gene Product | Phytohormone | Function Characteristic | Regulation Mode | Reference |
|---|---|---|---|---|---|
| OsHPL3 | Hydroperoxide lyase | JA, SA | Activates JA-mediated defense responses | Negative | [63,64] |
| OsLOX10 | Lipoxygenase | JA, SA | Upregulates ROS levels and gene expression of the JA and SA signaling pathways | Positive | [65] |
| OsOPR10 | 12-oxygen-phytodienoate reductase | JA, SA | Upregulates ROS levels and gene expression of the JA and SA signaling pathways | Positive | [66] |
| OsWRKY30 | WRKY transcription factor | JA, SA | Promotes JA accumulation to improve resistance | Positive | [67] |
| OsWRKY45-2 | WRKY transcription factor | JA | Upregulates JA level and the expression of defense-related genes | Positive | [4] |
| C3H12 | CCCH-type zinc finger nucleic acid-binding protein | JA | Positively regulates resistance to Xoo through the JA signaling pathway | Positive | [70] |
| OsPAD4 | Phytoalexin deficient 4 | JA | Participates in Xoo defense signaling via JA dependency | Positive | [71] |
| rrsRLK | Cytoplasmic RLK | JA | Regulates ROS levels and JA-related gene expression | Negative | [72] |
| OsPHD1 | UDP-glucose epimerase | JA | Disruption of OsPHD1 upregulates defense-related genes to activate the defense response | Negative | [73] |
| OsCOI1 | JA receptor | JA | Regulates JA and GA, which jointly mediate rice disease resistance | Positive | [75,76] |
| OsJAZ8 | JA ZIM-domain protein | JA | Negatively regulates JA-induced resistance to Xoo | Negative | [77] |
| OsWRKY72 | WRKY transcription factor | JA, ABA | Inhibition of AOS1 transcription reduces JA levels, resulting in susceptibility to Xoo | Negative | [78] |
| OsSAPK10 | Stress-activated protein kinase | JA, ABA | Phosphorylates WRKY72, alleviating the inhibition of JA synthesis | Positive | [78] |
| OsAOS1 | Allene oxide synthase | JA | Increases endogenous JA levels and thus improves resistance | Positive | [78] |
| OsAOS2 | Allene oxide synthase | JA, SA | May mediate resistance by influencing JA levels | Positive | [79] |
| OsMYC2 | JAZ-interacting transcription factor | JA | The resistance is mediated through JID-dependent interaction with the OsJAZ protein | Positive | [80] |
| RERJ1 | JA-inducible transcription factor | JA, SA | Interacts with OsMYC2 and OsNPR1 to regulate hormonal signaling | Positive | [81] |
| OsMED25 | Mediator subunit 25 | JA, Auxin | Negatively regulates a subset of OsMYC2-independent JA defense signaling | Negative | [83] |
| OsSRO1a | Similar to RCD one1a | JA | Negatively regulates the OsMYC2-mediated JA signaling pathway | Negative | [84] |
| OsJMTF1 | MYB transcription factor | JA, IAA | Promotes the biosynthesis of lignin and antibacterial monoterpene, γ-terpinene | Positive | [85] |
| OsCCD4b | 9-cis-epoxycarotenoid dioxygenase | JA, ABA | Promotes the synthesis of β-Cyc and downregulates ABA levels | Positive | [86] |
| OsEIL3 | Ethylene-insensitive3-like3 transcription factor | JA, SA, ET | Activates JA and SA biosynthesis and signaling pathways to regulate resistance | Positive | [89] |
2.3. Role of ET in Rice Resistance to BLB
2.4. Role of Auxin in Rice Resistance to BLB
2.5. Role of ABA in Rice Resistance to BLB
3. Mutual Regulatory Mechanism of Phytohormones in Rice Resistance to BLB
4. Application of Modern Breeding Techniques in Cultivating Rice Resistant to BLB
5. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elert, E. Rice by the numbers: A good grain. Nature 2014, 514, S50–S51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hallerman, E.; Peng, Y.; Li, Y. Development of Bt Rice and Bt Maize in China and Their Efficacy in Target Pest Control. Int. J. Mol. Sci. 2016, 17, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wang, S. Toward an understanding of the molecular basis of quantitative disease resistance in rice. J. Biotechnol. 2012, 159, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Liu, H.; Qiu, D.; Zhou, Y.; Li, X.; Xu, C.; Wang, S. A Pair of Allelic WRKY Genes Play Opposite Roles in Rice-Bacteria Interactions. Plant Physiol. 2009, 151, 936–948. [Google Scholar] [CrossRef]
- Rajarajeswari, N.V.L.; Muralidharan, K. Assessments of farm yield and district production loss from bacterial leaf blight epidemics in rice. Crop Prot. 2006, 25, 244–252. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of Bacterial Leaf Blight of Rice and Profiling of Secondary Metabolites Produced by Rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef]
- Naqvi, S.A.H. Bacterial leaf blight of rice: An overview of epidemiology and management with special reference to Indian sub-continent. Pak. J. Agric. Res. 2019, 32, 359–380. [Google Scholar] [CrossRef]
- Li, D.Q.; Zhong, Q.F.; Zeng, M.; Chen, Y.; Wang, B.; Cheng, Z.Q. Progress in Mapping, Cloning and Application of Resistance Genes to Bacterial Blight Disease in Rice. China Rice 2017, 23, 19–27. [Google Scholar]
- Abrol, D.; Shankar, U. Pesticides, Food Safety and Integrated Pest Management. Integr. Pest Manag. 2014, 3, 167–199. [Google Scholar]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance Genes and their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa L.)—An Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Xu, Z.; Yan, J.; Wang, Y.; Wang, Y.; Cheng, G.; Zou, L.; Chen, G. TALE-induced immunity against the bacterial blight pathogen Xanthomonas oryzae pv. oryzae in rice. Phytopathol. Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Lu, Y.; Tsuda, K. Intimate Association of PRR- and NLR-Mediated Signaling in Plant Immunity. Mol. Plant Microbe Interact. 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Wang, P.; Liu, J.; Lyu, Y.; Huang, Z.; Zhang, X.; Sun, B.; Li, P.; Jing, X.; Li, H.; Zhang, C. A Review of Vector-Borne Rice Viruses. Viruses 2022, 14, 2258. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, H.; Li, Y.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol. 2011, 155, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Antiviral Roles of Abscisic Acid in Plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Zhao, S.; Hong, W.; Wu, J.; Wang, Y.; Ji, S.; Zhu, S.; Wei, C.; Zhang, J.; Li, Y. A viral protein promotes host SAMS1 activity and ethylene production for the benefit of virus infection. eLife 2017, 6, e27529. [Google Scholar] [CrossRef]
- Guo, Q.; Major, I.T.; Howe, G.A. Resolution of growth-defense conflict: Mechanistic insights from jasmonate signaling. Curr. Opin. Plant Biol. 2018, 44, 72–81. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Le Thanh, T.; Thumanu, K.; Wongkaew, S.; Boonkerd, N.; Teaumroong, N.; Phansak, P.; Buensanteai, N. Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J. Plant Interact. 2016, 12, 108–120. [Google Scholar] [CrossRef]
- Mohan Babu, R.; Sajeena, A.; Vijaya Samundeeswari, A.; Sreedhar, A.; Vidhyasekaran, P.; Seetharaman, K.; Reddy, M.S. Induction of systemic resistance to Xanthomonas oryzae pv. oryzae by salicylic acid in Oryza sativa (L.). J. Plant Dis. Prot. 2003, 110, 419–431. [Google Scholar] [CrossRef]
- Pajerowska-Mukhtar, K.M.; Emerine, D.K.; Mukhtar, M.S. Tell me more: Roles of NPRs in plant immunity. Trends Plant Sci. 2013, 18, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhong, S.; Li, Q.; Zhu, Z.; Lou, Y.; Wang, L.; Wang, J.; Wang, M.; Li, Q.; Yang, D.; et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 2007, 5, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Hwang, D.J. Isolation and characterization of the rice NPR1 promoter. Plant Biotechnol. Rep. 2010, 4, 29–35. [Google Scholar] [CrossRef]
- Feng, J.X.; Cao, L.; Li, J.; Duan, C.J.; Luo, X.M.; Le, N.; Wei, H.; Liang, S.; Chu, C.; Pan, Q.; et al. Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. Eur. J. Plant Pathol. 2011, 131, 221–235. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Sun, L.; Li, Q.; Mao, B.; He, Z. The Systemic Acquired Resistance Regulator OsNPR1 Attenuates Growth by Repressing Auxin Signaling through Promoting IAA-Amido Synthase Expression. Plant Physiol. 2016, 172, 546–558. [Google Scholar] [CrossRef]
- Jiang, G.; Yin, D.; Shi, Y.; Zhou, Z.; Li, C.; Liu, P.; Jia, Y.; Wang, Y.; Liu, Z.; Yu, M.; et al. OsNPR3.3-dependent salicylic acid signaling is involved in recessive gene xa5-mediated immunity to rice bacterial blight. Sci. Rep. 2020, 10, 6313. [Google Scholar] [CrossRef]
- Bai, W.; Chern, M.; Ruan, D.; Canlas, P.E.; Sze-To, W.H.; Ronald, P.C. Enhanced disease resistance and hypersensitivity to BTH by introduction of an NH1/OsNPR1 paralog. Plant Biotechnol. J. 2011, 9, 205–215. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Yang, C.; Wang, L.; Qi, L.; Guo, Z.; Chen, X. Salicylic Acid Is Required for Broad-Spectrum Disease Resistance in Rice. Int. J. Mol. Sci. 2022, 23, 1354. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Yao, W.; Yin, Z.; Wang, Y.; Huang, Z.; Zhou, J.; Liu, J.; Lu, X.; Wang, F.; et al. CRISPR/Cas9-mediated simultaneous mutation of three salicylic acid 5-hydroxylase (OsS5H) genes confers broad-spectrum disease resistance in rice. Plant Biotechnol. J. 2023, 21, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhang, Y.; Tian, X.; Li, W.; Meng, H.; Zhou, Y.; Wang, Z.; Chen, Z.; Zhang, K.; Wang, M. Increased cytoplasmic Mg2+ level contributes to rice salicylic acid accumulation and broad-spectrum resistance. Plant Physiol. 2024, 195, 2515–2519. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, J.; Zheng, C.; Zheng, E.; Liang, W.; Tan, X.; Xu, R.; Yan, C.; Yang, Y.; Yi, K.; et al. OsTGAL1 suppresses the resistance of rice to bacterial blight disease by regulating the expression of salicylic acid glucosyltransferase OsSGT1. Plant Cell Environ. 2022, 45, 1584–1602. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Satou, J.; Iwata, M.; Uozumi, N.; Koga, J.; Kawano, T.; Koshiba, T.; Anzai, H.; Mitomi, M. Contribution of salicylic acid glucosyltransferase, OsSGT1, to chemically induced disease resistance in rice plants. Plant J. 2009, 57, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Panthapulakkal Narayanan, S.; Lung, S.C.; Liao, P.; Lo, C.; Chye, M.L. The overexpression of OsACBP5 protects transgenic rice against necrotrophic, hemibiotrophic and biotrophic pathogens. Sci. Rep. 2020, 10, 14918. [Google Scholar] [CrossRef]
- Gao, M.; He, Y.; Yin, X.; Zhong, X.; Yan, B.; Wu, Y.; Chen, J.; Li, X.; Zhai, K.; Huang, Y.; et al. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 2021, 184, 5391–5404.e17. [Google Scholar] [CrossRef]
- Menke, F.L.H.; van Pelt, J.A.; Pieterse, C.M.J.; Klessig, D.F. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 2004, 16, 897–907. [Google Scholar] [CrossRef][Green Version]
- Shen, X.; Yuan, B.; Liu, H.; Li, X.; Xu, C.; Wang, S. Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J. 2010, 64, 86–99. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Liu, Q.; Tu, R.; Zhou, X.; Zhang, Y.; Wu, W.; Yu, P.; Chen, D.; Zhan, X.; et al. Reduction of OsMPK6 activity by a R89K mutation induces cell death and bacterial blight resistance in rice. Plant Cell Rep. 2021, 40, 835–850. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Q.; Cao, Y.; Zhang, Y.; Chen, D.; Lou, X.; Cheng, S.; Cao, L. The OsMPK15 Negatively Regulates Magnaporthe oryza and Xoo Disease Resistance via SA and JA Signaling Pathway in Rice. Front. Plant Sci. 2019, 10, 752. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhou, M.; Yoo, H.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 9166–9173. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Mao, X.; Zhang, H.; Zhang, Y.; Fu, M.; Sun, Z.; Kuai, P.; Lou, Y.; Fang, Y. Lamin-like Proteins Negatively Regulate Plant Immunity through NAC WITH TRANSMEMBRANE MOTIF1-LIKE9 and NONEXPRESSOR OF PR GENES1 in Arabidopsis thaliana. Mol. Plant. 2017, 10, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yu, J.; Wu, Y.; Yao, X.; Mao, C.; Meng, X.; Ming, F. Rice transcription factor OsNAC2 maintains the homeostasis of immune responses to bacterial blight. Plant Physiol. 2024, 195, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A Rice NAC Transcription Factor Promotes Leaf Senescence via ABA Biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2020, 18, 429–442. [Google Scholar] [CrossRef]
- Yu, J.; Mao, C.; Zhong, Q.; Yao, X.; Li, P.; Liu, C.; Ming, F. OsNAC2 Is Involved in Multiple Hormonal Pathways to Mediate Germination of Rice Seeds and Establishment of Seedling. Front. Plant Sci. 2021, 12, 699303. [Google Scholar] [CrossRef]
- Bu, H.F.; Wang, X.Q.; Ren, Z.Q.; Xiao, J.H.; Zhang, N.; Yang, H.Z. Research Progress on Plant WRKY Transcription Factors Family Genes. J. Shanxi Agric. Sci. 2020, 48, 1158–1163. [Google Scholar]
- Ryu, H.S.; Han, M.; Lee, S.K.; Cho, J.I.; Ryoo, N.; Heu, S.; Lee, Y.H.; Bhoo, S.H.; Wang, G.L.; Hahn, T.R.; et al. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006, 25, 836–847. [Google Scholar] [CrossRef]
- Shimono, M.; Koga, H.; Akagi, A.; Hayashi, N.; Goto, S.; Sawada, M.; Kurihara, T.; Matsushita, A.; Sugano, S.; Jiang, C.J.; et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 2012, 13, 83–94. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.; Guo, Z. Alternative Splicing of Rice WRKY62 and WRKY76 Transcription Factor Genes in Pathogen Defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef]
- Peng, Y.; Bartley, L.E.; Chen, X.; Dardick, C.; Chern, M.; Ruan, R.; Canlas, P.E.; Ronald, P.C. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2008, 1, 446–458. [Google Scholar] [CrossRef]
- Choi, C.; Hwang, S.H.; Fang, I.R.; Kwon, S.; Park, S.I.; Ahn, I.; Kim, J.B.; Hwang, D.J. Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 2015, 208, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Xiao, J.; Ding, X.; Xiong, M.; Cai, M.; Cao, Y.; Li, X.; Xu, C.; Wang, S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007, 20, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cheng, H.; Li, X.; Xiao, J.; Xu, C.; Wang, S. Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiol. 2013, 163, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Abe, H.; Arimura, G. Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol. 2015, 56, 16–27. [Google Scholar] [CrossRef]
- Miyamoto, K.; Shimizu, T.; Okada, K. Transcriptional regulation of the biosynthesis of phytoalexin: A lesson from specialized metabolites in rice. Plant Biotechnol. 2014, 31, 377–388. [Google Scholar] [CrossRef]
- Shimizu, T.; Lin, F.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyamoto, K.; Miyamoto, K.; Minami, E.; Nishizawa, Y.; Iino, M.; Nojiri, H.; Yamane, H.; Okada, K. OsJAR1 contributes mainly to biosynthesis of the stress-induced jasmonoyl-isoleucine involved in defense responses in rice. Biosci. Biotechnol. Biochem. 2013, 77, 1556–1564. [Google Scholar] [CrossRef]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquiterpene synthase that produces an antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, H.; Matsunaga, A.; Suzuki, G.; Gomi, K. Monoterpene geraniol produced by rice terpene synthase 21 suppresses the expression of cell-division related genes in the rice bacterial pathogen, Xanthomonas oryzae pv. Oryzae. Physiol. Mol. Plant Pathol. 2021, 115, 101673. [Google Scholar] [CrossRef]
- Tong, X.; Qi, J.; Zhu, X.; Mao, B.; Zeng, L.; Wang, B.; Li, Q.; Zhou, G.; Xu, X.; Lou, Y.; et al. The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. Plant J. 2012, 71, 763–775. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Tang, J.; Wang, W.; Zhang, F.; Wang, G.; Chu, J.; Yan, C.; Wang, T.; Chu, C.; et al. Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice. PLoS ONE 2012, 7, e50089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Shen, X.L.; Zhou, L.S.; Lin, Q.X.; Wang, Z.L.; Chen, J.; Feng, H.J.; Zhang, Z.W.; Zhang, X.T.; Lu, G.D. OsLOX10 Positively Regulates Defense Responses of Rice to Rice Blast and Bacterial Blight. Chin. J. Rice Sci. 2022, 36, 348–356. [Google Scholar]
- Xu, D.J.; Lin, Q.X.; Li, Z.K.; Zhuang, X.Q.; Ling, Y.; Lai, M.L.; Chen, X.T.; Lu, G.D. OsOPR10 Positively Regulates Rice Blast and Bacterial Blight Resistance. Chin. J. Rice Sci. 2024, 38, 364–374. [Google Scholar]
- Peng, X.; Hu, Y.; Tang, X.; Zhou, P.; Deng, X.; Wang, H.; Guo, Z. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta 2012, 236, 1485–1498. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. Functional Involvement of a Mitogen Activated Protein Kinase Module, OsMKK3-OsMPK7-OsWRK30 in Mediating Resistance against Xanthomonas oryzae in Rice. Sci. Rep. 2016, 6, 37974. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Zhao, Y.; Wang, S.; Yuan, M. OsMAPK6 phosphorylates a zinc finger protein OsLIC to promote downstream OsWRKY30 for rice resistance to bacterial blight and leaf streak. J. Integr. Plant Biol. 2022, 64, 1116–1130. [Google Scholar] [CrossRef]
- Deng, H.; Liu, H.; Li, X.; Xiao, J.; Wang, S. A CCCH-type zinc finger nucleic acid-binding protein quantitatively confers resistance against rice bacterial blight disease. Plant Physiol. 2012, 158, 876–889. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, H.; Li, X.; Xiao, J.; Wang, S. Rice OsPAD4 functions differently from Arabidopsis AtPAD4 in host-pathogen interactions. Plant J. 2014, 78, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Park, J.C.; Cho, M.H.; Yang, J.; Kim, C.Y.; Jung, K.H.; Jeon, J.S.; An, G.; Lee, S.W. Lack of a Cytoplasmic RLK, Required for ROS Homeostasis, Induces Strong Resistance to Bacterial Leaf Blight in Rice. Front. Plant Sci. 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiang, X.; Zhang, Y.; Cao, Y.; Wang, B.; Zhang, Y.; Wang, C.; Jiang, M.; Duan, W.; Chen, D.; et al. Disruption of OsPHD1, Encoding a UDP-Glucose Epimerase, Causes JA Accumulation and Enhanced Bacterial Blight Resistance in Rice. Int. J. Mol. Sci. 2022, 23, 751. [Google Scholar] [CrossRef] [PubMed]
- Devoto, A.; Ellis, C.; Magusin, A.; Chang, H.S.; Chilcott, C.; Zhu, T.; Turner, J.G. Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 2005, 58, 497–513. [Google Scholar] [CrossRef]
- Yang, D.L. The Phytohormonal Signaling Pathways in Rice Immune Responses and Jasmonate Signaling Pathway Represses Gibberellin Signaling Pathway. Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 2009. [Google Scholar]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef]
- Yamada, S.; Kano, A.; Tamaoki, D.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012, 53, 2060–2072. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Y.; Tang, L.; Tong, X.; Wang, L.; Liu, L.; Huang, S.; Zhang, J. SAPK10-Mediated Phosphorylation on WRKY72 Releases Its Suppression on Jasmonic Acid Biosynthesis and Bacterial Blight Resistance. iScience 2019, 16, 499–510. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhuang, X.Q.; Wang, Z.L.; Lin, Q.X.; Li, Z.K.; Xu, D.J.; Chen, X.T. Effects of OsAOS2 gene on rice resistances to Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2024; online ahead of print. [Google Scholar]
- Uji, Y.; Taniguchi, S.; Tamaoki, D.; Shishido, H.; Akimitsu, K.; Gomi, K. Overexpression of OsMYC2 Results in the Up-Regulation of Early JA-Rresponsive Genes and Bacterial Blight Resistance in Rice. Plant Cell Physiol. 2016, 57, 1814–1827. [Google Scholar] [CrossRef]
- Valea, I.; Motegi, A.; Kawamura, N.; Kawamoto, K.; Miyao, A.; Ozawa, R.; Takabayashi, J.; Gomi, K.; Nemoto, K.; Nozawa, A.; et al. The rice wound-inducible transcription factor RERJ1 sharing same signal transduction pathway with OsMYC2 is necessary for defense response to herbivory and bacterial blight. Plant Mol. Biol. 2022, 109, 651–666. [Google Scholar] [CrossRef]
- Meng, F.; Yang, C.; Cao, J.; Chen, H.; Pang, J.; Zhao, Q.; Wang, Z.; Qing Fu, Z.; Liu, J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020, 62, 1552–1573. [Google Scholar] [CrossRef]
- Suzuki, G.; Fukuda, M.; Lucob-Agustin, N.; Inukai, Y.; Gomi, K. The Mutation of Rice MEDIATOR25, OsMED25, Induces Rice Bacterial Blight Resistance through Altering Jasmonate- and Auxin-Signaling. Plants 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, K.; Onohata, T.; Yariuchi, R.; Tanaka, S.; Akimitsu, K.; Gomi, K. The overexpression of OsSRO1a, which encodes an OsNINJA1- and OsMYC2-interacting protein, negatively affects OsMYC2-mediated jasmonate signaling in rice. Plant Cell Rep. 2020, 39, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Uji, Y.; Suzuki, G.; Fujii, Y.; Kashihara, K.; Yamada, S.; Gomi, K. Jasmonic acid (JA)-mediating MYB transcription factor1, JMTF1, coordinates the balance between JA and auxin signalling in the rice defence response. Physiol. Plant. 2024, 176, e14257. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Takeda, A.; Kiryu, M.; Gomi, K. Jasmonic Acid-Induced β-Cyclocitral Confers Resistance to Bacterial Blight and Negatively Affects Abscisic Acid Biosynthesis in Rice. Int. J. Mol. Sci. 2023, 24, 1704. [Google Scholar] [CrossRef] [PubMed]
- Leon-Reyes, A.; Van der Does, D.; De Lange, E.S.; Delker, C.; Wasternack, C.; Van Wees, S.C.M.; Ritsema, T.; Pieterse, C.M.J. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 2010, 232, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal Behav. 2013, 8, e24260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Shi, C.M.; Xu, G.; Wang, N.; Zuo, S.; Ning, Y.; Kang, H.; Liu, W.; Wang, R.; et al. Antagonistic control of rice immunity against distinct pathogens by the two transcription modules via salicylic acid and jasmonic acid pathways. Dev. Cell. 2024, 59, 1609–1622. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.; Sheen, J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009, 14, 270–279. [Google Scholar] [CrossRef]
- Feng, G.; Sanderson, B.; Keefover-Ring, K.; Liu, J.; Ma, T.; Yin, T.; Smart, L.B.; DiFazio, S.P.; Olson, M.S. Pathways to sex determination in plants: How many roads lead to Rome? Curr. Opin. Plant Biol. 2020, 54, 61–68. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ahammed, G.J.; Wu, C.; Fan, S.Y.; Zhou, Y.H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Delauré, S.L.; De Bolle, M.F.; Cammue, B.P.A. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Jeong, A.R.; Kwon, O.K.; Park, C.J. Oryza-Specific Orphan Protein Triggers Enhanced Resistance to Xanthomonas oryzae pv. oryzae in Rice. Front. Plant Sci. 2022, 13, 859375. [Google Scholar] [CrossRef]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Wang, N.N.; Zhao, Y.D.; Wang, X.L. The transcription factor OsEIL4 positively regulates rice resistance to rice blast and bacterialblight. Acta Phytopathol. Sin. 2024; online ahead of print. [Google Scholar]
- Shen, X.; Liu, H.; Yuan, B.; Li, X.; Xu, C.; Wang, S. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant Cell Environ. 2011, 34, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yang, Y.; He, Z. Roles of plant hormones and their interplay in rice immunity. Mol. Plant. 2013, 6, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid amido synthetase GH3-8 suppresses expansin expression andpromotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Domingo, C.; Andrés, F.; Tharreau, D.; Iglesias, D.J.; Talón, M. Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol. Plant Microbe Interact. 2009, 22, 201–210. [Google Scholar] [CrossRef]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 36–46. [Google Scholar] [CrossRef]
- Li, W.; Shao, M.; Yang, J.; Zhong, W.; Okada, K.; Yamane, H.; Qian, G.; Liu, F. Oscyp71Z2 involves diterpenoid phytoalexin biosynthesis that contributes to bacterial blight resistance in rice. Plant Sci. 2013, 207, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Lei, L.; Liu, Z.; Zhou, S.; Yang, C.; Zhu, X.; Guo, H.; Zhang, F.; Peng, M.; Zhang, M.; et al. Selection of a subspecies-specific diterpene gene cluster implicated in rice disease resistance. Nat. Plants 2020, 6, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, F.; Wang, J.; Fan, F.; Zhu, J.; Yang, J.; Liu, F.; Zhong, W. Overexpressing CYP71Z2 enhances resistance to bacterial blight by suppressing auxin biosynthesis in rice. PLoS ONE 2015, 10, e0119867. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Wu, J.; Lii, Y.; Li, Y.; Jin, H. Contribution of small RNA pathway components in plant immunity. Mol. Plant Microbe Interact. 2013, 26, 617–625. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, X.; Liu, D.; Xu, W.; Wise, R.; Shen, Q.H. The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet. 2014, 10, e1004755. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, M.; Wang, Z.M.; Fang, R.X.; Wang, X.J.; Jia, Y.T. Dynamic and Coordinated Expression Changes of Rice Small RNAs in Response to Xanthomonas oryzae pv. oryzae. J. Genet Genom. 2015, 42, 625–637. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Peng, T.; Sun, H.Z.; Teotia, S.; Wen, H.L.; Du, Y.X.; Zhang, J.; Li, J.Z.; Tang, G.L.; Xue, H.W.; et al. miR1432-OsACOT (Acyl-CoA thioesterase) module determines grain yield via enhancing grain filling rate in rice. Plant Biotechnol. J. 2019, 17, 712–723. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Q.; Li, Y.; Zhai, W.; Jiang, G.; Li, C. Inducible Enrichment of Osa-miR1432 Confers Rice Bacterial Blight Resistance through Suppressing OsCaML2. Int. J. Mol. Sci. 2021, 22, 11367. [Google Scholar] [CrossRef]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-tasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef] [PubMed]
- Vemanna, R.S.; Bakade, R.; Bharti, P.; Kumar, M.K.P.; Sreeman, S.M.; Senthil-Kumar, M.; Makarla, U. Cross-Talk Signaling in Rice During Combined Drought and Bacterial Blight Stress. Front. Plant Sci. 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant-pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Im, J.H.; Song, G.; Nam, S.; Park, S.R. OsWRKY114 Inhibits ABA-Induced Susceptibility to Xanthomonas oryzae pv. oryzae in Rice. Int. J. Mol. Sci. 2022, 23, 8825. [Google Scholar] [CrossRef]
- Xu, J.; Audenaert, K.; Hofte, M.; De Vleesschauwer, D. Abscisic Acid Promotes Susceptibility to the Rice Leaf Blight Pathogen Xanthomonas oryzae pv oryzae by Suppressing Salicylic Acid-Mediated Defenses. PLoS ONE 2013, 8, e67413. [Google Scholar]
- Zhang, D.; Tian, C.; Yin, K.; Wang, W.; Qiu, J.L. Postinvasive Bacterial Resistance Conferred by Open Stomata in Rice. Mol. Plant Microbe Interact. 2019, 32, 255–266. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kuroda, M.; Yamakawa, H.; Ashizawa, T.; Hirayae, K.; Kurimoto, L.; Shinya, T.; Shibuya, N. Suppression of a phospholipase D gene, OsPLDβ1, activates defense responses and increases disease resistance in rice. Plant Physiol. 2009, 150, 308–319. [Google Scholar] [CrossRef]
- Li, G.; Lin, F.; Xue, H.W. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res. 2007, 17, 881–894. [Google Scholar] [CrossRef]
- Lu, H.; Shen, Z.; Xu, Y.; Wu, L.; Hu, D.; Song, R.; Song, B. Immune Mechanism of Ethylicin-Induced Resistance to Xanthomonas oryzae pv. oryzae in Rice. J. Agric. Food Chem. 2023, 71, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; An, H.K.; Seol, Y.J.; Park, S.R.; Im, J.H. Rice transcription factor WRKY114 directly regulates the expression of OsPR1a and Chitinase to enhance resistance against Xanthomonas oryzae pv. oryzae. Biochem. Biophys. Res. Commun. 2020, 533, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Chern, M.; Bartley, L.E.; Han, M.; Jung, K.H.; Lee, I.; Walia, H.; Richter, T.; Xu, X.; Cao, P.; et al. Towards establishment of a rice stress response interactome. PLoS Genet. 2011, 7, e1002020. [Google Scholar] [CrossRef]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, S.; Huang, W.; Yang, J.; Dong, J.; Zhang, S.; Zhao, J.; Yang, T.; Mao, X.; Zhu, X.; et al. NAC transcription factor ONAC066 positively regulates disease resistance by suppressing the ABA signaling pathway in rice. Plant Mol. Biol. 2018, 98, 289–302. [Google Scholar] [CrossRef]
- Sugio, A.; Yang, B.; Zhu, T.; White, F.F. Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAγ1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA 2007, 104, 10720–10725. [Google Scholar] [CrossRef]
- Liu, C.; Ou, S.; Mao, B.; Tang, J.; Wang, W.; Wang, H.; Cao, S.; Schläppi, M.R.; Zhao, B.; Xiao, G.; et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 2018, 9, 3302. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Venturi, V.; He, Y.W.; Kojima, M.; Sakakibari, H.; Höfte, M.; De Vleesschauwer, D. Phytohormone-mediated interkingdom signaling shapes the outcome of rice-Xanthomonas oryzae pv. oryzae interactions. BMC Plant Biol. 2015, 15, 10. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Song, W.; Yang, Z.; Li, L.; Ma, Q.; Tan, X.; Wei, Z.; Li, Y.; Li, J.; et al. Different viral effectors suppress hormone-mediated antiviral immunity of rice coordinated by OsNPR1. Nat. Commun. 2023, 14, 3011. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.J.; Van Wees, S.C.M. Ethylene: Traffic Controller on Hormonal Crossroads to Defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Khan, M.A. Molecular breeding of rice for improved disease resistance, a review. Australas. Plant Pathol. 2015, 44, 273–282. [Google Scholar] [CrossRef]
- Tan, X.J.; Wang, Z.H.; Wu, Y.Y.; Zheng, E.S.; Xu, R.M.; Chen, J.P.; Wang, X.M.; Yan, C.Q. Application progress of gene editing techniques in rice disease-resistant genes and breeding research. Acta Agric. Zhejiangensis 2021, 33, 1982–1990. [Google Scholar]
- Peng, X.Q.; Wang, M.L. Research advances on resistance genes to bacterial blight disease in rice. Plant Physiol. J. 2022, 58, 472–482. [Google Scholar]
- Pradhan, S.K.; Barik, S.R.; Nayak, D.K.; Pradhan, A.; Pandit, E.; Nayak, P.; Das, S.R.; Pathak, H. Genetics, Molecular Mechanisms and Deployment of Bacterial Blight Resistance Genes in Rice. Crit. Rev. Plant Sci. 2020, 39, 360–385. [Google Scholar] [CrossRef]
- Ullah, I.; Jamil, S.; Iqbal, M.Z.; Shaheen, H.L.; Hasni, S.M.; Jabeen, S.; Mehmood, A.; Akhter, M. Detection of bacterial blight resistance genes in basmati rice landraces. Genet Mol. Res. 2012, 11, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Yugander, A.; Sundaram, R.M.; Singh, K.; Senguttuvel, P.; Ladhalakshmi, D.; Kemparaju, K.B.; Madhav, M.S.; Prasad, M.S.; Hariprasad, A.S.; Laha, G.S. Improved versions of rice maintainer line, APMS 6B, possessing two resistance genes, Xa21 and Xa38, exhibit high level of resistance to bacterial blight disease. Mol. Breed. 2018, 38, 100. [Google Scholar] [CrossRef]
- Hairmansis, A.; Warsono; Supartopo; Yullianida; Nasution, A.; Utami, D.W.; Suwarno. Introgression of bacterial blight resistance gene Xa7 into popular Indonesian rice varieties through backcross and molecular breeding. IOP Conf. Ser. Earth Environ. Sci. 2019, 457, 012050. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, C.L.; Li, G.F.; Zhang, X.K.; Liang, Y.T.; Sun, L.Q.; Zhao, K.J. Genetic Analysis on Bacterial Blight Resistance of Xa23-Transgenic Rice. Acta Agron. Sinica. 2008, 34, 1679–1687. [Google Scholar] [CrossRef]
- Yu, L.J.; Zhang, G.L.; Ding, X.W.; Gao, Y.; Xie, Y.F. Progress in Identification and Application of Resistance Genes to Bacterial Blight. Plant Physiol. J. 2012, 48, 223–231. [Google Scholar]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Cao, Y.; Jin, X.; Fu, Z.; Li, J.; Mo, X.; He, Y.; Tang, J.; Huang, S. Engineering Resistance to Bacterial Blight and Bacterial Leaf Streak in Rice. Rice 2021, 14, 38. [Google Scholar] [CrossRef]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 Mediated Genome Editing in Super Basmati Rice for Resistance Against Bacterial Blight by Targeting the Major Susceptibility Gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef]
- Wu, G.H.; Fu, X.Y. Editing rice negative regulation resistance gene OsEDR1 by CRISPR/Cas9 and analysis of its function. Chin. J. Appl. Environ. Biol. 2019, 25, 1375–1380. [Google Scholar]
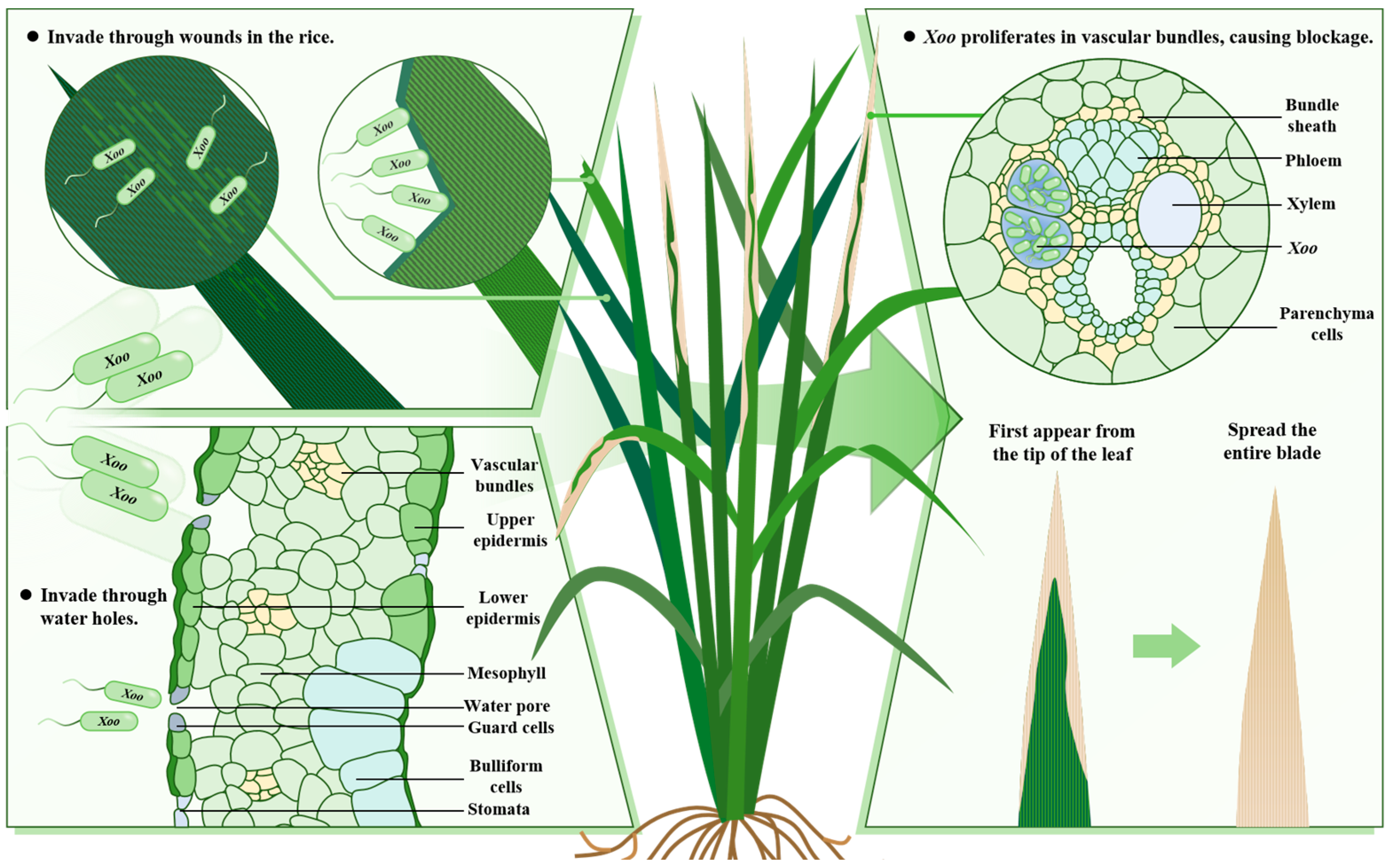
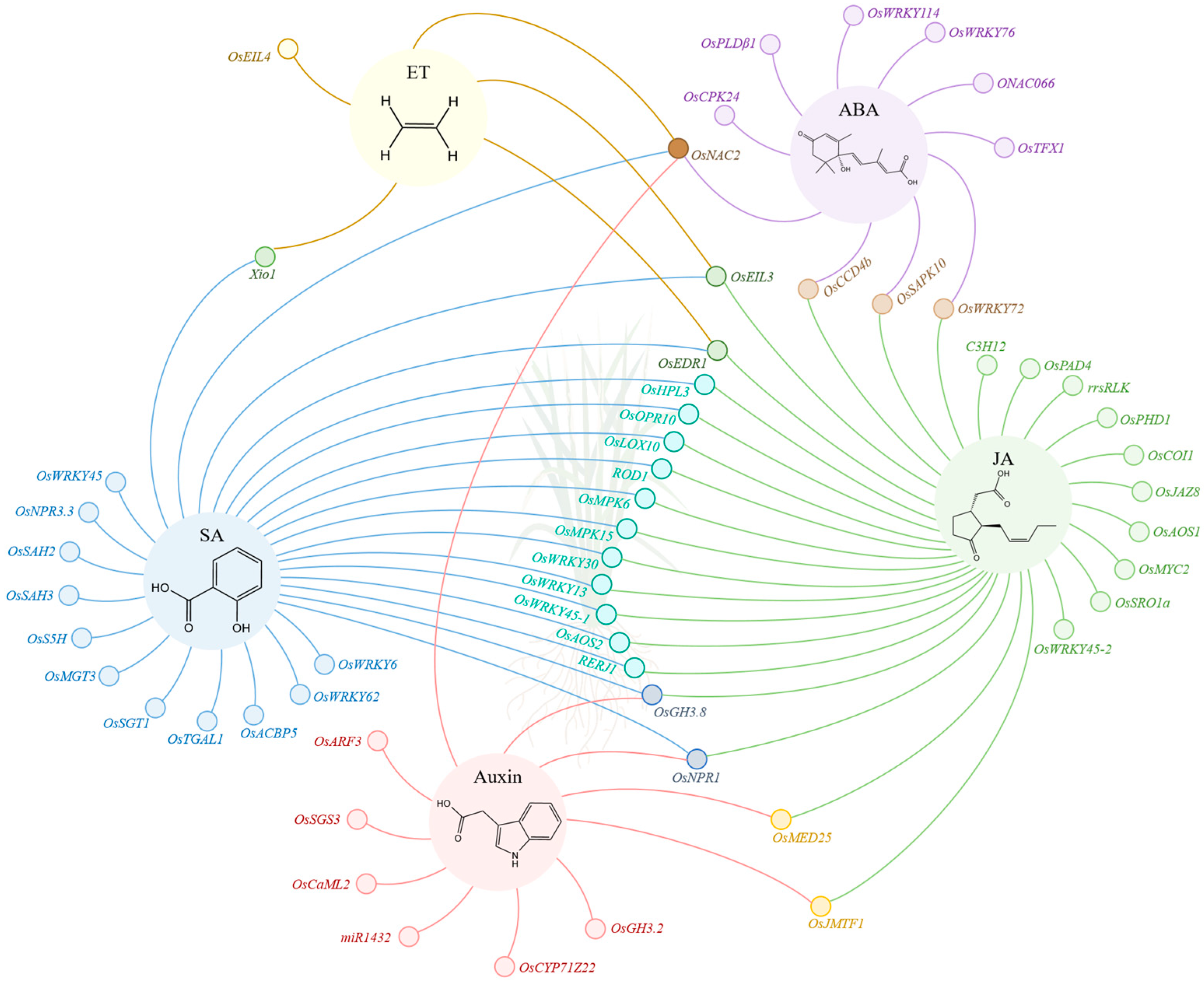
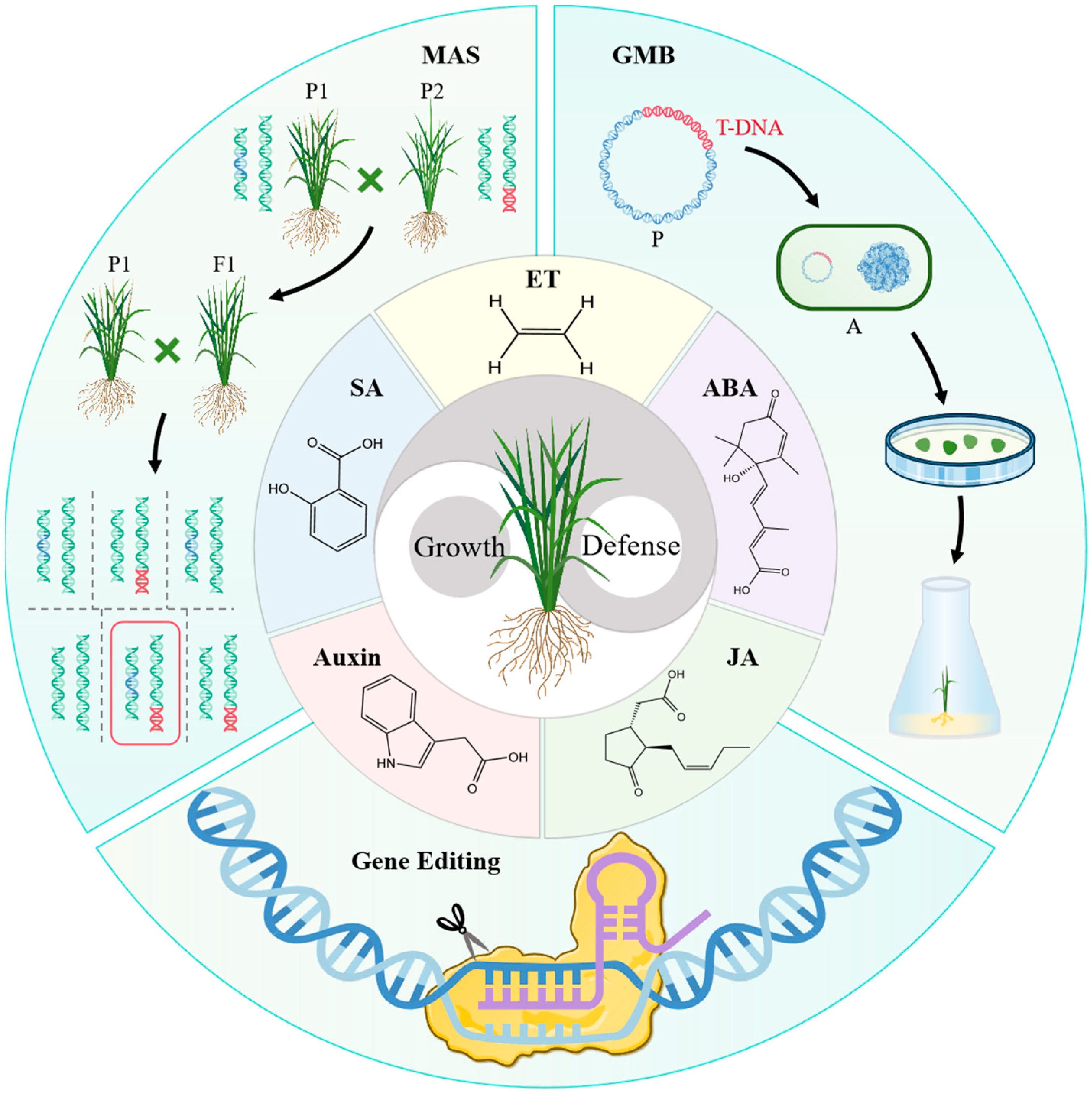
| Gene | Gene Product | Phytohormone | Function Characteristic | Regulation Mode | Reference |
|---|---|---|---|---|---|
| OsNPR1 | Non-expresser of pathogenesis-related 1 protein | SA, JA, Auxin | Binds to SA, then interacts with related TGA TF | Positive | [24,27] |
| OsNPR3.3 | Non-expresser of pathogenesis-related 3.3 protein | SA | Participates in the xa5-mediated immune response, and interacts with TGAL11 | Positive | [28] |
| OsSAH2 | SA hydroxylase | SA | Negatively regulates the SA signaling pathway and BLB resistance | Negative | [30] |
| OsSAH3 | SA hydroxylase | SA | Negatively regulates the SA signaling pathway and BLB resistance | Negative | [30] |
| OsS5H | SA hydroxylase | SA | Negatively regulates SA level and BLB resistance | Negative | [31] |
| OsMGT3 | Chloroplast-localized Mg2+ transporter | SA | Negatively regulates SA level and BLB resistance | Negative | [32] |
| OsSGT1 | SA glucosyltransferase | SA | Activated by OsTGAL1 to negatively regulate resistance to Xoo | Negative | [34] |
| OsTGAL1 | bZIP transcription factor | SA | Negatively regulates resistance to Xoo by activating the effect of OsSGT1 on SA metabolism | Negative | [34] |
| OsACBP5 | Acyl-CoA-binding protein | SA | Resistance is generated through an SA-induced defense response | Positive | [35] |
| ROD1 | Resistance of rice to diseases 1 | SA, JA | Degrades ROS to inhibit the defense response | Negative | [36] |
| OsMPK6 | Mitogen-activated protein kinase | SA, JA | Regulates ROS level and the gene expression of JA and SA signaling pathways | Negative | [38,39] |
| OsMPK15 | Mitogen-activated protein kinase | SA, JA | Regulates ROS level and the gene expression of JA and SA signaling pathways | Negative | [40] |
| OsNAC2 | NAC transcription factor | SA, ABA, ET, Auxin | Inhibits SA signal transduction and interacts with OsEREBP1 | Negative | [43,44,45,46] |
| OsWRKY45-1 | WRKY transcription factor | SA, JA | Regulates SA and JA levels and the expression of defense-related genes | Negative | [4] |
| OsWRKY45 | WRKY transcription factor | SA | Plays a positive role in BTH-induced BLB resistance and confers extremely strong resistance to BLB when overexpressed | Positive | [49] |
| OsWRKY62 | WRKY transcription factor | SA | Negatively regulates xa21-mediated resistance to BLB | Negative | [50,51] |
| OsWRKY6 | WRKY transcription factor | SA | Activates putative SA synthase OsICS1 to activate the SA signaling pathway | Positive | [52] |
| OsWRKY13 | WRKY transcription factor | SA, JA | Activate SA signaling pathway and inhibits JA synthesis | Positive | [53] |
| Gene | Gene Product | Phytohormone | Function Characteristic | Regulation Mode | Reference |
|---|---|---|---|---|---|
| Xio1 | Oryza-specific orphan protein | ET, SA | Produces ROS and H2O2 to participate in the defense response | Positive | [94] |
| OsEIL4 | Ethylene-insensitive3-like4 | ET | Positively regulates the expression of ET synthesis-related genes and defense-related genes | Positive | [96] |
| OsEDR1 | Mitogen-activated protein kinase | ET, SA, JA | Induces ACC and ACS to promote ET accumulation and inhibit SA and JA levels | Negative | [97] |
| Gene | Gene Product | Phytohormone | Function Characteristic | Regulation Mode | Reference |
|---|---|---|---|---|---|
| OsGH3.8 | IAA-amido synthetase | Auxin, SA, JA | Prevents the accumulation of IAA and inhibits the expression of expansins | Positive | [99] |
| OsGH3.2 | IAA-amido synthetase | Auxin | Prevents the accumulation of IAA and inhibits the expression of expansins | Positive | [15] |
| OsCYP71Z22 | Cytochrome P450 | Auxin | Prevents the accumulation of IAA and inhibits the expression of expansins | Positive | [105,106,107] |
| miR1432 | microRNA | Auxin | Disease resistance is mediated by targeting the negative regulatory gene OsCaML2 | Positive | [112,113] |
| OsCaML2 | Calmodulin-like protein 2 | Auxin | Co-mediates resistance as a target of miR1432 | Negative | [113] |
| OsSGS3 | Suppressor of gene silencing3 | Auxin | Regulates resistance through the OsSGS3-tasiRNA- OsARF3 module | Negative | [114] |
| OsARF3 | Auxin response factors | Auxin | Regulates resistance through the OsSGS3-tasiRNA- OsARF3 module | Positive | [114] |
| Gene | Gene Product | Phytohormone | Function Characteristic | Regulation Mode | Reference |
|---|---|---|---|---|---|
| OsPLDβ1 | Phospholipase D | ABA | Regulates ROS levels and the expression of defense-related genes | Negative | [122] |
| OsCPK24 | Calcium-dependent protein kinase 24 | ABA | Ethylicin activates OsCPK24 by influencing the ABA signaling pathway, thereby regulating rice resistance to BLB | Positive | [124] |
| OsWRKY114 | WRKY transcription factor | ABA | Promotes the SA signaling pathway and downregulates the expression of ABA-related genes | Positive | [126] |
| OsWRKY76 | WRKY transcription factor | ABA | Regulates defense-related genes and phytoalexin levels to regulate resistance | Negative | [50,127,128] |
| ONAC066 | NAC transcription factor | ABA | Exerts its functions in disease resistance by modulating the ABA signaling pathway, sugar, and amino acid accumulations in rice | Positive | [129] |
| OsTFX1 | bZIP transcription factor | ABA | The induction of OsTFX1 depends on the type III effector gene pthXo6 | Negative | [130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Xu, Y.; Rao, Y. Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones. Plants 2024, 13, 2541. https://doi.org/10.3390/plants13182541
Zhong Q, Xu Y, Rao Y. Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones. Plants. 2024; 13(18):2541. https://doi.org/10.3390/plants13182541
Chicago/Turabian StyleZhong, Qianqian, Yuqing Xu, and Yuchun Rao. 2024. "Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones" Plants 13, no. 18: 2541. https://doi.org/10.3390/plants13182541
APA StyleZhong, Q., Xu, Y., & Rao, Y. (2024). Mechanism of Rice Resistance to Bacterial Leaf Blight via Phytohormones. Plants, 13(18), 2541. https://doi.org/10.3390/plants13182541





