Production of Terpene Trilactones from Cell and Organ Cultures of Ginkgo biloba
Abstract
:1. Introduction
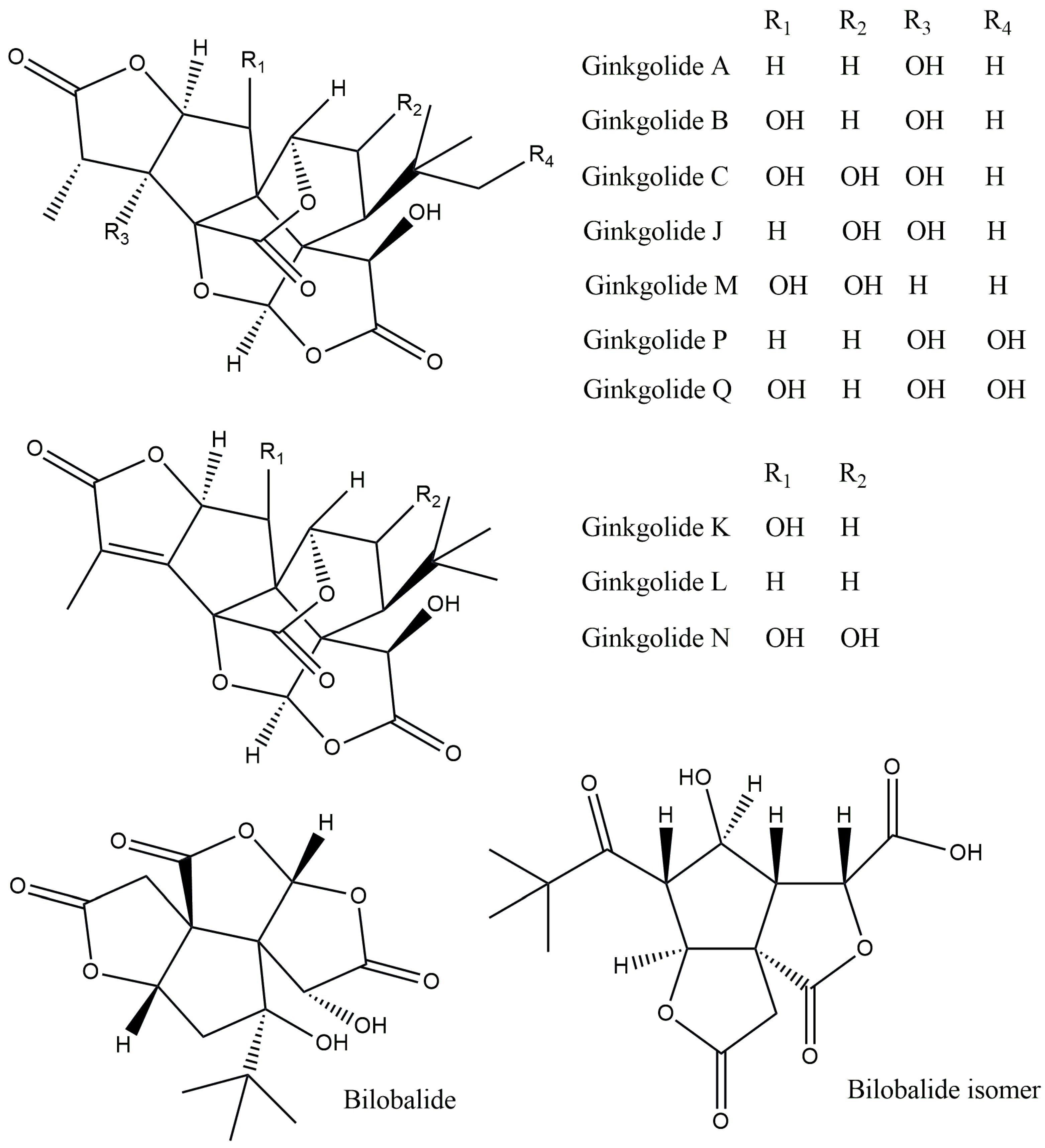
| Name of the Compound | Biological Activities | References |
|---|---|---|
| Diterpenes | ||
| Ginkgolide A | Anti-inflammatory and immunostimulant | [5] |
| Ginkgolide B | Stimulation of the central nervous system | [7] |
| Ginkgolide C | Antilipidemic and anticancer | [9] |
| Ginkgolide M | Central nervous system activity | [10] |
| Ginkgolide J | Anti-dementia | [11] |
| Ginkgolide P | No data | [4] |
| Ginkgolide K | Antioxidant, immunomodulatory, and neuroprotective | [13,17] |
| Ginkgolide Q | No data | [18] |
| Ginkgolide L | No data | [19] |
| Ginkgolide N | Inhibition of cholinesterase activity | [14] |
| Sesquiterpenes | ||
| Bilobalide | Anti-inflammatory, anti-ischemic, antioxidant, cardioprotective, and neuroprotective | [15] |
| Bilobalide isomer | No data | [20] |
2. Production of Terpene Trilactones in Plant Tissue Cultures
2.1. Selection of Cell Lines
2.2. Optimization of Nutrient Medium
2.3. Effect of Plant Growth Regulators
2.4. Impact of Nitrogen and Phosphate Levels
2.5. Influence of Sucrose Concentration
2.6. Inoculum Density, Light/Dark, and Temperature Effects
| Type of Culture | Medium Composition | Strategy Followed | Response | Total Ginkgolide Content | References |
|---|---|---|---|---|---|
| Cell cultures | Murashige and Skoog (MS) medium with 1 mg/L naphthaleneaceticacid (NAA), 0.1 mg/L kinetin and 30 g/L sucrose | Immobilized cells were cultured in 2 and 6 L bioreactors and shake flasks | The biomass yield was 14.24 and 14.82 g/DW/L for the 2 and 6 L immobilized cultures | 7, 17, 19, and 7 ng/g DW amounts of ginkgolide A were obtained in a shake flask (500 mL), shake flask (1200 mL), 2 L, and 6 L immobilized bioreactor cultures, respectively | [43] |
| The extracellular levels of phosphate, nitrate, ammonium, and carbohydrates were studied | |||||
| Callus and cell cultures | Modified MS medium with 30 g/L sucrose | The effect of different media was tested on induction callus, callus growth, and cell suspensions | The MS media with 1.0 mg/L and 0.1 mg/L NAA was excellent for callus induction | Not reported | [39,41] |
| The effect of NAA (0.5, 1, 2, 4, and 8 mg/L) was studied | The growth of the cells was good on MS medium with 1.0 mg/L NAA, 30 g/L sucrose, 1.75 mM phosphate, and a 1:5 molar ratio of NH4+ to NO3−. | ||||
| The effect of the concentration of sucrose (20, 30, 40, and 60 g/L) was studied | |||||
| The effect of the molar ratio of ammonium and nitrate ions (5/1, 3/1, 1/1, 1/2, 1/3, 1/5, 0/6 was studied | |||||
| The effect of the molar concentration of KH2PO4 (0, 0.25, 0.75, 1.25, 1.75, 2.25 mM) was studied | |||||
| Cell culture | MS medium with 2 mg/L NAA and 0.2 mg/L kinetin or 2 mg/L benzyladenine (BA) | Cell cultures transformed by Agrobacteriumrhizogenes | Transformed cell cultures possessed ginkgolides and bilobalide | Transformed cells contained 147, 83, 137, 87, and 200 µg/g DW of ginkgolide A, B, C, J, and bilobalide. | [38] |
| Cell suspension derived from the prothallus | Cell cultures of prothallus-derived cells contained higher ginkgolide levels | Prothallus-derived cells possessed 270, 160, 213, 70, and 160 µg/g DW of ginkgolide A, B, C, J, and bilobalide. | |||
| Callus from a zygotic embryo | No ginkgolides or bilobalides with microspore-derived cells and calli from immature zygotic embryos | ||||
| Cell suspension derived from microspores | |||||
| Embryo germination | MS medium with 1.0 mg/L thiamine chloride, 100 mg/L myoinositol, and 30 g/L sucrose | Comparison of ginkgolides in seeds, embryos, albumen, seedlings, and plantlets germinated in vitro | Plantlets germinated in vitro possessed highest ginkgolide levels | 1250 and 844 µg/g DW ginkgolide A and ginkgolide B | [45] |
| Cell and hairy root cultures | MS medium with 30 g/L sucrose or 20 g/L glucose‘ | Cell cultures were established by using different strains | Transformed roots possessed terpenes at the same concentration as whole plant leaves; however, they had a very slow growth rate | From 0.2 to 6.0 mg/g DW | [37] |
| Transformation of ginkgo tissue using Agrobacterium rhizogenes | |||||
| Cell culture | MS medium with 20 µM NAA and 100 mg/L myoinositol | Comparison of cell lines derived from male and female plants with that of in vivo leaves, stem bark, and stems | Variability in ginkgolide and bilobalide contents of ginkgo lines, sexuality, and the plant parts | 619, 976, and 198 g/g DW of GK-A, GK-B, and bilobalide were obtained in the light-exposed female line as compared to 184, 32, and 587 g/g DW of GK-A and GK-B in the continuous-dark-grown female line | [35] |
| The effect of light irradiation (16 h photoperiod) vs. continuous dark incubation of cultures on ginkgolide and bilobalide accumulation | Ginkgolide (GK)-A was the major constituent in suspension cultures of both male and female tree lines. Furthermore, accumulation was in the following order: GK-A > bilobalide > GK-B. More bilobalide was recorded in the male line than in the female line | ||||
| Light provoked a higher accumulation of GK-A and GK-B; however, the concentration of bilobalide decreased | |||||
| Cell culture | MS medium with 20 µM NAA and 100 mg/L myoinositol | The effect of MS medium supplemented with 20 µM NAA and 100 mg/L myoinositol was tested | MS medium with 20 µM NAA was good for cell growth and ginkgolide production | 6.5, 0.5, and 3.5 mg/L ginkgolide A, ginkgolide B, and bilobalide | [42] |
| The effect of 1, 3, 5, and 7% sucrose levels was tested | Of the various levels of sucrose tested, 3% sucrose enhanced cell growth; however, the media supplemented with 5 and 7% sucrose increased bilobalide and ginkgolide A production | ||||
| The effect temperatures of 20, 24, 28, 32, and 36 °C on cell growth and ginkgolides was tested | Among the various temperatures tested, 25 °C was good for biomass accumulation, and 36 °C increased bilobalide and ginkgolide A production | ||||
| Balloon-type bubble bioreactors | Among the various temperatures tested, 25 °C was good for biomass accumulation, and 36 °C increased bilobalide and ginkgolide A production | ||||
| In a 5 L balloon-type bubble bioreactor containing 2.5 L medium, good metabolite production was realized |
3. Application of Immobilization, Elicitation, and Precursor Feeding Strategies
| Type of Culture | Medium Composition | Strategy Followed | Response | Total Ginkgolide Content | References |
|---|---|---|---|---|---|
| Cell culture/ elicitation | MS medium with 3% sucrose and 3.5 mg/L NAA | 50, 200, and 800 mM KCl were used as elicitors and added to 14-day-old cell suspension cultures and treated for 12, 24, 48, and 72 h | 800 mM KCl severed inhibited the cell growth. However, it was responsible for 1.9- and 4.0-times higher accumulation of ginkgolide A and ginkgolide B | Elicitor was responsible for 1.9 (15 mg/g DW) and 4.0 times (8 mg/g DW) higher accumulation of ginkgolide A and ginkgolide B | [47] |
| Immobilized cell culture/elicitation | MS medium with 3% sucrose, 2 mg/L NAA, and 0.1 mg/L kinetin | Immobilization of cells using jute fibers | Immobilization cells lead 1.4 times higher accumulation of biomass | 78, 79, 71, and 7.5 mg/g DW bilobalide, ginkgolide A, ginkgolide B, and ginkgolide C, respectively | [48] |
| Eliciting with methyl jasmonate (MJ) and salicylic acid (SA) alone or in combination (0.01, 0.1 mM) | The combined elicitation (0.1 mM MJ + 0.1 SA) was responsible for 1.78-, 1.95-, 2.05-, and 2.95-fold more bilobalide, and ginkgolides A, B, and C respectively, compared to control | ||||
| Cell culture/effect of levopimaradiene (diterpene resin acid) | MS medium with 3% sucrose and 50 mg/L ascorbic acid. Two types of cell lines were used viz. dedifferentiated cells (DDCs) and cambial meristematic cells (CMCs). For the DDCs culture, 2 mg/L NAA and 2 mg/L indole butyric acid were added, while for the CMCs culture, 2 mg/L NAA and 2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) were added | Cell cultures were treated with levopimaradiene (LP) at 20, 40, 60, 80, 100, and 120 mg/L in 13-day-old cell cultures | The productions of ginkgolide A (GA) and ginkgolide B (GB) were 1.61- and 1.32-fold larger than that of the control groups when G. biloba DDCs were treated with LP, and the productions of ginkgolide C (GC) and BB reached 234 and 161 μg/L after being treated with LP for 60 h. | 234 and 161 μg/L ginkgolide C and bilobalide with 60 h LP treatment in DDCs | [49] |
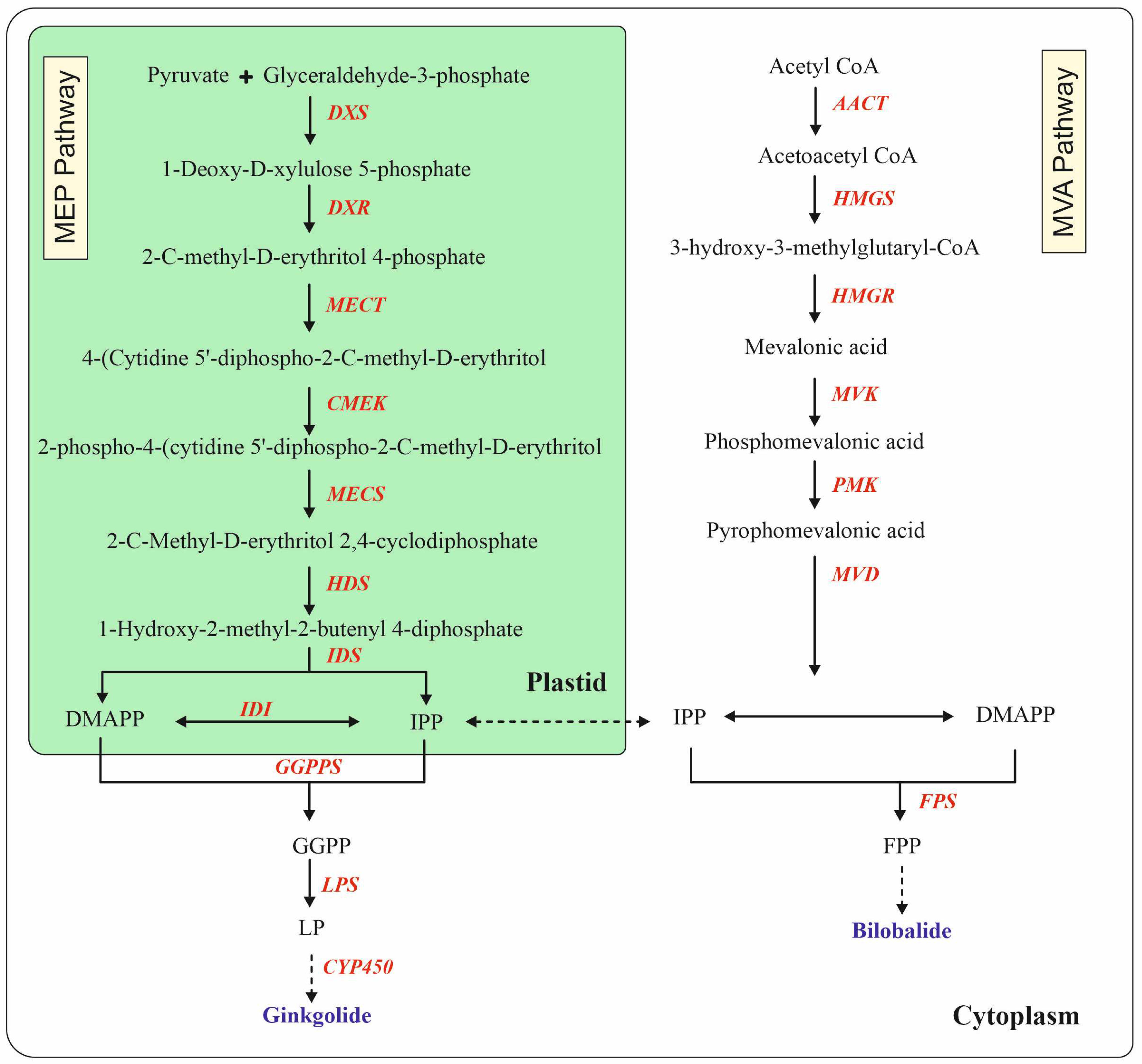
| The transcript levels of DXS, MECT, HDS, HDR, GGPP, and LPS (GAPDH was used as a housekeeping gene) were monitored by qRT-PCR | The production of GA, GB, GC, and BB was 2.03-, 1.43-, 1.22-, and 1.19-fold larger than that of the control groups in LP-treated CMCs | ||||
| Immobilized cell culture/ elicitation | Half-strength MS medium with 2 mg/L NAA, 0.5 mg/L kinetin, 0.1 g/L casein hydrolysate, and 0.8 g/L polyvinyl pyrrolidone | Cell suspension (2 mg) in 20 mL 1/2 MS medium with chitosan (CH, 50, 100, 200, 300, 400, and 500 mg/L) or yeast extract (YE, 50, 100, 200, 300, 400, and 500 mg/L) or methyl jasmonate (MJ, 0.01, 0.05,0.1, 0.3, and 0.5 mM) or salicylic acid (0.01, 0.05,0.1, 0.3 and 0.5 mM) was tested as elicitors. | The ginkgolide B (GB) intercellular and extracellular yields were 108.9/112.4 mg/L with 0.5 mM MJ treatment; whereas GB intercellular and extracellular yields were 7.4.4/82.1 mg/L with 0.3 mM SA treatment. Thus, increment of GB yield 371%/404% and 222%/268% with MJ and SA treatments | 114 mg/L GB could be produced with cotton immobilization of cells and 0.5 mM MJ treatment | [50] |
| Based on the above experiment, 0.5 and 0.3 mM MJ and SA were found to be the optimum concentrations for GB production; therefore, the subsequent cell cultures were established as above, and the cells were immobilized with 1 cm3 cubes of sponge, cotton fiber (pore size 500 µm in diameter), and a loofah (2–3 mm in diameter) for 14 days | The immobilization of cells with three materials showed that cotton and loofah were good in terms of cell quality, viability, and GB production | ||||
| Precursor feeding | |||||
| Cell culture | MS medium with 3.5 mg/L NAA and 3% sucrose | Cultures were supplemented with 0.01 mM precursors, such as geranyl pyrophosphate (GPP), geranylgeranyl pyrophosphate (GGPP), isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), farnesyl pyrophosphate (FPP), acetyl- CoA, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), glyceraldehyde-3 phosphate (GA-3P), mevalonate (MVA), and sodium pyruvate (SP) after 2 weeks of initial cultures and incubated for a further 5 days | Feeding cultures with IPP as a precursor was the most effective | IPP treatment enhanced bilobalide (BB), ginkgolide A (GA), and ginkgolide B (GB) by 10-, 2.3-, and 6.2-fold in the cells. | [51] |
| The IPP treatment also stimulated the excretion of GA and GB by 5.7- and 7.2-fold. | |||||
4. Metabolic Engineering for Biosynthesis of Terpene Trilactones
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Zheng, S. The missing link in Ginkgo evolution. Nature 2003, 423, 821–822. [Google Scholar] [CrossRef]
- Lin, H.Y.; Li, W.H.; Lin, C.F.; Wu, H.R.; Zhao, Y.P. International biological flora: Ginkgo biloba. J. Ecol. 2022, 110, 951–982. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A treasure of functional phytochemicals with multimedicinal applications. Evid. Based Complement. Alternat. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef]
- Biernacka, P.; Adamska, I.; Felisiak, K. The potential of Ginkgo biloba as a source of biologically active compounds—A review of the recent literature and patents. Molecules 2023, 23, 3993. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Quispe, C.; Jamaddar, S.; Hossain, R.; Ray, P.; Mondal, M.; Mohamed, Z.A.; Jaafaru, M.S.; Salehi, B.; Islam, M.T.; et al. Therapeutic promises of ginkgolide A: A literature-based review. Biomed. Phamarcother. 2020, 132, 110908. [Google Scholar] [CrossRef]
- Chen, D.; Sun, S.; Cai, D.; Kong, G. Induction of mitochondrial-dependent apoptosis in T24 cells by selenium (Se-) containing polysaccharide from Ginkgo biloba L. leaves. Int. J. Biol. Macromol. 2017, 101, 126–130. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, S.; Liu, P.; Liu, W.; Wang, Q.; Liu, Y.; Tan, H.; Chen, X.; Shi, X.; Wang, O.; et al. Polymeric nanoparticles-based brain delivery with improved therapeutic efficacy of ginkgolide B in Parkinson’s disease. Int. J. Nanomed. 2020, 15, 10453–10467. [Google Scholar] [CrossRef]
- DeFeudis, F.V.; Papadopoulos, V.; Drieu, K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundam. Clin. Pharmacol. 2003, 17, 405–417. [Google Scholar] [CrossRef]
- Yang, M.H.; Baek, S.H.; Um, J.Y.; Ahn, K.S. Anti-neoplastic effects of ginkgolide C through modulating c-Met phosphorylation in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2020, 21, 8303. [Google Scholar] [CrossRef]
- Bolshakov, S.; Dzyuba, S.V.; Decatue, J.; Nakanishi, K.A. Concise synthesis ginkgolide M, a minor component of a terpene trilactone faction from Ginkgo biloba roots. J. Nat. Prod. 2006, 69, 429–431. [Google Scholar] [CrossRef]
- Vitolo, O.; Gong, B.; Cao, Z.; Ishii, H.; Jaracz, S.; Nakanishi, K.; Arancio, O.; Dzyuba, S.V.; Lefort, R.; Shelanski, M. Protection against β-amyloid induced abnormal synaptic function and cell death by ginkgolide. J. Neurobiol. Aging 2009, 30, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zou, W.; Chen, M.; Cao, L.; Ding, J.; Xiao, W.; Hu, G. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur. J. Pharmacol. 2018, 833, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Chai, Z.; Song, L.J.; Wang, Q.; Song, G.B.; Wang, J.; Yu, J.Z.; Xiao, B.G.; Ma, C.G. The neuroprotective effects and transdifferentiation of astrocytes into dopaminergic neurons of ginkgolide K on Parkinson’ disease mice. J. Neuroimmunol. 2022, 364, 577806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.D.; Zhang, L.H.; Zhang, X.T.; Fang, C.S.; Ma, J.R. Protective effect of ginkgolide N against glutamate-induced injury in PC12 cells. J. Chin. Med. Mater. 2015, 38, 1694–1698. [Google Scholar]
- Lu, J.; Xie, L.; Liu, K.; Zhang, X.; Wang, X.; Dai, X.; Liang, Y.; Cao, Y.; Li, X. Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity, and safety. Phyother. Res. 2021, 35, 6114–6130. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Scheeren, H.A.; Rantio, T.; Ch. Melger, W.; Lelyveld, G.P. Determination of ginkgolides and bilobalide in Ginkgo biloba leaves and phytopharmaceuticals. J. Chromatogr. 1991, 543, 375–387. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, J.; Meng, X.; Xu, F.; Zhang, W.; Liao, Y.; Qu, J. Molecular cloning, characterization, and functional analysis of acetyl-Co-A C-acetyltransferase and mevalonate kinase genes involved in terpene trilactone biosynthesis from Ginkgo biloba. Molecules 2017, 22, 74. [Google Scholar] [CrossRef]
- Liao, H.J.; Zheng, Y.F.; Li, H.Y.; Peng, G.P. Two new ginkgolides from the leaves of Ginkgo biloba. Planta Med. 2011, 77, 1818–1821. [Google Scholar] [CrossRef]
- Wang, J.X.; Liu, X.G.; Gan, Z.Y.; Dong, X.; Lou, F.C.; Li, P.; Yang, H. Pharmacokinetics and tissue distribution study of ginkgolide L in rats by ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B 2015, 1006, 30–36. [Google Scholar] [CrossRef]
- Dong, H.L.; Lin, S.; Wu, Q.L.; Su, R.X.; Wu, Z.L.; Dong, H.Y.; Li, H.L.; Zhang, W.D. A new bilobalide isomer and two cis-coumaroylated flavanol from Ginkgo biloba leaves. Fitoterapia 2020, 142, 104516. [Google Scholar] [CrossRef]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; Wiley: London, UK, 2009. [Google Scholar]
- Cordoba, E.; Salmi, M.; Leon, P. Unravelling the regulatory mechanism that modulate the MEP pathway in higher plants. J. Exp. Bot. 2009, 60, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Song, Q.; Ye, J.; Wang, L.; Xu, F. Characterization, function, and transcriptional profiling analysis of 3-hydroxy-3-methylglutaryl-CoA synthase gene (GbHMGS1) towards stresses and exogenous hormone treatments in Ginkgo biloba. Molecules 2017, 22, 1706–1725. [Google Scholar] [CrossRef]
- Rao, S.; Meng, X.; Liao, Y.; Yu, T.; Cao, J.; Tan, J.; Xu, F.; Cheng, S.Y. Characterization and functional analysis of two novel 3-Hydroxy-3-methylglutaryl-CoA synthase genes (GbHMGR2 and GbHMGR3) from Ginkgo biloba. Sci. Rep. 2019, 9, 14109. [Google Scholar] [CrossRef]
- Shepmann, H.G.; Pang, J.; Matsuda, S.P. Cloning can characterization of Ginkgo biloba levopomaradiene synthase, which catalyzes the first committed step in ginkgolide biosynthesis. Arch. Biochem. Biophys. 2001, 392, 263–269. [Google Scholar] [CrossRef]
- Forman, V.; Luo, D.; Geu-Flores, F.; Lemcke, R.; Nelson, D.R.; Kampranis, S.C.; Staerk, D.; Moller, B.L.; Patraki, I. A gene cluster in Ginkgo biloba encodes unique multifunctional cytochrome P450s that initiate ginkgolide biosynthesis. Nat. Commun. 2022, 13, 5143. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Light as an elicitor for enhanced production of secondary metabolites in plant cell, tissue and organ cultures. Plant Growth Regul. 2024, in press. [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Anthocyanin production from plant cell and organ cultures in vitro. Plants 2024, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Anthraquinone production from cell and organ cultures of Rubia species: An overview. Metabolites 2023, 13, 39. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Production of anthraquinones from cell and organ cultures of Morinda species. Appl. Microbiol. Biotechnol. 2023, 107, 2061–2071. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites in cell and organ cultures: Current status and future outlooks. Plant Growth Regul. 2023, in press. [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Paek, K.Y. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem. Rev. 2016, 15, 129–145. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, S.J.; Jung, H.Y.; Kang, Y.M.; Kan, S.M.; Theertha Prasad, D.; Kim, S.W.; Choi, M.S. Variation of ginkgolides and bilobalide contents in leaves and cell cultures of Ginkgo biloba L. Biotechnol. Bioprocess Eng. 2004, 9, 35–40. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Balz, J.P.; Courtois, D.; Drieu, J.; Drieu, K.; Reynoird, J.P.; Sohier, C.; Teng, B.P.; Touche, A.; Petiard, V. Production of ginkgolides and bilobalide by Ginkgo biloba plants and tissue cultures. Planta Med. 1999, 65, 620–626. [Google Scholar] [CrossRef]
- Laurain, D.; Tremouillaux-Guiller, J.; Chenieux, J.C.; van Been, T.A. Production of ginkgolide and bilobalide in transformed and gametophyte derived cell cultures of Ginkgo biloba. Phytochemistry 1997, 46, 127–130. [Google Scholar] [CrossRef]
- Jeon, M.H.; Sung, S.H.; Jeon, S.; Huh, H.; Kim, J.; Kim, Y.C. Cultures of Ginkgo biloba, effect of nutritional and hormonal factors on the growth of cultured cells derived from Ginkgo biloba. Arch. Pham. Res. 1993, 16, 244–250. [Google Scholar] [CrossRef]
- Schenk, R.Y.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Jeon, M.H.; Sung, S.H.; Huh, H.; Kim, Y.C. Ginkgolide B production in cultured cells derived from Ginkgo biloba L. leaves. Plant Cell Rep. 1995, 14, 501–504. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, S.J.; Kang, Y.M.; Jung, H.Y.; Theertha Prasad, D.; Kim, S.W.; Chung, Y.G.; Choi, M.S. Production of ginkgolides and bilobalide from optimized the Ginkgo biloba cell culture. Biotechnol. Bioprocss Eng. 2004, 9, 41–46. [Google Scholar] [CrossRef]
- Carrier, D.J.; Chauret, N.; Mancini, M.; Coulombe, P.; Neufeld, R.; Weber, M.; Archambault, J. Detection of ginkgolide A in Ginkgo biloba cell cultures. Plant Cell Rep. 1991, 10, 256–259. [Google Scholar] [CrossRef]
- Huh, H.; Staba, E.J. Ontogenic aspects of ginkgolide production in Ginkgo biloba. Planta Med. 1993, 59, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kamoda, S.; Terada, T.; Saburi, Y. Ginkgolide production in relation to organogenesis in Ginkgo biloba. J. Wood Sci. 1998, 44, 375–378. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Min, J.Y.; Park, D.J.; Jeong, M.J.; Song, H.J.; Heo, C.M.; Kim, H.G.; Yang, J.K.; Lee, C.H.; Karigar, C.S.; et al. Potassium chloride elicits enhancement of bilobalide and ginkgolides production by Ginkgo biloba cell cultures. For. Sci. Technol. 2010, 6, 49–54. [Google Scholar] [CrossRef]
- Sukito, A.; Tachibana, S. Effect of methyl jasmonate and salicylic acid synergism on enhancement of bilobalide and ginkgolide production by immobilized cell cultures of Ginkgo biloba. Bioresour. Bioprocess. 2016, 3, 24. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Qian, M.; Liang, C.; Zi, J.; Yu, R. Effect of levopimaradiene on terpene trilactone biosynthesis and gene expression profiling in Ginkgo biloba cells. Nat. Prod. Commun. 2017, 12, 1007–1010. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, T.Y.; Fu, W.C.; Su, W.T. Effects of organic elicitors on the recycled production of ginkgolide B in immobilized cell cultures of Ginkgo biloba. J. Funct. Biomater. 2023, 14, 95. [Google Scholar] [CrossRef]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Park, D.J.; Jung, H.N.; Karigar, C.S.; Ha, Y.L.; Kim, S.W.; Choi, M.S. Effect of supplementing terpenoid biosynthetic precursors on the accumulation of bilobalide and ginkgolides. J. Biotechnol. 2006, 123, 85–92. [Google Scholar] [CrossRef]
- Ikram, N.K.B.K.; Zhan, X.; Pan, X.W.; King, B.C.; Simonsen, H.T. Stable heterologous expression of biologically active terpenoids in green plants. Front. Plant Sci. 2015, 6, 129. [Google Scholar] [CrossRef]
- Pang, Y.; Shen, G.; Berges, T.; Cardier, H.; Wu, W.; Sun, X.; Tang, K. Molecular cloning, characterization and heterologous expression in Saccharomyces cerevisiae of a mevalonate diphosphate decarboxylase cDNA from Ginkgo biloba. Physiol. Plant. 2006, 127, 19–27. [Google Scholar] [CrossRef]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, C.; Lu, W. Heterologous production of levopimaric acid in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, X.; Zhou, X.; Liu, X.; Yi, Y.; Su, D.; Zhang, W.; Liao, Y.; Ye, J.; Xu, F. The Ginkgo biloba miroRNA 160-ERF4 module participates in terpene trilactone biosynthesis. Plant Physiol. 2024, in press. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murthy, H.N.; Yadav, G.G.; Paek, K.Y.; Park, S.-Y. Production of Terpene Trilactones from Cell and Organ Cultures of Ginkgo biloba. Plants 2024, 13, 2575. https://doi.org/10.3390/plants13182575
Murthy HN, Yadav GG, Paek KY, Park S-Y. Production of Terpene Trilactones from Cell and Organ Cultures of Ginkgo biloba. Plants. 2024; 13(18):2575. https://doi.org/10.3390/plants13182575
Chicago/Turabian StyleMurthy, Hosakatte Niranjana, Guggalada Govardhana Yadav, Kee Yoeup Paek, and So-Young Park. 2024. "Production of Terpene Trilactones from Cell and Organ Cultures of Ginkgo biloba" Plants 13, no. 18: 2575. https://doi.org/10.3390/plants13182575






