Challenges of Salinity Intrusion and Drought Stress on Olive Tree Cultivation on Mljet Island
Abstract
1. Introduction
2. Results
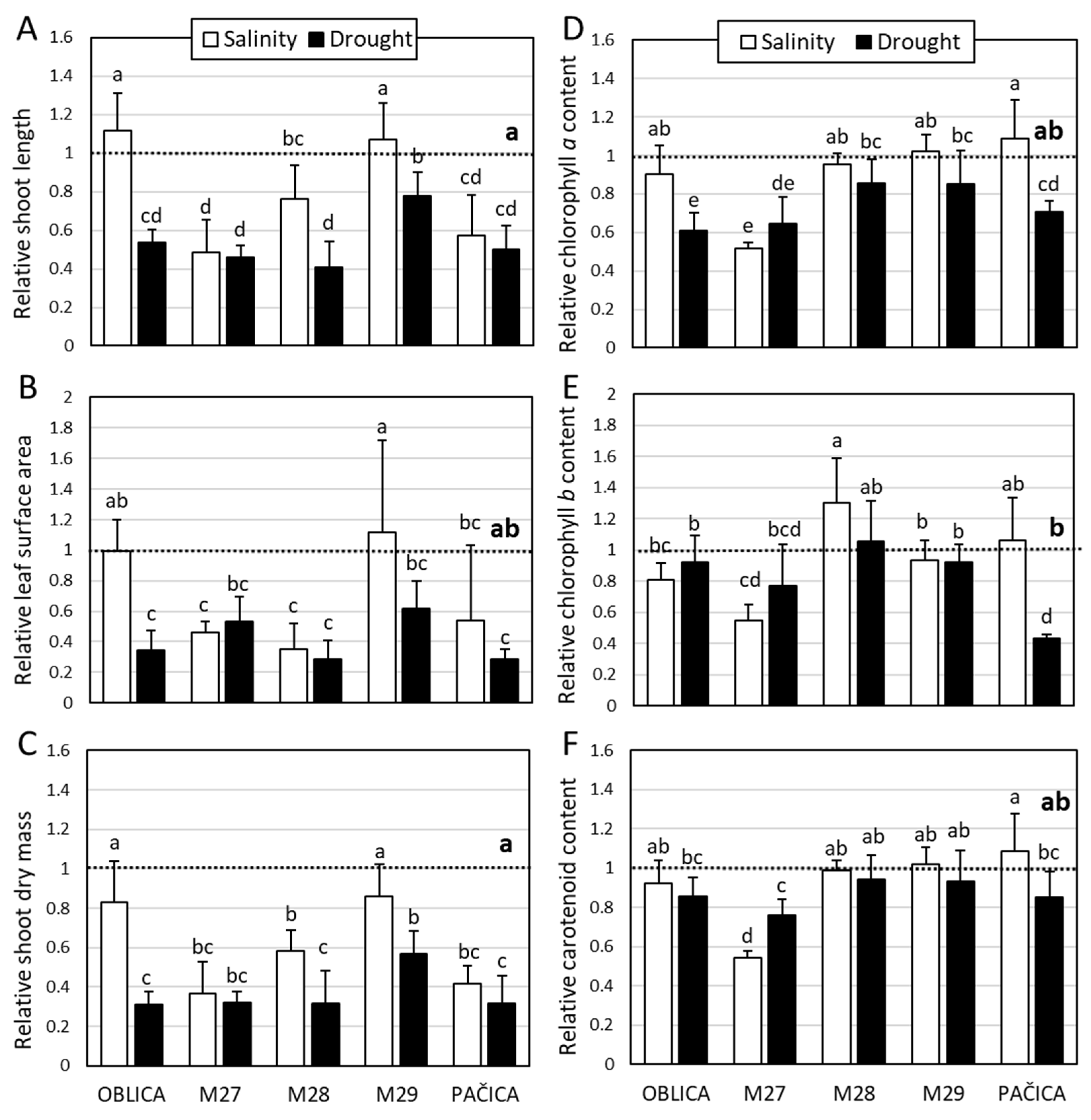
2.1. Influence of Salinity and Drought on Growth and Photosynthetic Pigments
2.2. Influence of Salinity and Drought on Leaf and Root Levels of Na+ and Cl−
2.3. Influence of Salinity and Drought on Leaf and Root Levels of K+, Mg2+ and Ca2+
2.4. Influence of Salinity and Drought on Biochemical Indicators
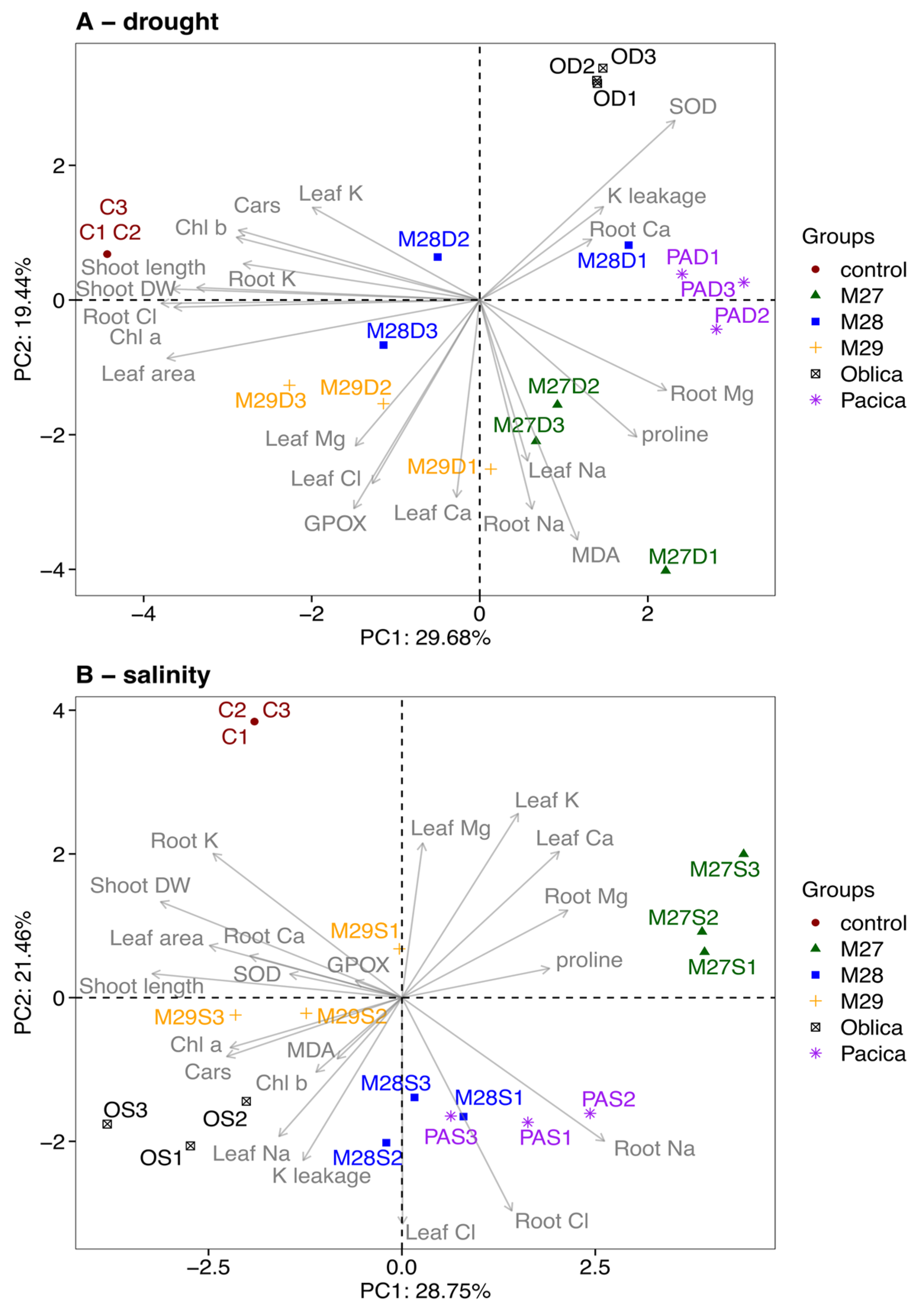
2.5. Evaluation of the Tolerance of Olive Genotypes to Salinity and Drought Using PCA
3. Discussion
3.1. Influence of Salinity and Drought on Growth and Photosynthetic Pigments
3.2. Influence of Salinity and Drought on Leaf and Root Levels of Na+ and Cl−
3.3. Influence of Salinity and Drought on Leaf and Root Levels of K+, Mg2+ and Ca2+
3.4. Influence of Salinity and Drought on Biochemical Indicators
4. Materials and Methods
4.1. Acquisition of Plant Material
4.2. Plant Cultivation
4.3. Experimental Design and Treatment Application
4.4. Morphometric Measurements and Ionic Composition Analysis
4.5. Biochemical Parameter Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nodilo, M. Prirodna baština otoka Mljeta—Temelj razvoja zdravstvenog turizma. Šumarski List 2012, 136, 377–384. Available online: https://hrcak.srce.hr/86974 (accessed on 12 April 2024).
- Nodilo, M. Vrt u Benediktinskom samostanu Sv. Marije na Mljetu. Šumarski List 2011, 135, 153–159. Available online: https://hrcak.srce.hr/67625 (accessed on 12 April 2024).
- Mljet Tourist Board. Island of Mljet: Nature and Tourism. Available online: https://www.mljet.hr (accessed on 12 April 2024).
- Gould, I.; De Waegemaeker, J.; Tzemi, D.; Wright, I.; Pearson, S.; Ruto, E.; Karrasch, L.; Christensen, L.S.; Aronsson, H.; Eich-Greatorex, S. Salinization threats to agriculture across the North Sea Region. In Future of Sustainable Agriculture in Saline Environments; Negacz, K., Vellinga, P., Barrett-Lennard, E., Choukr-Allah, R., Elzenga, T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 71–92. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Jacob, B. Agriculture and water quality interactions: A global overview. In SOLAW Background Thematic Report-TR08; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011; Available online: http://www.fao.org/3/a-bl092e.pdf (accessed on 10 April 2024).
- de Louw, P.G.B.; Eeman, S.; Siemon, B.; Voortman, B.R.; Gunnink, J.; van Baaren, E.S.; Oude Essink, G.H.P. Shallow rainwater lenses in deltaic areas with saline seepage. Hydrol. Earth Syst. Sci. 2011, 15, 3659–3678. [Google Scholar] [CrossRef]
- Metternicht, G. Soils: Salinization. In International Encyclopedia of Geography: People, the Earth, Environment and Technology; Richardson, D., Beach, T., Bendix, J., Dunford, M., Gao, S., Grove, K., Kalra, Y., Kim, J., Kobayashi, A., Kwan, M.-P., et al., Eds.; American Cancer Society: New York, NY, USA, 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Harper, R.J.; Dell, B.; Ruprecht, J.K.; Sochacki, S.J.; Smettem, K.R.J. Chapter 7—Salinity and the reclamation of salinized lands. In Soils and Landscape Restoration; Stanturf, J.A., Callaham, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 193–208. [Google Scholar] [CrossRef]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Change 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Hoerling, M.; Eischeid, J.; Perlwitz, J.; Quan, X.; Zhang, T.; Pegion, P. On the increased frequency of Mediterranean drought. J. Clim. 2012, 25, 2146–2161. [Google Scholar] [CrossRef]
- Gouveia, C.M.; Trigo, R.M.; Beguería, S.; Vicente-Serrano, S.M. Drought impacts on vegetation activity in the Mediterranean region: An assessment using remote sensing data and multi-scale drought indicators. Glob. Planet. Change 2017, 151, 15–27. [Google Scholar] [CrossRef]
- Strikić, F.; Svalina, T.; Šuste, M.; Gugić, J.; Tadić, J.; Storić, Z. Uzgoj maslina u zaleđu Vodica. Pomol. Croat. 2019, 23, 49–58. [Google Scholar] [CrossRef]
- Gambella, F.; Bianchini, L.; Cecchini, M.; Egidi, G.; Ferrara, A.; Salvati, L.; Colantoni, A.; Morea, D. Moving toward the north? The spatial shift of olive groves in Italy. Agric. Econ. 2021, 67, 129–135. [Google Scholar] [CrossRef]
- Tattini, M.; Traversi, M.L. On the mechanism of salt tolerance in olive (Olea europaea L.) under low- or high-Ca2+ supply. Environ. Exp. Bot. 2009, 65, 72–81. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Chartzoulakis, K.S. Salinity and olive: Growth, salt tolerance, photosynthesis and yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Goreta, S.; Bučević-Popović, V.; Pavela-Vrančić, M.; Perica, S. Salinity induced changes in growth, superoxide dismutase activity, and ion content of two olive cultivars. J. Plant Nutr. Soil Sci. 2007, 170, 398–403. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Perica, S.; Goreta, S.; Selak, G.V. Growth, biomass allocation and leaf ion concentration of seven olive (Olea europaea L.) cultivars under increased salinity. Sci. Hortic. 2008, 117, 123–129. [Google Scholar] [CrossRef]
- Melgar, J.C.; Guidi, L.; Remorini, D.; Agati, G.; Degl’innocenti, E.; Castelli, S.; Baratto, M.C.; Faraloni, C.; Tattini, M. Antioxidant defences and oxidative damage in salt-treated olive plants under contrasting sunlight irradiance. Tree Physiol. 2009, 29, 1187–1198. [Google Scholar] [CrossRef]
- Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Radić Brkanac, S. Physiological and biochemical response of wild olive (Olea europaea subsp. europaea var. sylvestris) to salinity. Front. Plant Sci. 2021, 12, 712005. [Google Scholar] [CrossRef]
- Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Liber, Z.; Radić Brkanac, S. Comparative analysis of cultivated and wild olive genotypes to salinity and drought stress. Front. Plant Sci. 2024, 15, 1423761. [Google Scholar] [CrossRef]
- Klepo, T.; Benčić, Đ.; Liber, Z.; Belaj, A.; Strikić, F.; Kević, N.; Šatović, Z. Revealing the diversity and complex relationships of Croatian olive germplasm. Int. J. Mol. Sci. 2024, 25, 3170. [Google Scholar] [CrossRef]
- Tadić, J. Resistance of Wild and Cultivated Olive Genotypes to Increased Salinity and Drought. Ph.D. Thesis, University of Osijek, Osijek, Croatia, 4 November 2022. [Google Scholar]
- Mousavi, S.; Mariotti, R.; Valeri, M.C.; Regni, L.; Lilli, E.; Albertini, E.; Proietti, P.; Businelli, D.; Baldoni, L. Characterization of differentially expressed genes under salt stress in olive. Int. J. Mol. Sci. 2022, 23, 154. [Google Scholar] [CrossRef]
- Testi, L.; Orgaz, F.; Villalobos, F.J. Variations in bulk canopy conductance of an irrigated olive (Olea europaea L.) orchard. Environ. Exp. Bot. 2006, 55, 15–28. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Coppa, E.; Brunori, E.; Cristofori, V.; Rugini, E.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Shah, M.K.N.; et al. Response of olive shoots to salinity stress suggests the involvement of sulfur metabolism. Plants 2021, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Gucci, R.; Coradeschi, M.A.; Ponzio, C.; Everarard, J.D. Growth, gas exchange and ion content in Olea europaea plants during salinity and subsequent relief. Physiol. Plant. 1995, 95, 203–210. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, M.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Ben Youssef, N.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef]
- Ayaz, M.; Varol, N.; Yolcu, S.; Pelvan, A.; Kaya, Ü.; Aydoğdu, E.; Bor, M.; Özdemir, F.; Türkan, I. Three (Turkish) olive cultivars display contrasting salt stress-coping mechanisms under high salinity. Trees-Struct. Funct. 2021, 35, 1283–1298. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants: Cell to cell and long distance signaling. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trabelsi, L.; Gargouri, K.; Ben Hassena, A.; Mbadra, C.; Ghrab, M.; Ncube, B.; Van Staden, J.; Gargouri, R. Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agric. Water Manag. 2019, 213, 749–759. [Google Scholar] [CrossRef]
- Melgar, J.; Benlloch, M.; Fernández-Escobar, R. Calcium increases sodium exclusion in olive plants. Sci. Hortic. 2006, 109, 303–305. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.Y.; Sebastiani, L. Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and Salinity in Citriculture: Optimal Practices to Alleviate Salinity and Water Stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’amato, R.; Proietti, P. Behavior of four olive cultivars during salt stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Abdullah, F.B. Saline water irrigation effects on antioxidant defense system and proline accumulation in leaves and roots of field-grown olive. J. Agric. Food Chem. 2009, 57, 11484–11490. [Google Scholar] [CrossRef] [PubMed]
- Vlašić, A.; Strunje, B. Morfološki, Citološki i Fiziološki Sterilitet Sorta Masline; Institut za Jadranske Kulture i Melioraciju Krša, Split, Odjel za Voćarstvo i Maslinarstvo; NITP Slobodna Dalmacija: Split, Croatia, 1980. [Google Scholar]
- Zec, J. Sortiment masline u Dalmaciji. Biljn. Proizv. 1951, 1, 3–20. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 39. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidase. Meth. Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Radić, S.; Cvjetko, P.; Glavaš, K.; Roje, V.; Pevalek-Kozlina, B.; Pavlica, M. Oxidative stress and DNA damage in broad bean (Vicia faba L.) seedlings induced by thallium. Environ. Toxicol. Chem. 2009, 28, 189–196. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]


| Genotype | Treatment | Mg2+ (mg/L) | Ca2+ (mg/L) | K+ (mg/L) | K+ Leakage (mg/L) |
|---|---|---|---|---|---|
| Oblica | control * | 1.00 ± 0.038 a | 1.00 ± 0.063 a | 1.00 ± 0.189 a | 1.00 ± 0.171 bc |
| salinity | 0.73 ± 0.081 bc | 0.36 ± 0.016 b | 0.87 ± 0.038 bc | 2.90 ± 0.674 a | |
| drought | 0.55 0.009 d | 0.27 ± 0.011 b | 1.01 ± 0.059 a | 1.72 ± 0.020 b | |
| control | 1.00 ± 0.191 a | 1.00 ± 0.059 a | 1.00 ± 0.045 a | 1.00 ± 0.172 bc | |
| M27 | salinity | 0.80 ± 0.016 ab | 1.11 ± 0.397 a | 0.98 ± 0.026 a | 1.07 ± 0.393 bc |
| drought | 0.94 ± 0.025 a | 1.20 ± 0.167 a | 0.88 ± 0.023 abc | 0.97 ± 0.225 bc | |
| control | 1.00 ±0.448 a | 1.00 ± 0.119 a | 1.00 ± 0.024 a | 1.00 ± 0.218 bc | |
| M28 | salinity | 0.70 ±0.035 cd | 0.82 ± 0.198 ab | 0.95 ± 0.019 ab | 1.60 ± 0.613 b |
| drought | 0.97 ± 0.214 a | 1.14 ± 0.198 a | 1.01 ± 0.213 a | 0.60 ± 0.302 c | |
| control | 1.00 ± 0.291 a | 1.00 ± 0.100 a | 1.00 ± 0.047 a | 1.00 ± 0.155 bc | |
| M29 | salinity | 0.73 ± 0.027 bc | 0.83 ± 0.164 ab | 0.90 ± 0.012 ab | 1.83 ± 0.648 b |
| drought | 0.79 ± 0.030 ab | 1.09 ± 0.092 a | 0.88 ± 0.017 abc | 1.39 ± 0.288 bc | |
| Control | 1.00 ± 0.397 a | 1.00 ± 0.150 a | 1.00 ± 0.172 a | 1.00 ± 0. 581 bc | |
| Pačica | Salinity | 0.93 ± 0.041 a | 0.85 ± 0.071 ab | 0.93 ± 0.022 ab | 2.08 ± 0.652 ab |
| Drought | 0.94 ± 0.020 a | 1.31 ± 0.338 a | 0.79 ± 0.006 c | 1.60 ± 0.443 b |
| Genotype | Treatment | Mg2+ (mg/L) | Ca2+ (mg/L) | K+ (mg/L) |
|---|---|---|---|---|
| Oblica | control * | 1.00 ± 0.113 cd | 1.00 ± 0.168 d | 1.00 ± 0.011 a |
| salinity | 0.95 ± 0.162 cd | 0.89 ± 0.223 d | 0.90 ± 0.041 b | |
| drought | 0.93 ± 0.009 cd | 1.28 ± 0.089 c | 0.66 ± 0.039 e | |
| control | 1.00 ± 0.062 cd | 1.00 ± 0.048 d | 1.00 ± 0.0330 a | |
| M27 | salinity | 1.29 ± 0.055 ab | 0.56 ± 0.042 e | 0.71 ± 0.011 cde |
| drought | 1.40 ± 0.127 a | 1.01 ± 0.082 cd | 0.76 ± 0.062 c | |
| control | 1.00 ± 0.048 cd | 1.00 ± 0.049 d | 1.00 ± 0.034 a | |
| M28 | salinity | 0.95 ± 0.121 cd | 1.11 ± 0.054 cd | 0.69 ± 0.011 de |
| drought | 1.10 ± 0.066 bc | 2.25 ± 0.182 a | 0.59 ± 0.056 f | |
| control | 1.00 ± 0.049 cd | 1.00 ± 0.036 d | 1.00 ± 0.036 a | |
| M29 | salinity | 0.86 ± 0.045 cd | 0.83 ± 0.024 d | 0.76 ± 0.032 c |
| drought | 0.78 ± 0.069 d | 1.05 ± 0.077 cd | 0.51 ± 0.015 g | |
| control | 1.00 ± 0.022 cd | 1.00 ± 0.072 d | 1.00 ± 0.024 a | |
| Pačica | salinity | 0.93 ± 0.129 cd | 0.38 ± 0.032 e | 0.73 ± 0.017 cd |
| drought | 1.43 ± 0.247 a | 1.65 ± 0.362 b | 0.55 ± 0.016 fg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Radić Brkanac, S. Challenges of Salinity Intrusion and Drought Stress on Olive Tree Cultivation on Mljet Island. Plants 2024, 13, 2549. https://doi.org/10.3390/plants13182549
Tadić J, Dumičić G, Veršić Bratinčević M, Vitko S, Radić Brkanac S. Challenges of Salinity Intrusion and Drought Stress on Olive Tree Cultivation on Mljet Island. Plants. 2024; 13(18):2549. https://doi.org/10.3390/plants13182549
Chicago/Turabian StyleTadić, Josip, Gvozden Dumičić, Maja Veršić Bratinčević, Sandra Vitko, and Sandra Radić Brkanac. 2024. "Challenges of Salinity Intrusion and Drought Stress on Olive Tree Cultivation on Mljet Island" Plants 13, no. 18: 2549. https://doi.org/10.3390/plants13182549
APA StyleTadić, J., Dumičić, G., Veršić Bratinčević, M., Vitko, S., & Radić Brkanac, S. (2024). Challenges of Salinity Intrusion and Drought Stress on Olive Tree Cultivation on Mljet Island. Plants, 13(18), 2549. https://doi.org/10.3390/plants13182549






