CaZingipain2 Acts Positively in Pepper (Capsicum annuum L.) Immunity against R. solanacearum
Abstract
:1. Introduction
2. Results
2.1. CaZingipain2 Upregulation in Pepper Plants upon R. Solanacearum Inoculation under WM Conditions
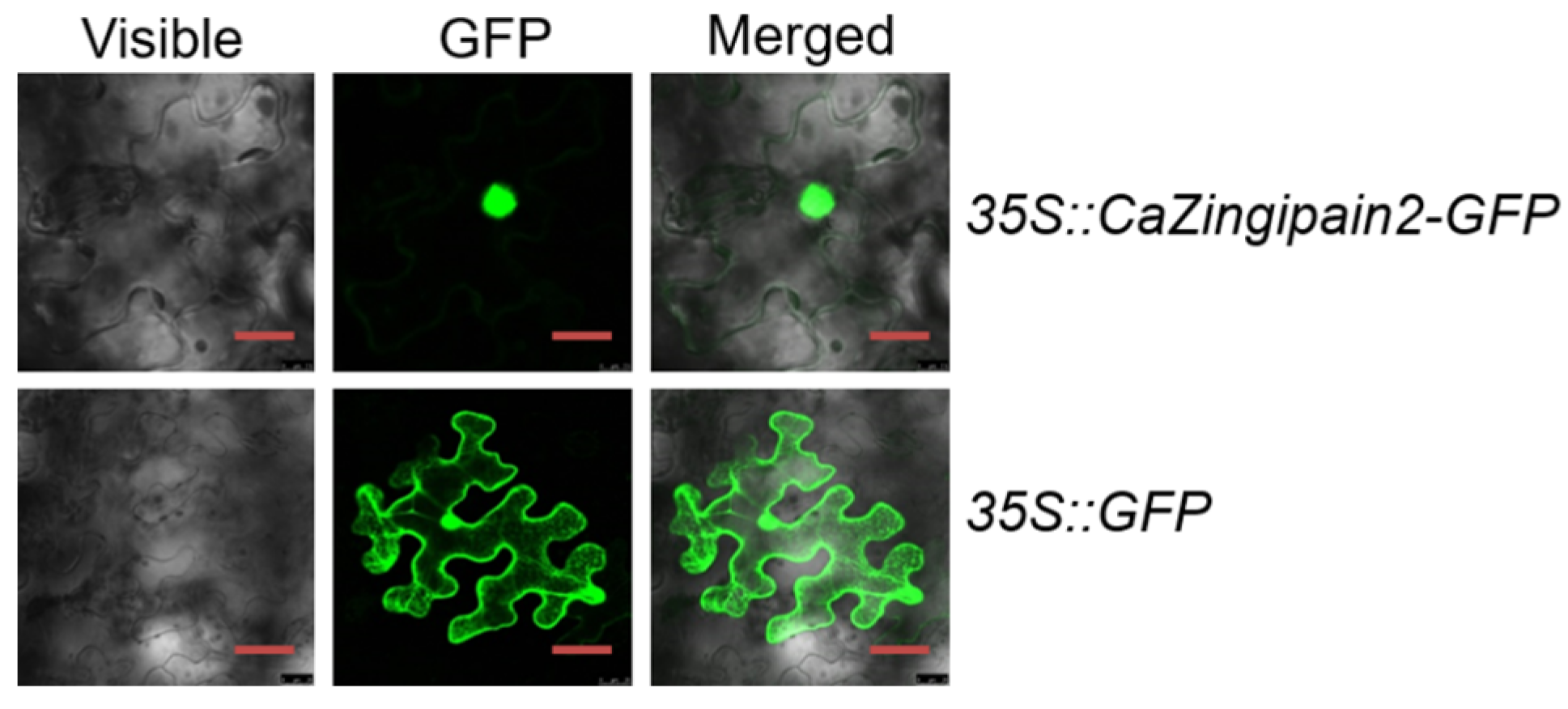
2.2. CaZingipain2 Localized to Nuclei in Epidermal Cells of Nicotiana benthamiana Leaves
2.3. CaZingipain2 Is Upregulated upon R. solanacearum Infection under RM and WM Conditions
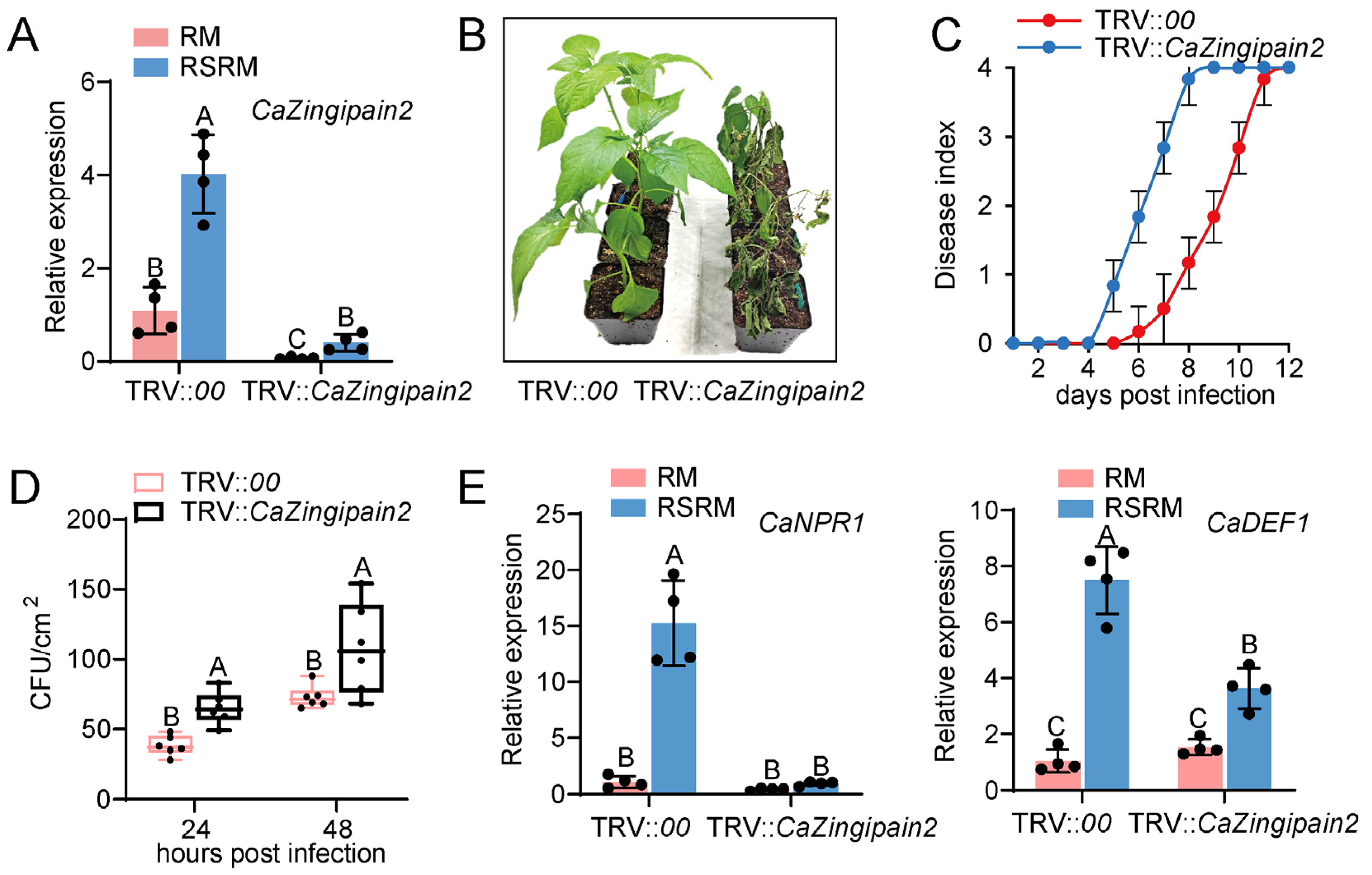
2.4. Silencing of CaZingipain2 Enhances Susceptibility of Pepper to R. solanacearum
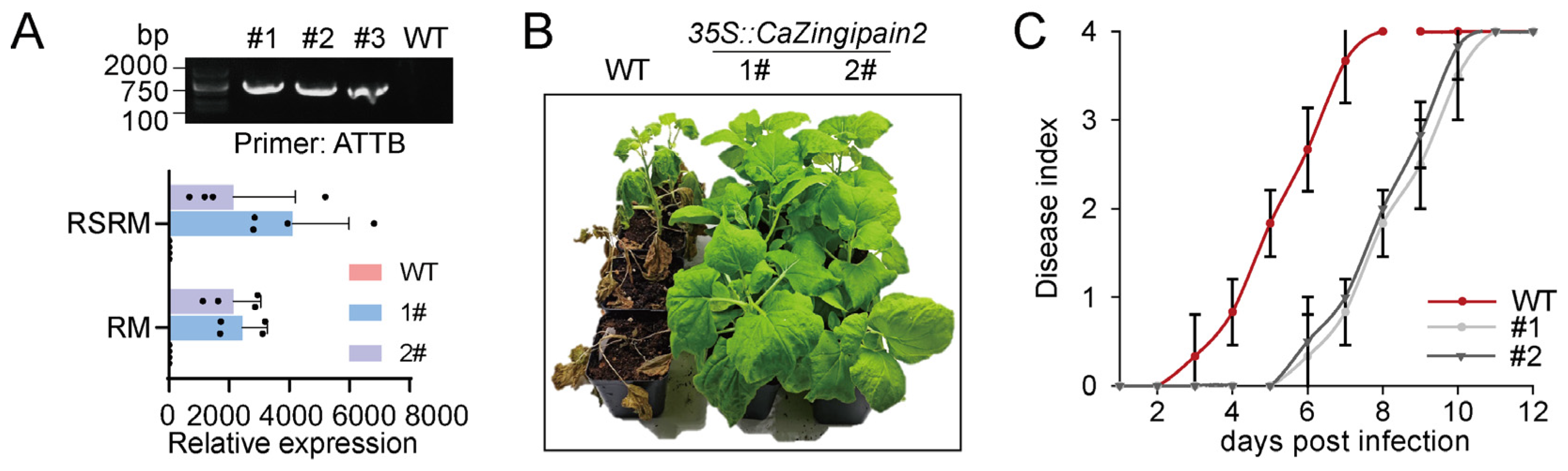
2.5. Overexpression of CaZingipain2 Promoted Nicotiana Benthamiana Immunity against R. solanacearum under RM and WM Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Sequence Analysis and Primer Design
4.3. Vector Construction
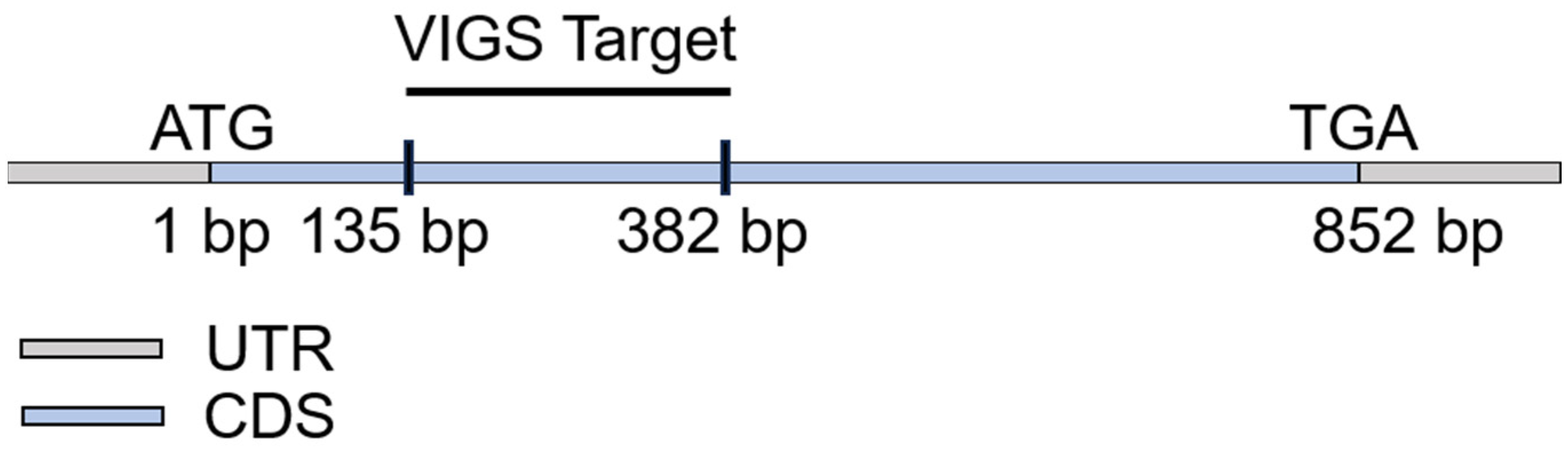
4.4. Virus−Induced Gene Silencing (VIGS) Assay
4.5. Subcellular Localization
4.6. Genetic Transformation of Nicotiana Benthamiana
4.7. Total RNA Extraction and Reverse Transcription
4.8. Fluorescent Quantitative PCR
4.9. Colony−Forming Unit (CFU) Determination
4.10. Disease Index
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Castroverde, C.D.M. Diversity, Function and Regulation of Cell Surface and Intracellular Immune Receptors in Solanaceae. Plants 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Chernin, L.; Höfte, M.M. Differential effectiveness of IC1270-induced systemic resistance against hemibiotrophic and necrotrophic leaf pathogens in rice. BMC Plant Biol. 2009, 9, 9. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Iswanto, A.B.B.; Son, G.H.; Kim, S.H. Recent Advances in Effector-Triggered Immunity in Plants: New Pieces in the Puzzle Create a Different Paradigm. Int. J. Mol Sci. 2021, 22, 4709. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chen, H.; Liu, F.; Fu, Z. PTI and ETI Convergent pathways with diverse elicitors. Trends Plant Sci. 2021, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Liu, H.J.; Hu, M.H.; Wang, Q.; Cheng, L.; Zhang, Z.B. Role of Papain-Like Cysteine proteases in plant development. Front. Plant Sci. 2018, 9, 1717. [Google Scholar] [CrossRef]
- Kedzior, M.; Seredynski, R.; Gutowicz, J. Microbial inhibitors of cysteine proteases. Med. Microbiol. Immun. 2016, 205, 275–296. [Google Scholar] [CrossRef]
- Misas-Villamil, J.C.; van der Hoorn, R.A.; Doehlemann, G. Papain-like cysteine proteases as hubs in plant immunity. New Phytol. 2016, 212, 902–907. [Google Scholar] [CrossRef]
- Shindo, T.; Misas-Villamil, J.C.; Horger, A.C.; Song, J.; van der Hoorn, R.A. A role in immunity for Arabidopsis cysteine protease RD21, the ortholog of the tomato immune protease C14. PLoS ONE 2012, 7, e29317. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, Q.; Chen, C.; Xu, T.; Jiang, Y.; Hu, W.; Liao, A.; Zhang, J.; Le, X.; Li, H.; et al. A root-knot nematode effector targets the Arabidopsis cysteine protease RD21A for degradation to suppress plant defense and promote parasitism. Plant J. 2024, 118, 1500–1515. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; He, B.; Hu, J.; Dai, Y.; Chen, W.; Yi, M.; Zhang, H.; Ye, Y.; Cui, Z.; et al. Targeting Magnaporthe oryzae effector MoErs1 and host papain-like protease OsRD21 interaction to combat rice blast. Nat. Plants 2024, 10, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Sayanta, B.; Gabriella, D.A.; Swayamjit, R.; Sydney, F.; Clare, L.C. The Potyviral Protein 6K1 Reduces Plant Proteases Activity during Turnip mosaic virus Infection. Viruses 2022, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, S.; van der Linde, K.; Lahrmann, U.; Acar, B.; Kaschani, F.; Colby, T.; Kaiser, M.; Ding, Y.Z.; Schmelz, E.; Huffaker, A.; et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat. Plants 2018, 4, 172–180. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, F.W.; Cheng, K.T.; Chang, C.H.; Hung, S.C.; Efferth, T.; Chen, Y.R. XCP1 cleaves Pathogenesis-related protein 1 into CAPE9 for systemic immunity in. Nat. Commun. 2023, 14, 4697. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ziv, A.; Levy, Y.; Citovsky, V.; Gafni, Y. The Tomato yellow leaf curl virus (TYLCV) V2 protein inhibits enzymatic activity of the host papain-like cysteine protease CYP1. Biochem. Bioph. Res. Commun. 2015, 460, 525–529. [Google Scholar] [CrossRef]
- Grudkowska, M.; Zagdanska, B. Multifunctional role of plant cysteine proteinases. Acta Biochim. Pol. 2004, 51, 609–624. [Google Scholar] [CrossRef]
- Hua, T.; Robitaille, M.; Roberts-Thomson, S.J.; Monteith, G.R. The intersection between cysteine proteases, Ca2+ signalling and cancer cell apoptosis. Biochim. Biophys Acta Mol. Cell Res. 2023, 1870, 119532. [Google Scholar] [CrossRef]
- Van der Hoorn, R.A.L. Plant proteases: From phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Hatsugai, N. The role of vacuole in plant cell death. Cell Death Differ. 2011, 18, 1298–1304. [Google Scholar] [CrossRef]
- Karnchanatat, A.; Tiengburanatam, N.; Boonmee, A. Zingipain, A cysteine protease from Zingiber ottensii Valeton rhizomes with antiproliferative activities against fungi and human malignant cell lines. Prep. Biochem. Biotech. 2011, 41, 138–153. [Google Scholar] [CrossRef]
- Rungsaeng, P.; Sangvanich, P.; Karnchanatat, A. Zingipain, a ginger protease with acetylcholinesterase inhibitory activity. Appl. Biochem. Biotech. 2013, 170, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Yusuke, S.; Eliza Po-Iian, L. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2019, 225, 87–104. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X. Life-or-death decisions in plant immunity. Curr. Opin. Immunol. 2022, 75, 102169. [Google Scholar] [CrossRef] [PubMed]
- Friederike, B.; Jaqueline, B.; Gesa, H.; Ipek, Y.; Jürgen, Z.; Jane, E.P. Natural variation in temperature-modulated immunity uncovers transcription factor bHLH059 as a thermoresponsive regulator in Arabidopsis thaliana. PLoS Genet. 2021, 17, 1009290. [Google Scholar] [CrossRef]
- Charles, R.-L.; Christina, A.M.R.; Christian Danve Marco, C.; Peter, M. The plant disease triangle facing climate change A molecular perspective. Trends Plant Sci. 2024, 29, 895–914. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, J.; Shang, H.; Chen, X.; Hu, X. The RLK protein TaCRK10 activates wheat high-temperature seedling-plant resistance to stripe rust through interacting with TaH2A.1. Plant J. For. Cell Mol. Biol. 2021, 108, 1241–1255. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Paek, K. Temperature-specific vsiRNA confers RNAi-mediated viral resistance at elevated temperature in Capsicum annuum. J. Exp. Bot. 2021, 72, 1432–1448. [Google Scholar] [CrossRef]
- Lijun, J.; Jian, L.; Jianlong, Z.; Yu, Y.; Yuhong, Y.; Yan, L.; Yang, J.; Zhenchuan, M.; Yunsheng, W.; Bingyan, X. Chromosome-scale genome assembly-assisted identification of Mi-9 gene in Solanum arcanum accession LA2157, conferring heat-stable resistance to Meloidogyne incognita. Plant Biotechnol. J. 2023, 21, 1496–1509. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.; Ding, A.; Wang, W.; Sun, Y. Ralstonia solanacearumInduced defense strategies of plants against. Front. Microbiol. 2023, 14, 1059799. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, W.; Cheng, S.; Zhang, H.; Zong, J.; Zhang, Z. Ralstonia solanacearum—A soil borne hidden enemy of plants: Research development in management strategies, their action mechanism and challenges. Front. Plant Sci. 2023, 14, 1141902. [Google Scholar] [CrossRef] [PubMed]
- Lowe-Power, T.; Hendrich, C.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.; Ricca, P.; Naidoo, J.; Cook, D.; et al. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Shen, L.; Wu, R.; Cao, J.; Tang, W.; Lu, Q.; Huang, Y.; Guan, D.; He, S. Solanaceous plants switch to cytokinin-mediated immunity against Ralstonia solanacearum under high temperature and high humidity. Plant Cell Environ. 2022, 45, 459–478. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Zhang, Y.; Shi, Y.; Shen, L.; Zhang, Q.; Qiu, A.; Guan, D.; He, S. Temperature-dependent action of pepper mildew resistance locus O 1 in inducing pathogen immunity and thermotolerance. J. Exp. Bot. 2024, 75, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.F.; Wang, Y.N.; Yu, L.; Eulgem, T.; Lai, Y.; Liu, Z.Q.; Wang, X.; Qiu, A.L.; Zhang, T.X.; Lin, J.; et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013, 36, 757–774. [Google Scholar] [CrossRef]
- Cai, H.; Yang, S.; Yan, Y.; Xiao, Z.; Cheng, J.; Wu, J.; Qiu, A.; Lai, Y.; Mou, S.; Guan, D.; et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J. Exp. Bot. 2015, 66, 3163–3174. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Z.; Yang, S.; Yang, T.; Liang, J.; Wen, J.; Liu, Y.; Li, J.; Shi, L.; Tang, Q.; et al. Pepper CabZIP63 acts as a positive regulator during Ralstonia solanacearum or high temperature-high humidity challenge in a positive feedback loop with CaWRKY40. J. Exp. Bot. 2016, 67, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Huang, Y.; Wang, H.; Wan, M.; Lv, J.; Cheng, X.; Chen, Y.; Cai, W.; Yang, S.; Shen, L.; et al. CabZIP23 Integrates in CabZIP63-CaWRKY40 Cascade and Turns CabZIP63 on Mounting Pepper Immunity against Ralstonia solanacearum via Physical Interaction. Int. J. Mol. Sci. 2022, 23, 2656. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, W.; Shen, L.; Cao, J.; Liu, C.; Hu, J.; Guan, D.; He, S. A CaCDPK29-CaWRKY27b module promotes CaWRKY40-mediated thermotolerance and immunity to Ralstonia solanacearum in pepper. New Phytol. 2022, 233, 1843–1863. [Google Scholar] [CrossRef]
- Yan, W.; Yuanchao, W.; Yiming, W. Apoplastic Proteases: Powerful Weapons against Pathogen Infection in Plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef]
- Wen, L.; Peng, L.; Yizhen, D.; Zijing, Z.; Junjian, S.; Ji, H.; Minhui, L.; Pinggen, X.; Zide, J.; Guanghui, K. Litchi aspartic protease LcAP1 enhances plant resistance via suppressing cell death triggered by the pectate lyase PlPeL8 from Peronophythora litchii. New Phytol. 2024, 242, 2682–2701. [Google Scholar] [CrossRef]
- Jiorgos, K.; Mariana, S.; Fatih, D.; Oliver, M.; Sonja, K.; Parvinderdeep, S.K.; Ruby, O.G.; Samantha, R.; Ana Lucía, B.-C.; Brian, C.M.; et al. Bioengineering secreted proteases convert divergent Rcr3 orthologs and paralogs into extracellular immune co-receptors. Plant Cell 2024, 36, 3260–3276. [Google Scholar] [CrossRef]
- Jiorgos, K.; Shivani, M.; Oliver, M.; Sonja, K.; Parvinderdeep, S.K.; Judith, K.P.; Renier, A.L.V.D.H. Evolution of a guarded decoy protease and its receptor in solanaceous plants. Nat. Commun. 2020, 11, 4393. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Wu, R.; Huang, Y.; Lu, Q.; Hui, W.; Huang, X.; Zhang, Y.; Wu, Q.; Cheng, X.; et al. Differential CaKAN3-CaHSF8 associations underlie distinct immune and heat responses under high temperature and high humidity conditions. Nat. Commun. 2023, 14, 4477. [Google Scholar] [CrossRef]
- Henri, D.; Alessandro, G.; Adrien, B.; Hua, C.-W.; Yves, M.; Fabienne, V.; Fabien, M.; Samantha, V.; Richard, B.; Marta, M. Reshaping the Primary Cell Wall: Dual Effects on Plant Resistance to Ralstonia solanacearum and Heat Stress Response. Mol. Plant Microbe Interact. 2024, 37, 619–634. [Google Scholar] [CrossRef]
- Chung, E.; Seong, E.; Kim, Y.C.; Chung, E.J.; Oh, S.K.; Lee, S.; Park, J.M.; Joung, Y.H.; Choi, D. A method of high frequency virus-induced gene silencing in chili pepper. Mol. Cells 2004, 17, 377–380. [Google Scholar] [CrossRef]
- Ferdinand, R.; Artur, D.C.M.; Margit, L.D.C.M.; Herta, S.; Diethard, M.; Veronika, H.; Hans, W.; Hermann, K. Coat protein mediated resistance to Plum Pox Virus in Nicotiana clevelandii and N. benthamiana. Plant Cell Rep. 1992, 11, 30–33. [Google Scholar] [CrossRef]
- Bardonnet, N.; Hans, F.; Serghini, M.A.; Pinck, L. Protection against virus infection in tobacco plants expressing the coat protein of grapevine fanleaf nepovirus. Plant Cell Rep. 1994, 13, 357–360. [Google Scholar] [CrossRef]




| Disease Level | Symptom Description |
|---|---|
| 0 | Plant normal, no symptoms. |
| 1 | Plant is slightly wilted, 1/4 of the leaves are wilted. |
| 2 | One−half of the leaves are wilted. |
| 3 | Three−fourths of the leaves are wilted. |
| 4 | Whole plant wilts, or plant dies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Wu, Z.; Qing, Y.; Duan, C.; Guo, Y.; Zhang, X.; Huang, R.; He, S.; Qiu, A. CaZingipain2 Acts Positively in Pepper (Capsicum annuum L.) Immunity against R. solanacearum. Plants 2024, 13, 2552. https://doi.org/10.3390/plants13182552
Wu R, Wu Z, Qing Y, Duan C, Guo Y, Zhang X, Huang R, He S, Qiu A. CaZingipain2 Acts Positively in Pepper (Capsicum annuum L.) Immunity against R. solanacearum. Plants. 2024; 13(18):2552. https://doi.org/10.3390/plants13182552
Chicago/Turabian StyleWu, Ruijie, Zhen Wu, Yalin Qing, Chenfeng Duan, Yiling Guo, Xujing Zhang, Ronghua Huang, Shuilin He, and Ailian Qiu. 2024. "CaZingipain2 Acts Positively in Pepper (Capsicum annuum L.) Immunity against R. solanacearum" Plants 13, no. 18: 2552. https://doi.org/10.3390/plants13182552






