The Effects of Auxin Transport Inhibition on the Formation of Various Leaf and Vein Patterns
Abstract
:1. Introduction
2. Results
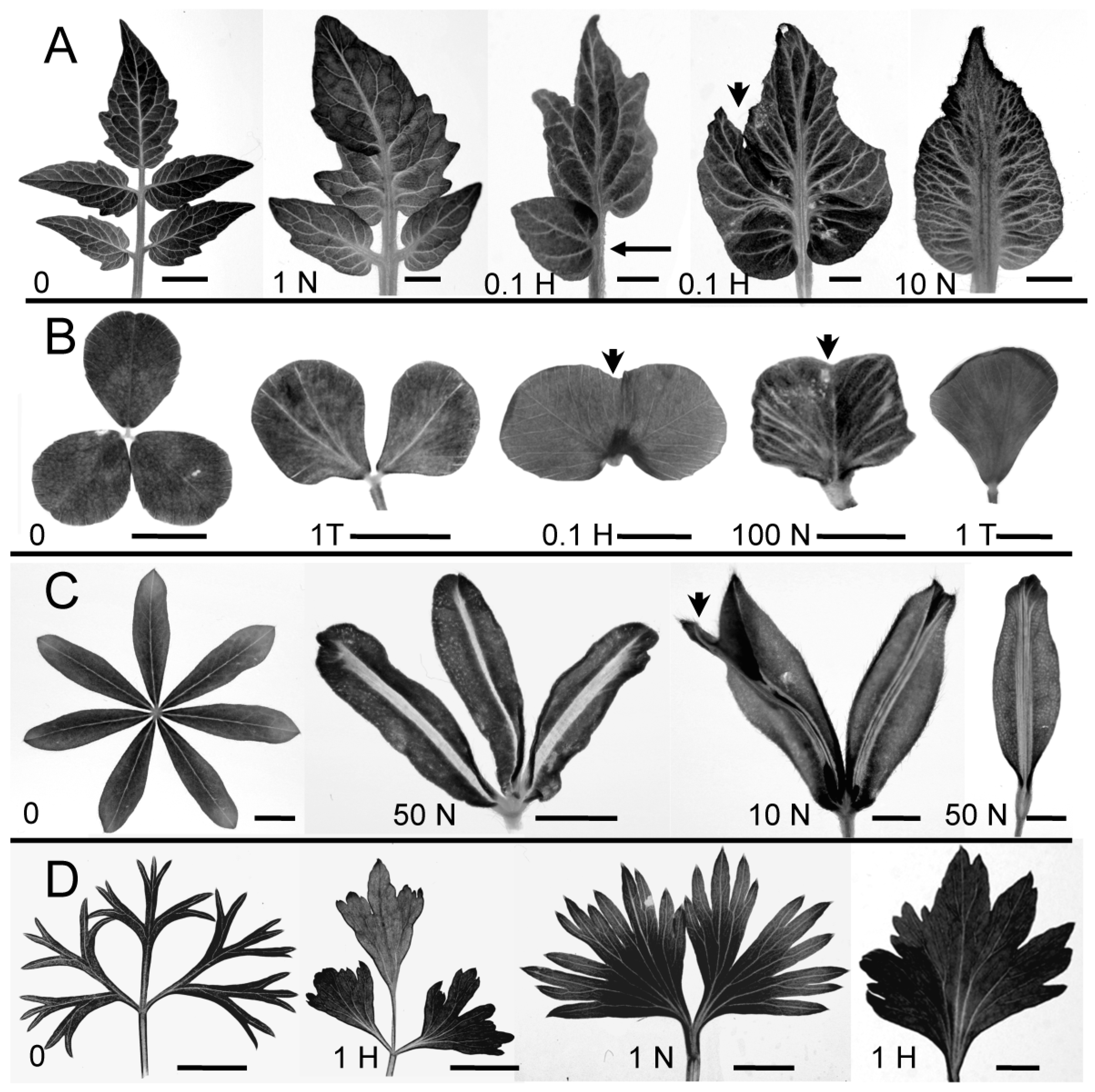
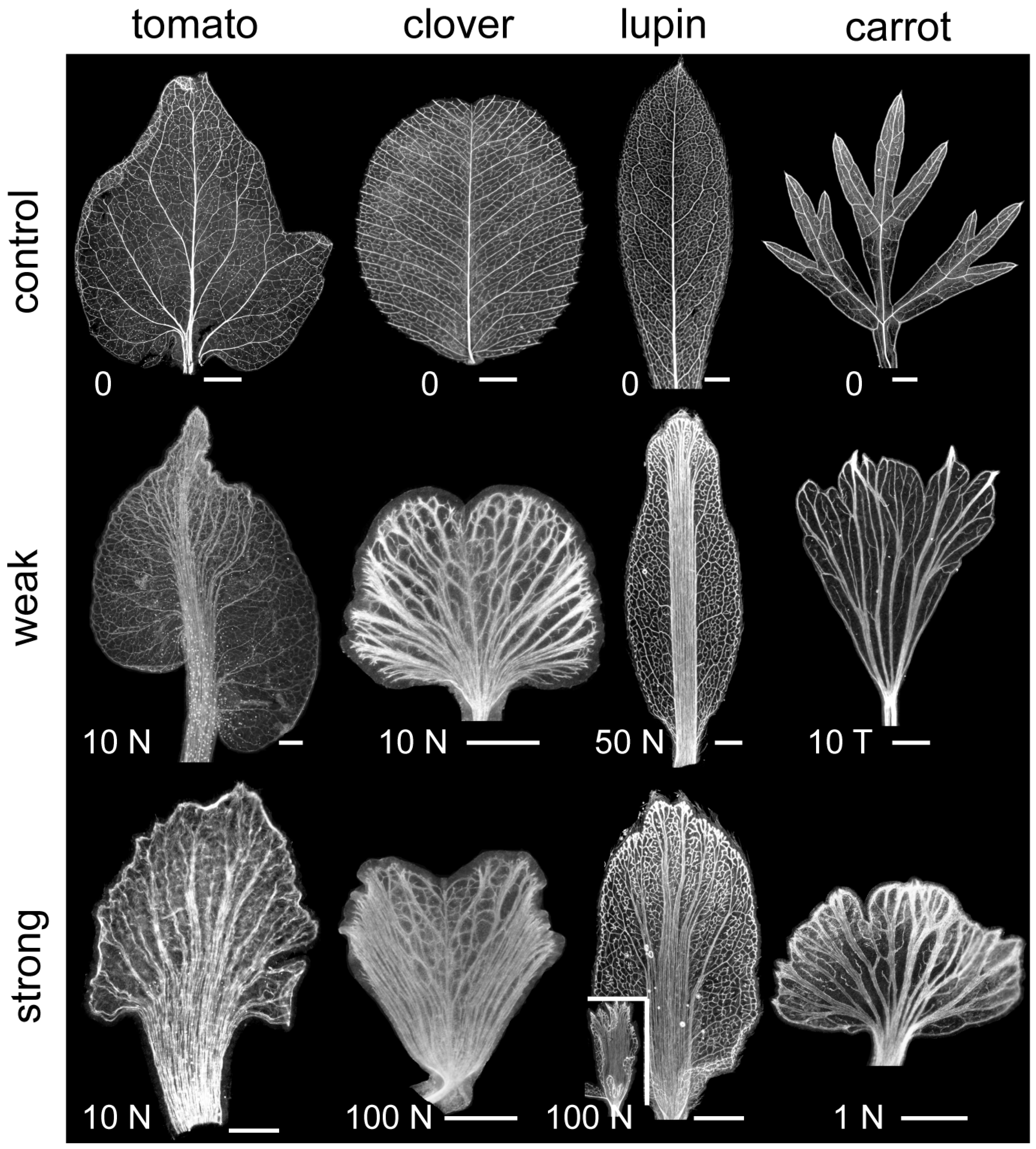
2.1. Polar Auxin Transport Is Involved in Compound Leaflet Morphogenesis in Different Leaf Complexity Types
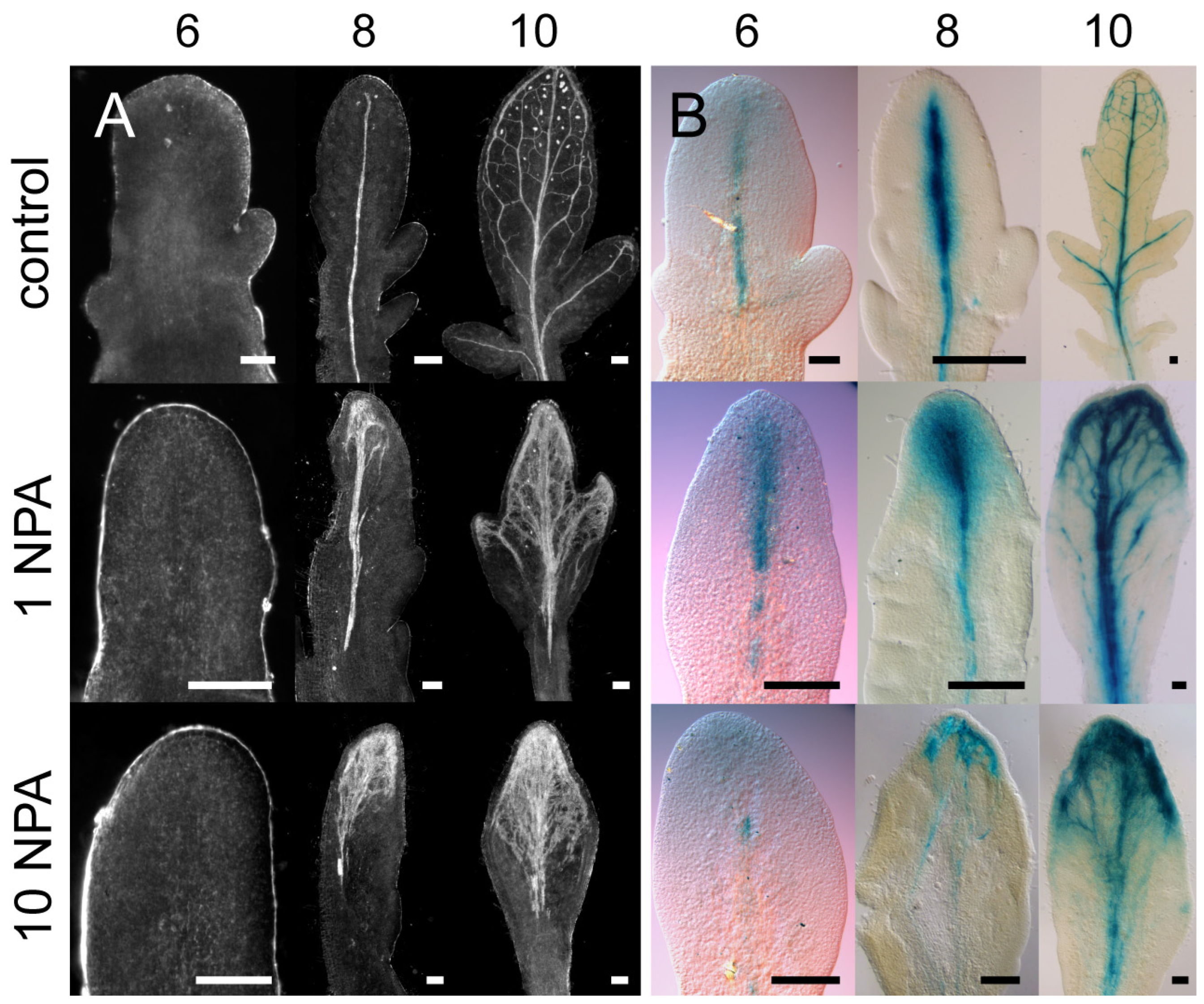
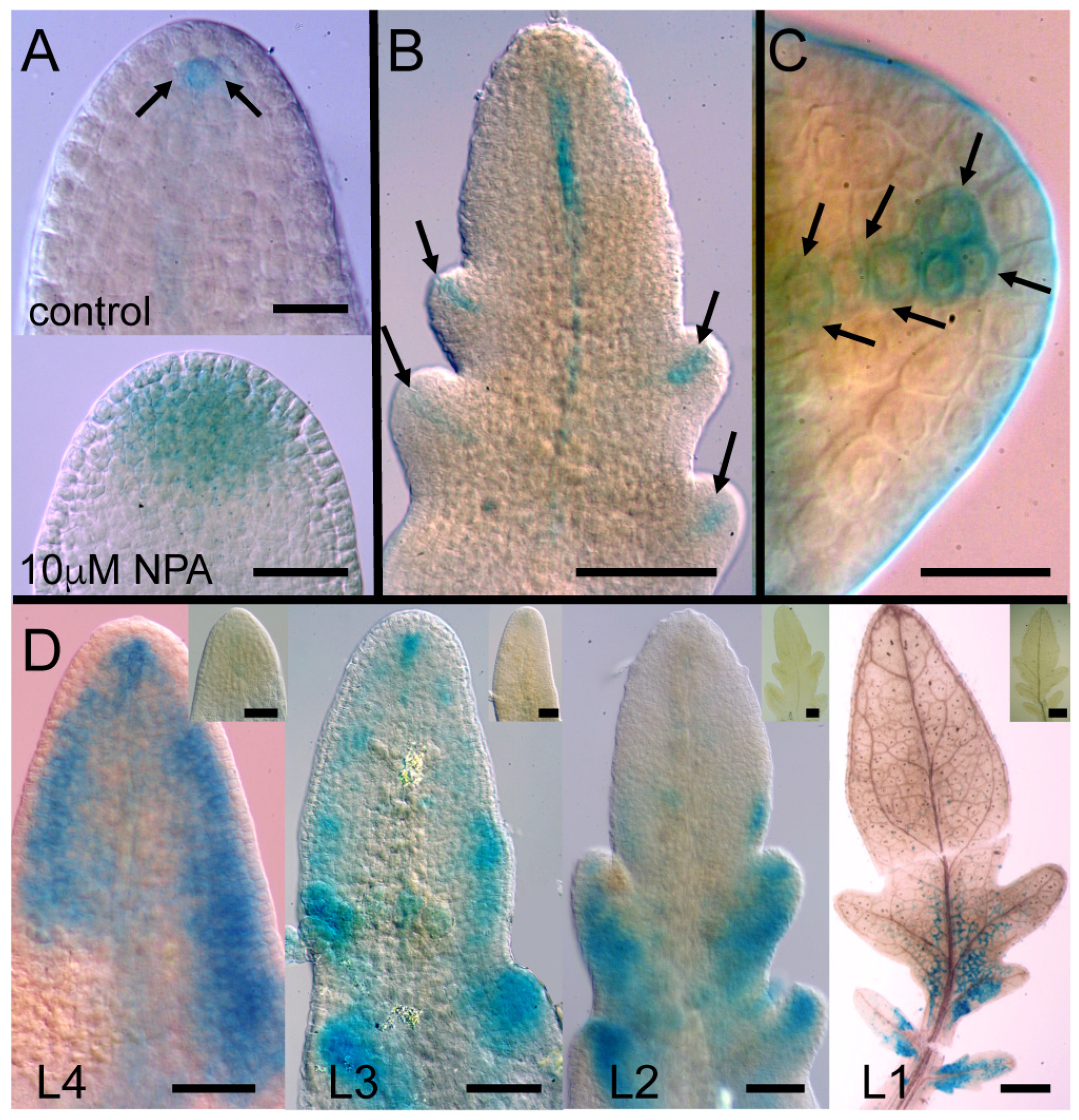
2.2. Polar Auxin Transport Inhibitors Induced More Parallel Leaf/Leaflet Venation Patterning
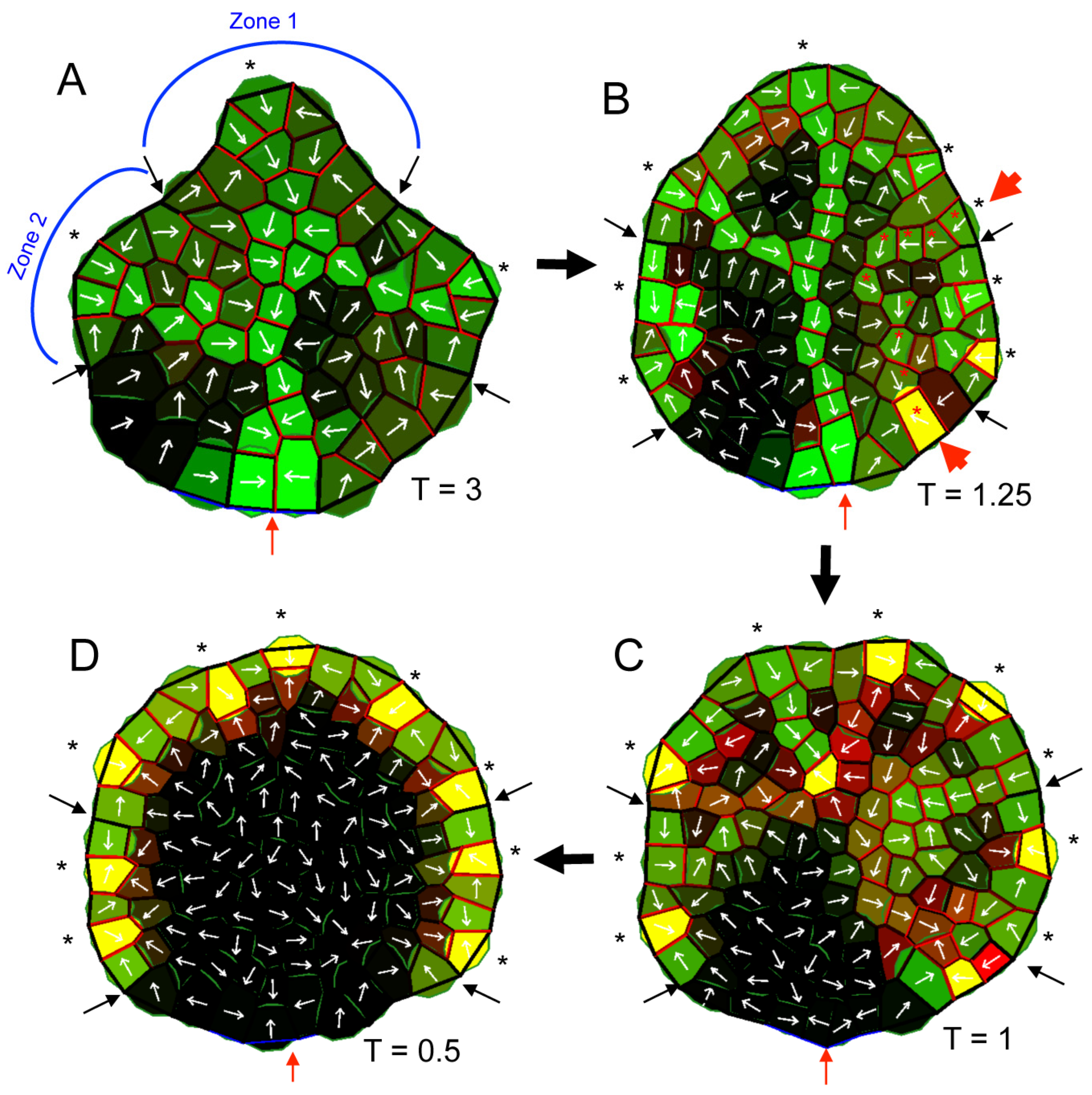
2.3. A PAT-Growth Model Generates the Observed Vein and Morphological Responses to PATi
2.4. Exogenous Auxin Application Altered Tomato Leaf Complexity but Not Venation Patterning
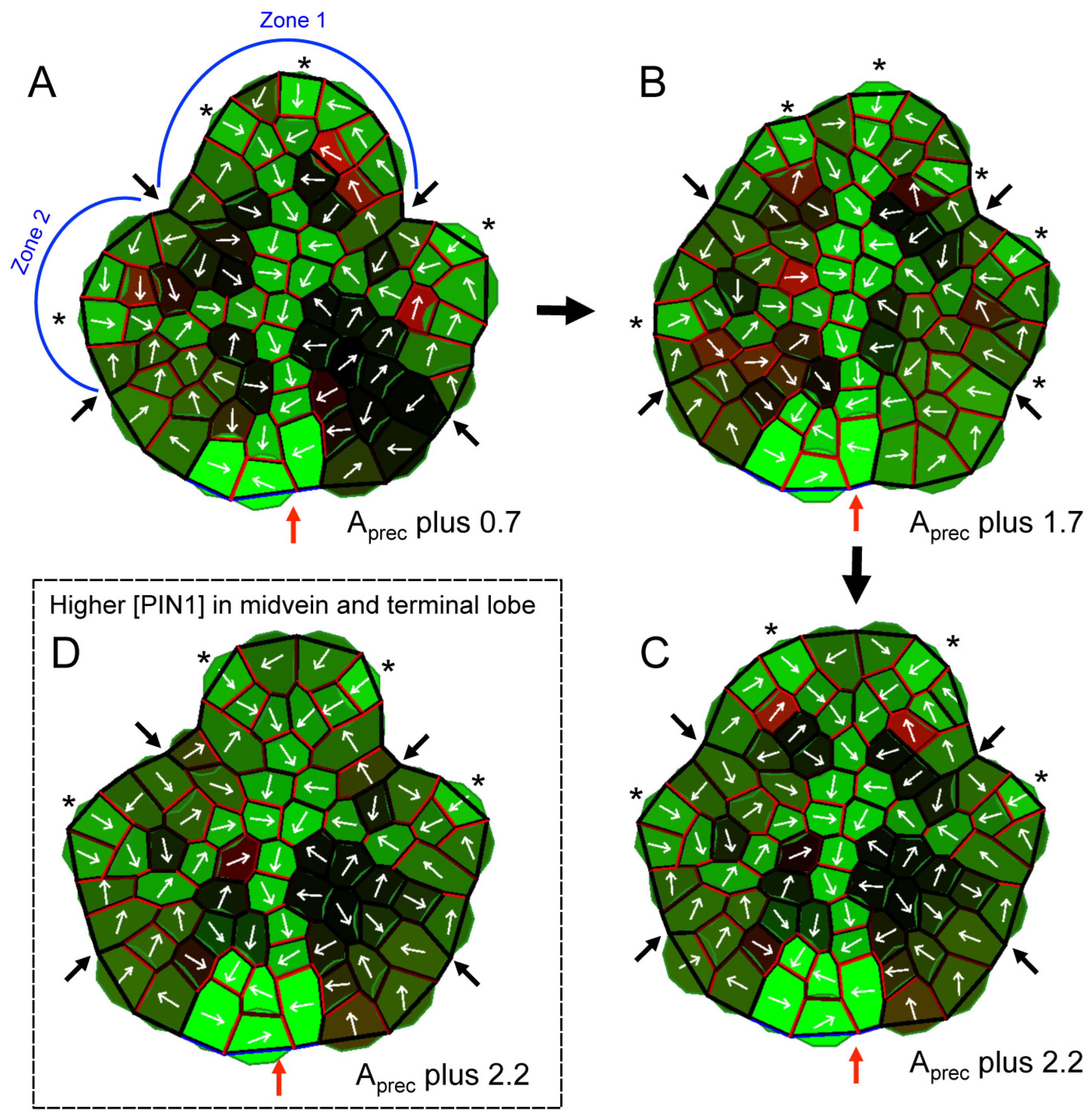
2.5. Modeling Suggests That Vein Developmental Stage Affects the Different Morphological and Venation Responses to Exogenous IAA Exposure
3. Discussion
3.1. PATi Reduced Complexity in Diverse Compound Leaf Types
3.2. PATi Produced Supernumary Veins with More Parallel Patterning
3.3. The Effects of Exogenous IAA Varies with Developmental Stage and Can Differ from PATi
3.4. A PAT-Growth Model for the Morphological and Venation Effects of PATi and Exogenous IAA
4. Materials and Methods
4.1. Plant Material and Growth Conditions
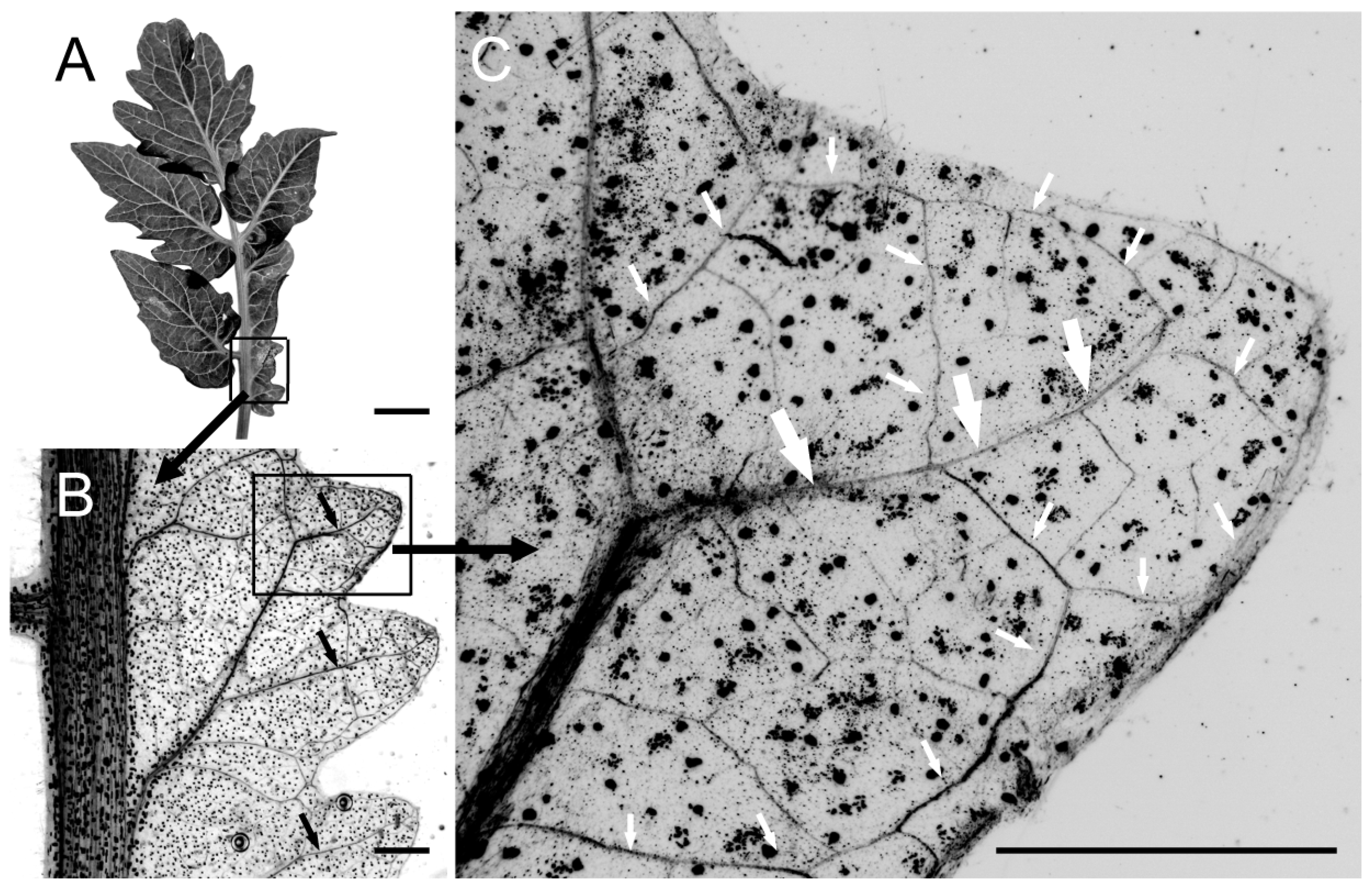
4.2. Analyses of Leaf Complexity and Venation Patterning
4.3. Computer Simulations of Leaf Venation and Growth
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ash, A.; Ellis, B.; Hickey, L.J.; Johnson, K.; Wilf, P.; Wing, S. Manual of Leaf Architecture–Morphological Description and Categorization of Dicotyledonous and Net-Veined Monocotyledonous Angiosperms. Leaf Architecture Working Group; Smithsonian Institution: Washington, DC, USA, 1999. [Google Scholar]
- Kerstetter, R.A.; Poethig, R.S. The Specification of Leaf Identity During Shoot Development. Ann. Rev. Cell Dev. Biol. 1998, 14, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Dengler, N.G. Comparison of Leaf Development in Normal (+/+), Entire (E/E), and Lanceolate (La/+) Plants of Tomato, Lycopersicon eculentum ‘Ailsa Craig’. Bot. Gaz. 1984, 145, 66–77. [Google Scholar] [CrossRef]
- Dengler, N.G.; Tsukaya, H. Leaf Morphogenesis in Dicotyledons: Current Issues. Int. J. Plant Sci. 2001, 162, 459–464. [Google Scholar] [CrossRef]
- Hagemann, W.; Gleissberg, S. Organogenetic Capacity of Leaves: The Significance of Marginal Blastozones in Angiosperms. Plant Syst. Evol. 1996, 199, 121–152. [Google Scholar] [CrossRef]
- Kaplan, D.R. Fundamental Concepts of Leaf Morphology and Morphogenesis: A Contribution to the Interpretation of Molecular Genetic Mutants. Int. J. Plant Sci. 2001, 162, 465–474. [Google Scholar] [CrossRef]
- Holtan, H.E.; Hake, S. Quantitative Trait Locus Analysis of Leaf Dissection in Tomato Using Lycopersicon pennellii Segmental Introgression Lines. Genetics 2003, 165, 1541–1550. [Google Scholar] [CrossRef]
- Maugarny-Calès, A.; Laufs, P. Getting Leaves into Shape: A Molecular, Cellular, Environmental and Evolutionary View. Development 2018, 145, dev161646. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Runions, A.; Vuolo, F.; Strauss, S.; Lymbouridou, R.; Routier-Kierzkowska, A.L.; Wilson-Sánchez, D.; Jenke, H.; Galinha, C.; Mosca, G.; et al. A Growth-Based Framework for Leaf Shape Development and Diversity. Cell 2019, 177, 1405–1418. [Google Scholar] [CrossRef]
- Nikolov, L.A.; Runions, A.; Das Gupta, M.; Tsiantis, M. Leaf Development and Evolution. Curr. Top. Dev. Biol. 2019, 31, 109–139. [Google Scholar]
- Van Volkenburgh, E. Leaf Expansion–An Integrating Plant Behaviour. Plant Cell Environ. 1999, 22, 1463–1473. [Google Scholar] [CrossRef]
- Dengler, N.; Kang, J. Vascular Patterning and Leaf Shape. Current Opinion in Plant Biol. 2001, 4, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Runions, A.; Tsiantis, M.; Prusinkiewicz, P. A Common Developmental Program can Produce Diverse Leaf Shapes. New Phytol. 2017, 216, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Scarpella, E.; Marcos, D.; Friml, J.; Berleth, T. Control of Leaf Vascular Patterning by Polar Auxin Transport. Genes Dev. 2006, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, C.L.; Schuetz, M.; Yu, Q.; Mattsson, J. Dynamics of MONOPTEROS and PIN-FORMED 1 Expression during Leaf Vein Pattern Formation in Arabidopsis thaliana. Plant J. 2007, 49, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef]
- Liu, C.-M.; Xi, Z.-H.; Chua, N.-H. Auxin Polar Transport is Essential for the Establishment of Bilateral Symmetry During Early Plant Embryogenesis. Plant Cell 1993, 5, 521–530. [Google Scholar] [CrossRef]
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin Regulates the Initiation and Radial Position of Plant Lateral Organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of Phyllotaxis by Polar Auxin Transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Scarpella, E.; Barkoulas, M.; Tsiantis, M. Control of Leaf and Vein Development by Auxin. Cold Spr. Harb. Persp. Biol. 2010, 2, a001511. [Google Scholar] [CrossRef]
- Barkoulas, M.; Hay, A.; Kougioumoutzi, E.; Tsiantis, M. A Developmental Framework for Dissected Leaf Formation in the Arabidopsis Relative. Cardamine Hirsute. Nat. Gen. 2008, 40, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Koenig, D.; Bayer, E.; Kang, J.; Kuhlemeier, C.; Sinha, N. Auxin Patterns in Solanum lycopersicum Leaf Morphogenesis. Development 2009, 136, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Smith, R.; Mandel, T.; Nakayama, N.; Sauer, M.; Prusinkiewicz, P.; Kuhlemeier, C. Integration of Transport-Based Models for Phyllotaxis and Midvein Formation. Genes Dev. 2009, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin Signaling in Arabidopsis Leaf Vascular Development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Sung, Z.R.; Berleth, T. Responses of Plant Vascular Systems to Auxin Transport Inhibition. Development 1999, 126, 2979–2991. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, J.; Hong, J.; Zhang, C.; Wei, H.; Ying, W.; Sun, C.; Sun, L.; Mao, Y.; Gao, Y.; et al. Structural Insights into Auxin Recognition and Efflux by Arabidopsis PIN1. Nature 2022, 609, 611–618. [Google Scholar] [CrossRef]
- Verna, C.; Janani Ravichandran, S.; Sawchuk, M.G.; Linh, N.M.; Scarpella, E. Coordination of Tissue Cell Polarity by Auxin Transport and Signaling. eLife 2019, 8, e51061. [Google Scholar] [CrossRef]
- Avasarala, S.; Yang, J.; Caruso, J.L. Production of Phenocopies of the Lanceolate Mutant in Tomato Using Polar Auxin Transport Inhibitors. J. Exp. Bot. 1996, 47, 709–712. [Google Scholar] [CrossRef]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delanlande, C.; Regad, F.; Chaabouni, S.; Latche, A.; Pech, J.C.; Bouzayen, M. The Tomato Aux/IAA Transcription Factor IAA9 is Involved in Fruit Development and Leaf Morphogenesis. Plant Physiol. 2005, 146, 1759–1772. [Google Scholar] [CrossRef]
- DeMason, D.A.; Chawla, R. Roles for Auxin during Morphogenesis of the Compound Leaves of Pea (Pisum sativum). Planta 2004, 218, 435–448. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, N.; Zhang, Q.; Endress, P.K.; Huang, J.; Ma, H. Resolution of Deep Eudicot Phylogeny and Their Temporal Diversification using Nuclear Genes from Transcriptomic and Genomic Datasets. New Phytol. 2017, 214, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; Liu, M.M.; Aoyama, T.; Bierfreund, N.M.; Braun, M.; Coudert, Y.; Dennis, R.J.; O’Connor, D.; Wang, X.Y.; White, C.D.; et al. Plasma Membrane-Targeted PIN Proteins Drive Shoot Development in a Moss. Curr. Biol. 2014, 24, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Bilsborough, G.D.; Runions, A.; Barkoulas, M.; Jenkins, H.W.; Hasson, A.; Galinha, C.; Laufs, P.; Hay, A.; Prusinkiewicz, P.; Tsiantis, M. Model for the Regulation of Arabidopsis thaliana Leaf Margin Development. Proc. Natl. Acad. Sci. USA 2011, 108, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Holloway, D.M.; Wenzel, C.L. Polar Auxin Transport Dynamics of Primary and Secondary Vein Patterning in Dicot Leaves. Silico Plants 2021, 3, diab030. [Google Scholar] [CrossRef]
- Ravichandran, S.J.; Linh, N.M.; Scarpella, E. The Canalization Hypothesis—Challenges and Alternatives. New Phytol. 2020, 227, 1051–1059. [Google Scholar] [CrossRef]
- Linh, N.M.; Scarpella, E. Leaf Vein Patterning is Regulated by the Aperture of Plasmodesmata Intercellular Channels. PLoS Biol. 2022, 20, e3001781. [Google Scholar]
- Lincoln, C.; Britton, J.H.; Estelle, M. Growth and Development of the axr1 Mutants of Arabidopsis. Plant Cell 1990, 2, 1071–1080. [Google Scholar]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA Proteins Repress Expression of Reporter Genes Containing Natural and Highly Active Synthetic Auxin Response Elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar]
- Luschnig, C.; Gaxiola, R.A.; Grisafi, P.; Fink, G.R. EIR1, a Root-Specific Protein Involved in Auxin Transport, is Required for Gravitropism in Arabidopsis thaliana. Genes Dev. 1998, 12, 2175–2187. [Google Scholar] [CrossRef]
- Park, S.H.; Morris, J.L.; Park, J.E.; Hirschi, K.D.; Smith, R.H. Efficient and Genotype-Independent Agrobacterium-Mediated Tomato Transformation. J. Plant Physiol. 2003, 160, 1253–1257. [Google Scholar] [CrossRef]
- Merks, R.M.; Guravage, M.; Inzé, D.; Beemster, G.T. VirtualLeaf: An Open-Source Framework for Cell-Based Modeling of Plant Tissue Growth and Development. Plant Physiol. 2011, 155, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Runions, A.; Prusinkiewicz, P. Auxin-Driven Patterning with Unidirectional Fluxes. J. Exp. Bot. 2015, 66, 5083–5102. [Google Scholar] [CrossRef] [PubMed]
- Antonovici, C.C.; Peerdeman, G.Y.; Wolff, H.B.; Merks, R.M.H. Modeling Plant Tissue Development using VirtualLeaf. In Methods in Molecular Biology; Lucas, M., Ed.; Humana: New York, NY, USA, 2022; pp. 165–198. [Google Scholar] [CrossRef]
- Merks, R.M.H.; Guravage, M.A. Building Simulation Models of Developing Plant Organs using VirtualLeaf. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 333–352. [Google Scholar] [CrossRef]
- Merks, R.M.; Van de Peer, Y.; Inzé, D.; Beemster, G.T. Canalization Without Flux Sensors: A Traveling-Wave Hypothesis. Trends Plant Sci. 2007, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]

| Plant | Leaf | Control | 0.1 H | 1 H | 0.1 N | 1 N | 10 N | 0.1 T | 1 T | 10 T |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 2.8 ± 0.6 (21) | 2.2 ± 0.8 (18) | 1.1 ± 0.3 (9) | 1.9 ± 0.7 (17) | 1.3 ± 0.6 (22) | 1.0 ± 0 (17) | 2.7 ± 0.8 (15) | 3.0 ± 0.6 (13) | 2.0 ± 0.9 (6) | |
| p-value | 8.0 × 10−3 ** | 3.0 × 10−10 ** | 1.4 × 10−4 ** | 1.1 × 10−9 ** | 1.1 × 10−11 ** | 7.6 × 10−1 | 3.7 × 10−1 | 7.9 × 10−2 | ||
| % with < median | 19 | 61 | 100 | 82 | 91 | 100 | 20 | 15 | 67 | |
| L2 | 3.5 ± 0.8 (22) | 2.5 ± 1.1 (17) | 1.1 ± 0.3 (11) | 2.7 ± 0.6 (17) | 1.8 ± 0.9 (22) | 1.0 ± 0 (17) | 3.6 ± 0.9 (14) | 3.3 ± 0.6 (14) | 2.3 ± 1.0 (6) | |
| tomato | p-value | 4.9 × 10−3 ** | 4.3 × 10−13 ** | 1.8 × 10−3 ** | 4.4 × 10−8** | 2.4 × 10−12 ** | 7.0 × 10−1 | 4.8 × 10−1 | 4.4 × 10−2 * | |
| % with < median | 5 | 47 | 100 | 24 | 73 | 100 | 7 | 0 | 33 | |
| L3 | 4.5 ± 0.9 (22) | 2.9 ± 0.9 (18) | 1.5 ± 0.5 (6) | 2.9 ± 0.6 (16) | 2.1 ± 1.0 (22) | 1.0 ± 0 (14) | 4.5 ± 1.0 (14) | 4.0 ± 1.0 (13) | 3.2 ± 0.4 (6) | |
| p-value | 2.5 × 10−6 ** | 1.8 × 10−7 ** | 1.4 × 10−7 ** | 1.1 × 10−10 ** | 1.2 × 10−14 ** | 7.1 × 10−1 | 1.4 × 10−1 | 7.5 × 10−3 * | ||
| % with < median | 32 | 94 | 100 | 100 | 100 | 100 | 21 | 62 | 100 | |
| L2 | 2.8 ± 0.6 (13) | 2.9 ± 0.3 (9) | 1.6 ± 0.5 (7) | 5 ± 0 (5) | 2.0 ± 0.8 (4) | 2.7 ± 0.6 (3) | 1.8 ± 0.5 (4) | |||
| p-value | 8.2 × 10−1 | 2.4 × 10−4 ** | ND | 8.5 × 10−9 ** | 1.3 × 10−1 | 6.6 × 10−1 | 1.1 × 10−2 * | ND | ||
| clover | % with < median | 8 | 11 | 100 | 0 | 75 | 33 | 100 | ||
| L3 | 3.0 ± 0 (13) | 2.4 ± 0.7 (9) | 1.9 ± 1.0 (8) | 3.0 ± 0 (5) | 2.5 ± 0.8 (6) | 3.0 ± 0 (3) | 2.3 ± 1.0 (4) | |||
| p-value | 5.1 × 10−2 | 1.5 × 10−2 * | ND | undefined | 2.0 × 10−1 | undefined | 2.1 × 10−1 | ND | ||
| % with < median | 0 | 44 | 63 | 0 | 33 | 0 | 50 | |||
| L2 | 6.6 ± 0.8 (47) | 6.3 ± 0.6 (3) | 4.0 ± 2.3 (57) | |||||||
| p-value | ND | ND | ND | 4.9 × 10−1 | 1.4 × 10−11 ** | ND | ND | ND | ||
| lupin | % with < median | 45 | 67 | 86 | ||||||
| L3 | 7.0 ± 0.7 (48) | 6.3 ± 0.6 (3) | 5.3 ± 2.4 (55) | |||||||
| p-value | ND | ND | ND | 1.7 × 10−1 | 5.2 × 10−6 ** | ND | ND | ND | ||
| % with < median | 25 | 67 | 69 | |||||||
| L1 | 5.6 ± 1.2 (17) | 5.2 ± 0.6 (13) | 4.2 ± 2.1 (8) | 5.4 ± 0.9 (5) | 1.7 ± 1.6 (6) | 5.4 ± 1.3 (9) | 5.0 ± 0 (9) | |||
| p-value | 1.9 × 10−1 | 1.3 × 10−1 | 7.1 × 10−1 | 1.0 × 10−3 ** | ND | 7.9 × 10−1 | 5.6 × 10−2 | ND | ||
| % with < median | 6 | 0 | 25 | 0 | 83 | 0 | 0 | |||
| L2 | 6.4 ± 1.4 (14) | 7.9 ± 1.0 (11) | 6.4 ± 2.5 (7) | 6.6 ± 1.7 (5) | 5.8 ± 1.8 (5) | 7.5 ± 1.4 (8) | 7.0 ± 1.2 (7) | |||
| carrot | p-value | 4.9 × 10−3 ** | 9.5 × 10−1 | 7.8 × 10−1 | 5.5 × 10−1 | ND | 9.1 × 10−2 | 2.9 × 10−1 | ND | |
| % with < median | 50 | 0 | 29 | 40 | 40 | 13 | 14 | |||
| L3 | 6.9 ± 1.1 (12) | 9.0 ± 1.0 (9) | 7.4 ± 2.6 (5) | 8.0 ± 1.1 (6) | 8.6 ± 0.9 (5) | |||||
| p-value | 2.4 × 10−4 ** | 7.1 × 10−1 | ND | ND | ND | 7.5 × 10−2 | 8.8 × 10−3 ** | ND | ||
| % with < median | 17 | 0 | 20 | 0 | 0 |
| Leaf | TT 1; Control | TT 1; 10% IAA | TT 2; Control | TT 2; 1% IAA | TT 2; 10% IAA | G; Control | G; 10% IAA |
|---|---|---|---|---|---|---|---|
| Leaf 2 | 3.5 ± 0.8 (48) | 3.1 ± 1.1 (59) | |||||
| p-value | 1.5 × 10−2 * | ND | ND | ND | ND | ND | |
| % < median | 6 | 22 | |||||
| % fused | 0 | 20 | |||||
| Leaf 3 | 4.5 ± 0.9 (48) | 3.4 ± 1.3 (59) | |||||
| p-value | 5.0 × 10−7 ** | ND | ND | ND | ND | ND | |
| % < median | 33 | 75 | |||||
| % fused | 0 | 36 | |||||
| Leaf 4 | 5.1 ± 0.6 (48) | 3.7 ± 1.5 (56) | 5.1 ± 0.4 (24) | 5.0 ± 0.2 (26) | 4.5 ± 0.9 (24) | 10.0 ± 1.2 (5) | 5.0 ± 1.9 (5) |
| p-value | 3.1 × 10−9 ** | 9.7 × 10−2 | 2.6 × 10−3 ** | 8.2 × 10−4 ** | |||
| % < median | 6 | 54 | 0 | 4 | 29 | 20 | 100 |
| % fused | 0 | 32 | 0 | 0 | 8 | 0 | 0 |
| Leaf 5 | 5.1 ± 0.5 (48) | 4.6 ± 0.9 (47) | 5.3 ± 0.5 (24) | 5.0 ± 0.2 (26) | 4.1 ± 1.2 (24) | 15.4 ± 1.9 (5) | 5.0 ± 2.5 (5) |
| p-value | 1.0 × 10−3 ** | 3.8 × 10−2 * | 1.0 × 10−4 * | 4.8 × 10−5 ** | |||
| % < median | 0 | 23 | 0 | 0 | 46 | 20 | 100 |
| % fused | 0 | 4 | 0 | 12 | 8 | 0 | 20 |
| Leaf 6 | 5.3 ± 0.7 (23) | 4.8 ± 1.2 (26) | 4.0 ± 1.4 (22) | 15.2 ± 1.6 (5) | 6.4 ± 3.5 (5) | ||
| p-value | ND | ND | 4.7 × 10−2 * | 2.9 × 10−4 ** | 1.3 × 10−3 ** | ||
| % < median | 4 | 12 | 45 | 40 | 100 | ||
| % fused | 0 | 27 | 23 | 0 | 20 | ||
| Leaf 7 | 5.6 ± 0.8 (24) | 4.4 ± 1.9 (25) | 3.3 ± 2.3 (15) | 16.0 ± 0 (5) | 10.4 ± 5.1 (5) | ||
| p-value | ND | ND | 4.2 × 10−3 ** | 8.9 × 10−4 ** | 3.5 × 10−2 * | ||
| % < median | 0 | 32 | 67 | 0 | 80 | ||
| % fused | 0 | 56 | 73 | 0 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzel, C.L.; Holloway, D.M.; Mattsson, J. The Effects of Auxin Transport Inhibition on the Formation of Various Leaf and Vein Patterns. Plants 2024, 13, 2566. https://doi.org/10.3390/plants13182566
Wenzel CL, Holloway DM, Mattsson J. The Effects of Auxin Transport Inhibition on the Formation of Various Leaf and Vein Patterns. Plants. 2024; 13(18):2566. https://doi.org/10.3390/plants13182566
Chicago/Turabian StyleWenzel, Carol L., David M. Holloway, and Jim Mattsson. 2024. "The Effects of Auxin Transport Inhibition on the Formation of Various Leaf and Vein Patterns" Plants 13, no. 18: 2566. https://doi.org/10.3390/plants13182566
APA StyleWenzel, C. L., Holloway, D. M., & Mattsson, J. (2024). The Effects of Auxin Transport Inhibition on the Formation of Various Leaf and Vein Patterns. Plants, 13(18), 2566. https://doi.org/10.3390/plants13182566






