Economic Evaluation of Conservation through Use of an Araucaria angustifolia Provenance and Progeny Test
Abstract
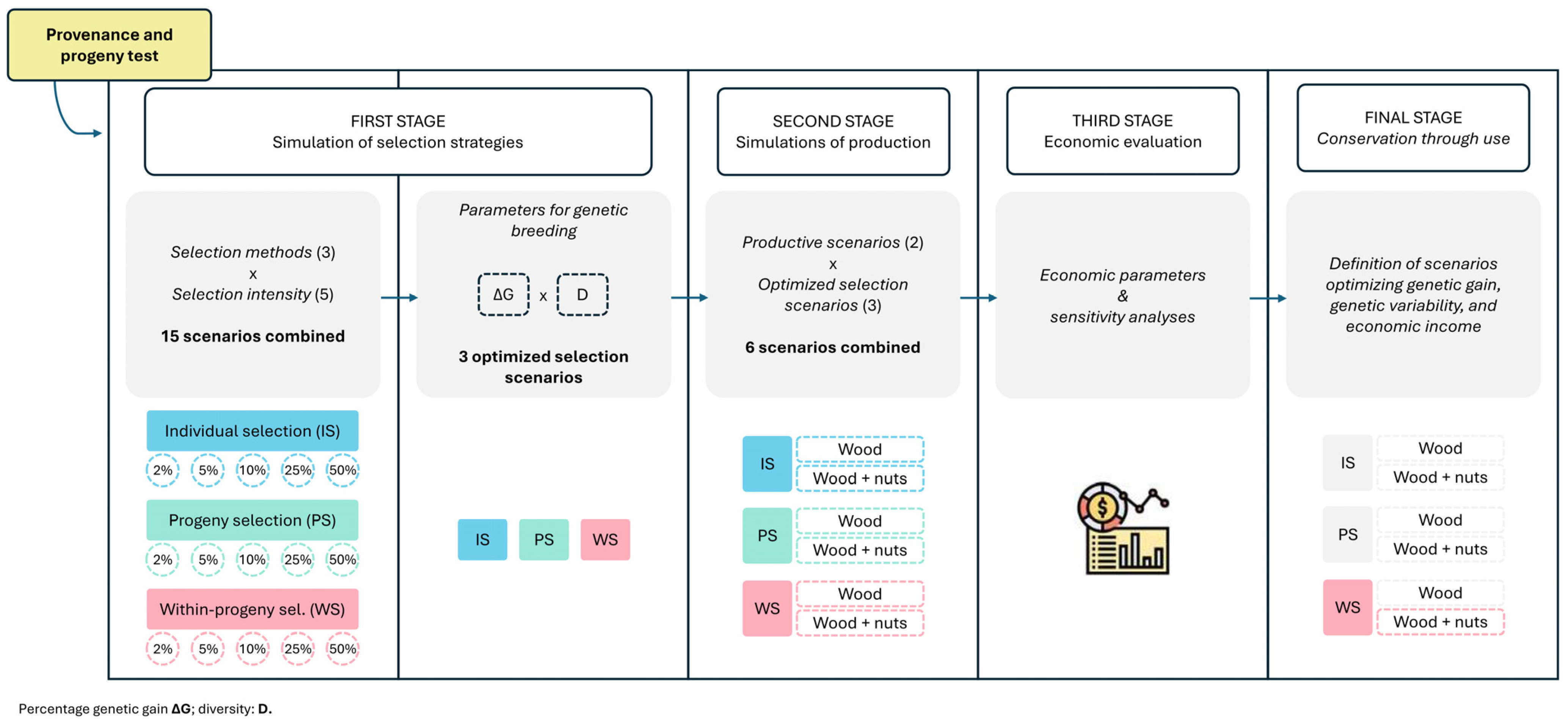
1. Introduction
2. Results
2.1. Scenario Selection Considering Selection Intensity, Gain, Diversity, and Productivity
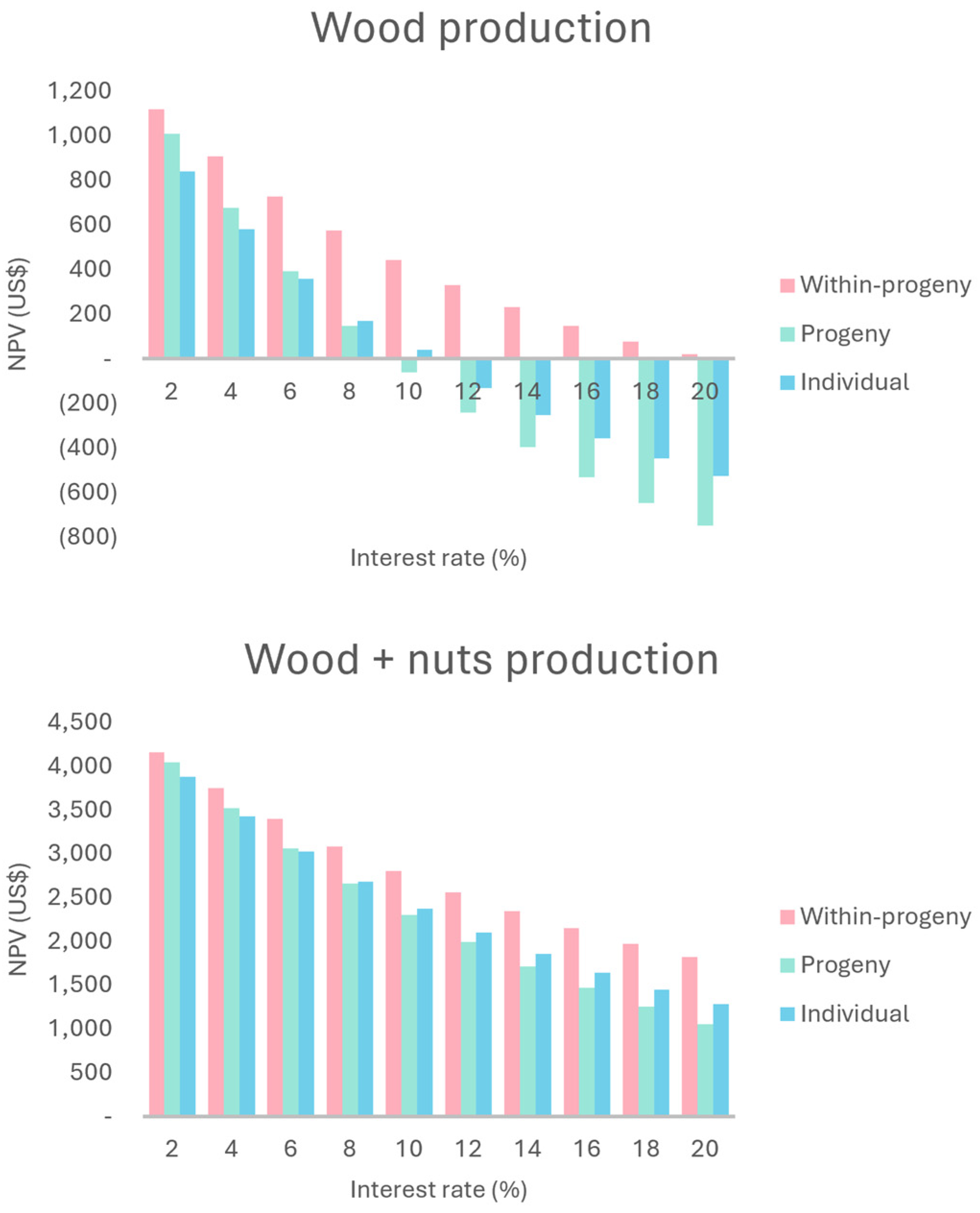
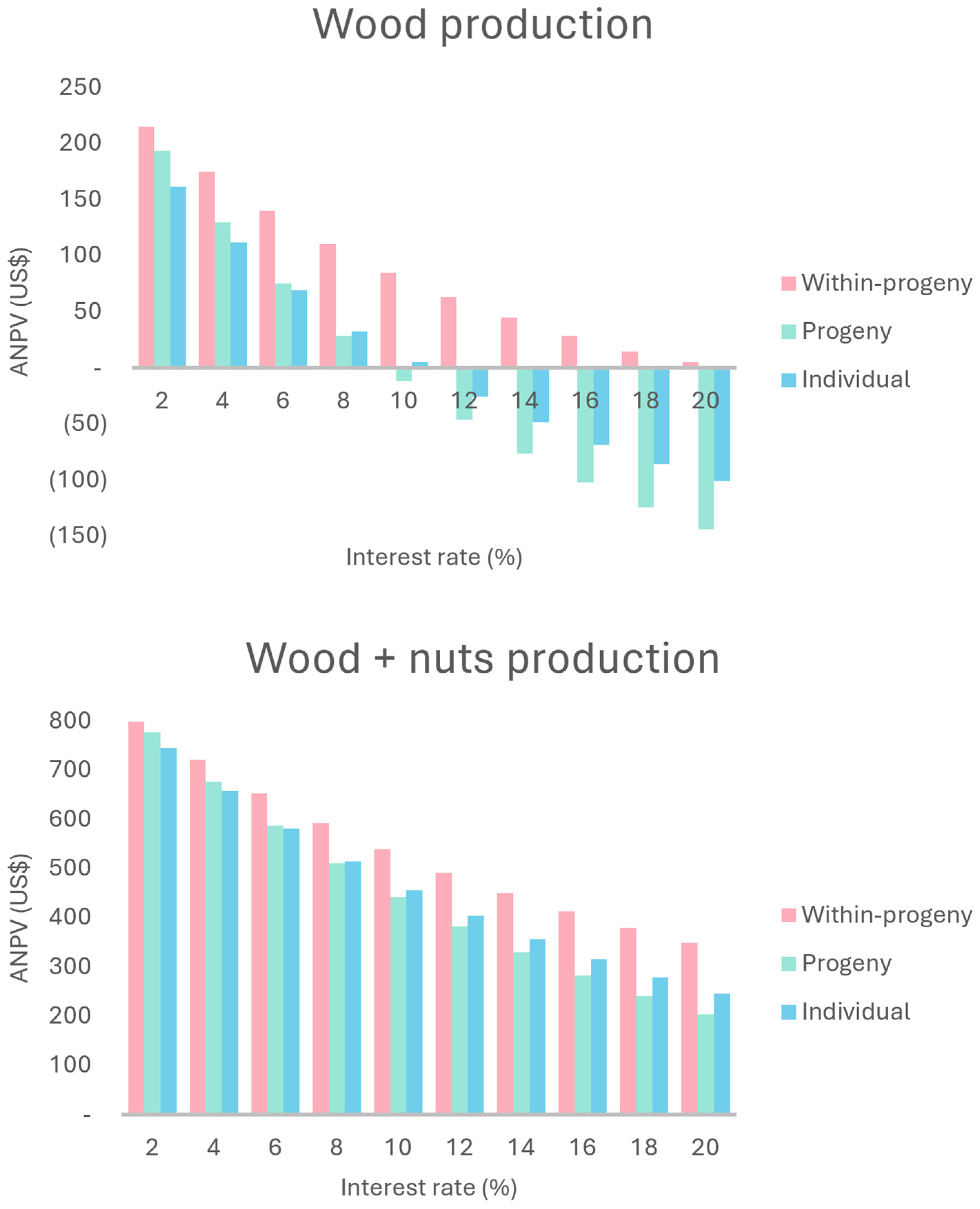
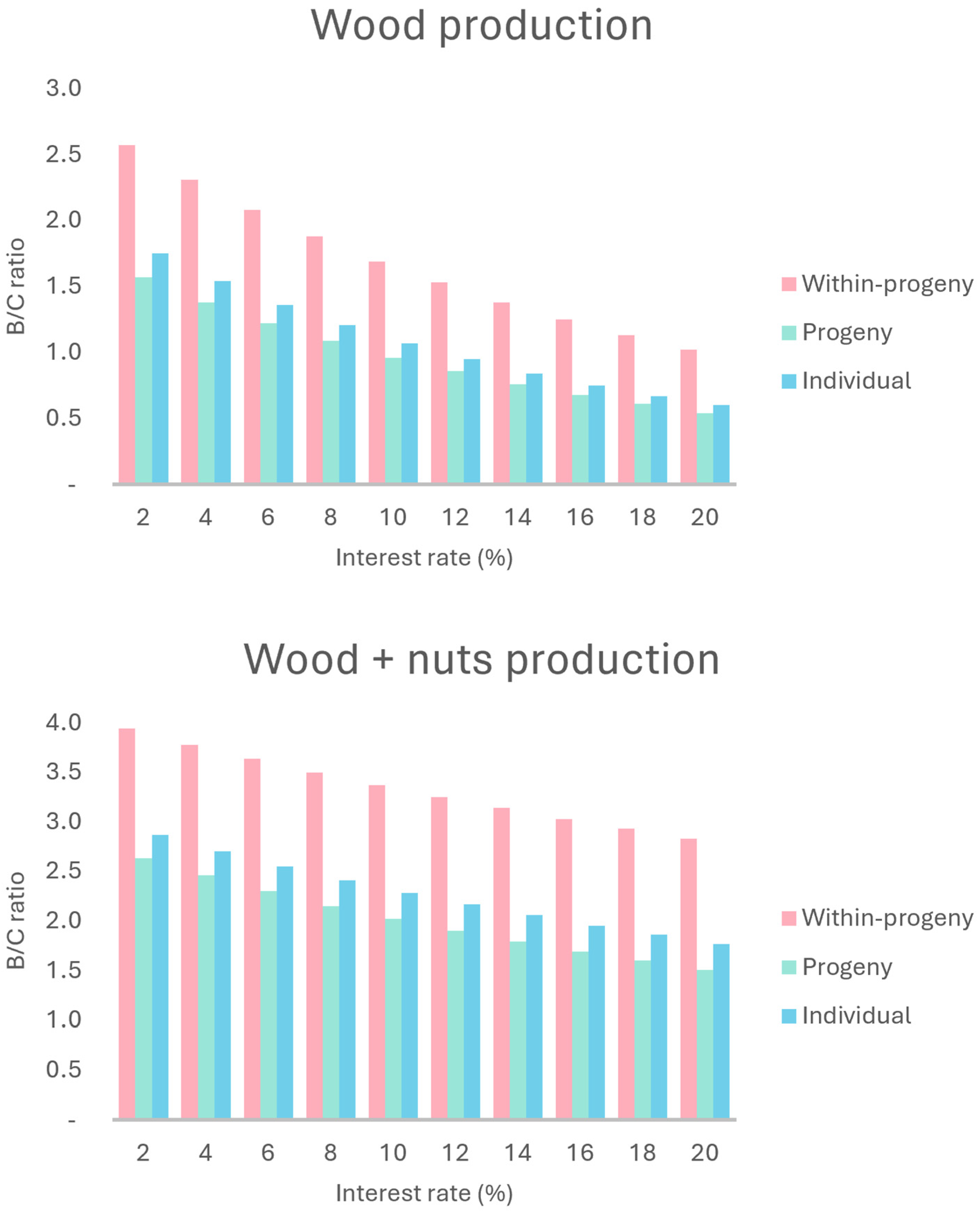
2.2. Economic Evaluation and Sensitivity Analysis of Production Scenarios
2.2.1. Wood Production
2.2.2. Wood + Nuts Production
3. Discussion
3.1. Optimal Point between Genetic Gain and Diversity
3.2. Economic Evaluation and Sensitivity Analysis of Wood Production Scenarios
3.3. Economic Evaluation and Sensitivity Analysis of Wood and Nuts Production Scenarios
4. Materials and Methods
4.1. Provenance and Progeny Test
4.2. Simulation of Selection Strategies
4.3. Simulations of Wood and Nuts Production
4.4. Economic Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levis, C.; Costa, F.R.; Bongers, F.; Peña-Claros, M.; Clement, C.R.; Junqueira, A.B.; Neves, E.G.; Tamanaha, E.K.; Figueiredo, F.O.G.; Salomão, R.P.; et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 2017, 355, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Levis, C.; Flores, B.M.; Moreira, P.A.; Luize, B.G.; Alves, R.P.; Franco-Moraes, J.; Lins, J.; Konings, E.; Peña-Claros, M.; Bongers, F.; et al. How people domesticated Amazonian forests. Front. Ecol. Evol. 2018, 5, 171. [Google Scholar] [CrossRef]
- Zechini, A.A.; Lauterjung, M.B.; Candido-Ribeiro, R.; Montagna, T.; Bernardi, A.P.; Hoeltgebaum, M.P.; dos Reis, M.S. Genetic conservation of Brazilian pine (Araucaria angustifolia) through traditional land use. Econ. Bot. 2018, 72, 166–179. [Google Scholar] [CrossRef]
- Sallai, R.C.; Salu, B.R.; Silva-Lucca, R.A.; Alves, F.L.; Napoleão, T.H.; Paiva, P.M.G.; Ferreira, R.S.; Sampaio, M.U.; Oliva, M.L.V. Biotechnological potential of Araucaria angustifolia pine nuts extract and the cysteine protease inhibitor AaCI-2S. Plants 2020, 9, 1676. [Google Scholar] [CrossRef]
- Castrillon, R.G.; Helm, C.V.; Mathias, A.L. Araucaria angustifolia and the pinhão seed: Starch, bioactive compounds and functional activity—A bibliometric review. Ciência Rural 2023, 53, e20220048. [Google Scholar] [CrossRef]
- Finger, C.A.G.; Costa, E.A.; Hess, A.F.; Liesenberg, V.; Bispo, P.C. Simulating sustainable forest management practices using crown attributes: Insights for Araucaria angustifolia trees in Southern Brazil. Forests 2023, 14, 1285. [Google Scholar] [CrossRef]
- Enright, N.J.; Hill, R.S. The Southern Conifers: An introduction. In Ecology of the Southern Conifers; Enright, N.J., Hill, R.S., Eds.; Melbourne University Press: Melbourne, Australia, 1995; pp. 1–9. [Google Scholar]
- de Sousa, V.A.; Reeves, P.A.; Reilley, A.; Aguiar, A.V.; Stefenon, V.M.; Richards, C.M. Genetic diversity and biogeographic determinants of population structure in Araucaria angustifolia (Bert.) O. Ktze. Conserv. Genet. 2020, 21, 217–229. [Google Scholar] [CrossRef]
- Inza, M.V.; Aguirre, N.C.; Torales, S.L.; Pahr, N.M.; Fassola, H.E.; Fornes, L.F.; Zelener, N. Genetic variability of Araucaria angustifolia in the Argentinean Parana Forest and implications for management and conservation. Trees 2018, 32, 1135–1146. [Google Scholar] [CrossRef]
- Wrege, M.S.; Sousa, V.A.; Fritzsons, E.; Soares, M.T.S.; Aguiar, A.V. Predicting Current and Future Geographical Distribution of Araucaria in Brazil for Fundamental Niche Modeling. Environ. Ecol. Res. 2016, 4, 269–279. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Santos, K.L.; Siminski, A. Present and future of the critically endangered Araucaria angustifolia due to climate change and habitat loss. Forestry 2020, 93, 401–410. [Google Scholar] [CrossRef]
- Sthapit, B.; Ramesh, V.; Villupanoor, P.; Rajan, S.; Arsanti, I.W.; Idris, S.; Rao, V.R. On-farm/In Situ Conservation of Tropical Fruit Tree Diversity: Emerging Concepts and Practices. Indian J. Plant Genet. Resour. 2016, 29, 285–288. [Google Scholar] [CrossRef]
- Reis, M.S.; Montagna, T.; Mattos, A.G.; Filippon, S.; Ladio, A.H.; Marques, A.d.C.; Zechini, A.A.; Peroni, N.; Mantovani, A. Domesticated landscapes in Araucaria Forests, Southern Brazil: A multispecies local conservation-by-use system. Front. Ecol. Evol. 2018, 6, 11. [Google Scholar] [CrossRef]
- Li, D.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Kashimshetty, Y.; Pelikan, S.; Rogstad, S.H. Effective seed harvesting strategies for the ex situ genetic diversity conservation of rare tropical tree populations. Biodivers. Conserv. 2017, 26, 1311–1331. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Domingues, C.R.D.S.; Poggere, G.C.; Motta, A.C.V.; Reis, A.R.D.; Moraes, M.F.D.; Prior, S.A. Elemental composition and nutritional value of Araucaria angustifolia seeds from subtropical Brazil. J. Food Sci. Technol. 2019, 56, 1073–1077. [Google Scholar] [CrossRef]
- Resende, R.; Tanno, P.; Silva Junior, O.B.; Aguiar, A.V.; Sousa, V.A.; Sebbenn, A.; Grattapaglia, D. Genetic analysis of growth curves of individual trees and country-wide provenances of Araucaria angustifolia shows huge potential for enhanced domestication, breeding and conservation of this iconic Brazilian conifer. In Embrapa Florestas-Resumo em Anais de Congresso (ALICE); Pesquisa Florestal Brasileira: Colombo, Brazil, 2019; Volume 39, p. 48. [Google Scholar]
- Sousa, V.A.; Fritzsons, E.; Pinto Júnior, J.E.; Aguiar, A.V. Araucária: Pesquisa e Desenvolvimento no Brasil, 1st ed.; Embrapa: Brasília, Brazil, 2021; p. 360. [Google Scholar]
- Mattos, P.; De Angeli Curto, R.; Muñoz Braz, E.; Péllico Netto, S. How do Araucaria angustifolia trees grow in overstocked stands? Dendrochronologia 2022, 74, 125976. [Google Scholar] [CrossRef]
- Hess, A.F.; Silveira, A.C.; Krefta, S.M.; Santos, D.V.; Filho, M.D.H.V.; Atanazio, K.A.; Schorr, L.P.B.; Souza, I.d.A.; Borsoi, G.A.; Stepka, T.F.; et al. Crown dynamics of Brazilian pine (Araucaria angustifolia) in Santa Catarina region of Brazil. Aust. J. Crop Sci. 2018, 12, 449. [Google Scholar] [CrossRef]
- Hess, A.F.; da Silveira, A.C.; Krefta, S.M.; Santos, D.V.; Vieira Filho, M.D.H.; Atanazio, K.A.; Schorr, L.P.B.; Souza, I.A.; Borsoi, G.A.; Stepka, T.F.; et al. Crown efficiency and pine cones production for Brazilian pine (Araucaria angustifolia (Bertol.) Kuntze) in South Brazil. J. Agric. Sci. 2019, 11, 247. [Google Scholar] [CrossRef]
- Frankham, R.; Bradshaw, C.J.; Brook, B.W. Genetics in conservation management: Revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol. Conserv. 2014, 170, 56–63. [Google Scholar] [CrossRef]
- Orellana, E.; Vanclay, J.K. Could native Araucaria forests be managed for timber production on small farms in southern Brazil? For. Ecol. Manage. 2018, 430, 1–9. [Google Scholar] [CrossRef]
- Routa, J.; Kilpeläinen, A.; Ikonen, V.P.; Asikainen, A.; Venäläinen, A.; Peltola, H. Effects of intensified silviculture on timber production and its economic profitability in boreal Norway spruce and Scots pine stands under changing climatic conditions. Forestry 2019, 92, 648–658. [Google Scholar] [CrossRef]
- David, H.C.; Arce, J.E.; Oliveira, E.B.D.; Netto, S.P.; Miranda, R.O.V.D.; Ebling, Â.A. Economic analysis and revenue optimization in management regimes of Pinus taeda. Rev. Ceres 2017, 64, 222–231. [Google Scholar] [CrossRef]
- IEA—Instituto de Economia Agrícola. Arrendamento em Dinheiro. 2017. Available online: https://infoiea.agricultura.sp.gov.br/nia1/precor.aspx?cod_tipo=5&cod_sis=12 (accessed on 29 January 2020).
- Setiawan, B.; Lahjie, A.; Yusuf, S.; Ruslim, Y. Model of Community Forest Land Management Production and Financial Simulation of Super Teak, Solomon Teak and Sungkai Trees in Samboja Kutai Kartanegara East Kalimantan, Indonesia. Energy Environ. Res. 2019, 9, 48. [Google Scholar] [CrossRef]
- Oliveira, E.B. Softwares Para Manejo e Análise Econômica de Plantios Florestais; Embrapa Florestas: Colombo, Brasil, 2011; p. 68. [Google Scholar]
- Instituto de Economia Agrícola. Valor de Terra Nua. 2017. Available online: http://ciagri.iea.sp.gov.br/bancoiea_TEste/Precor_TerraNua_SEFAZ.aspx (accessed on 29 January 2020).
- Susaeta, A.; Demers, C. The Optimal Forest Management of an Even-Aged Stand: The Biological Rotation versus the Land Expectation Value: FOR355/FR424. EDIS 2019, 2019, 4. [Google Scholar] [CrossRef]
- Machado, J.A.R.; Bacha, C.J.C. Análise da rentabilidade econômica dos reflorestamentos com essências nativas brasileiras: O caso do Estado de São Paulo. Rev. Econ. Sociol. Rural. 2002, 40, 581–604. [Google Scholar] [CrossRef]
- Souza, I.; Hess, A.F.; Costa, E.A.; Silveira, A.C.; Schorr, L.P.B.; Atanazio, K.A. Development of models to aid decision-making in the management of Araucaria angustifolia (Bertol.) Kuntze. Floresta 2020, 50, 1854–1863. [Google Scholar] [CrossRef]
- Susaeta, A.; Gong, P. Economic viability of longleaf pine management in the Southeastern United States. For. Policy Econ. 2019, 100, 14–23. [Google Scholar] [CrossRef]
- Setiawan, B.; Lahjie, A.M.; Yusuf, S.; Ruslim, Y. Assessing the feasibility of forest plantation of native species: A case study of Agathis dammara and Eusideroxylon zwageri in Balikpapan, East Kalimantan, Indonesia. Biodiversitas 2019, 20, 2453–2461. [Google Scholar] [CrossRef]
- Farinha, A.O.; Durpoix, C.; Valente, S.; Sousa, E.; Roques, A.; Branco, M. The stone pine, Pinus pinea L., a new highly rewarding host for the invasive Leptoglossus occidentalis. NeoBiota 2018, 41, 1–18. [Google Scholar] [CrossRef]
- Maier, T.F.; Benini, R.D.M.; Fachini, C.; Santana, P.J.A.D. Financial analysis of enrichment model using timber and non-timber products of secondary remnants in the Atlantic Forest. Rev. Árvore 2018, 42, 6. [Google Scholar] [CrossRef]
- Hultkrantz, L.; Mantalos, P. Hedging with trees: Tail-hedge discounting of long-term forestry returns. J. For. Econ. 2018, 30, 52–57. [Google Scholar] [CrossRef]
- Jin, X.; Pukkala, T.; Li, F.; Dong, L. Optimal management of Korean pine plantations in multifunctional forestry. J. For. Res. 2017, 28, 1027–1037. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D. Techno-economic analysis of a biomass gasification power plant dealing with forestry residues blends for electricity production in Portugal. J. Clean. Prod. 2019, 212, 741–753. [Google Scholar] [CrossRef]
- Machado, J.A.R.; Aguiar, A.V.; Pontinha, A.A.S.; Souza, B.M.; Sebbenn, A.M.; Hallsworth, J.E.; Freitas, M.L.M. Enhancing genetic fitness while maintaining genetic variability of Araucaria. Sci. For. 2021, 49, e3572. [Google Scholar] [CrossRef]
- Herrmann, T.M. Indigenous Knowledge and Management of Araucaria Araucana Forest in the Chilean Andes: Implications for Native Forest Conservation. Biodivers. Conserv. 2006, 15, 647–662. [Google Scholar] [CrossRef]
- Kainer, K.A.; Wadt, L.H.O.; Staudhammer, C.L. The evolving role of Bertholletia excelsa in Amazonia: Contributing to local livelihoods and forest conservation. Desenvolv. Meio Ambiente 2018, 48, 477–497. [Google Scholar] [CrossRef]
- Sebbenn, A.M. Tamanho amostral para conservação ex situ de espécies arbóreas com sistema misto de reprodução. Rev. Inst. Florest. 2003, 15, 147–162. [Google Scholar] [CrossRef]
- Rossi, M. Mapa Pedológico do Estado de São Paulo: Revisado e Ampliado; Inclui Mapas; Instituto Florestal: São Paulo, Brazil, 2017; Volume 1, p. 118. [Google Scholar]
- Sanquetta, C.R.; Dolci, M.C.; Corte, A.P.D.; Sanquetta, M.N.I.; Pelissari, A.L. Estimação de volumes de Araucaria angustifolia (Bertol.) O. Kuntze por fatores de forma em classes diamétricas e modelos de regressão. In Enciclopédia Biosfera; Centro Científico Conhecer: Goiânia, Brazil, 2016; Volume 13, pp. 588–597. [Google Scholar]
- Mattos, J.R. O Pinheiro Brasileiro, 2nd ed.; Artes Gráficas Princesa: Lajes, Brazil, 1994; p. 225. [Google Scholar]
- Paludo, G.F.; Mantovani, A.; Klauberg, C.; Reis, M.S. Estrutura demográfica e padrão espacial de uma população natural de Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae) em Santa Catarina. Rev. Árvore 2009, 33, 1109–1121. [Google Scholar] [CrossRef]
- Zanon, M.L.B.; Finger, C.A.G.; Schneider, P.R. Proporção da dióicia e distribuição diamétrica de árvores masculinas e femininas de Araucaria angustifolia (Bert.) Kuntze, em povoamentos implantados. Ciência Florest. 2009, 19, 425–431. [Google Scholar] [CrossRef][Green Version]
- David, A.; Pike, C.; Stine, R. Comparison of selection methods for optimizing genetic gain and gene diversity in a red pine (Pinus resinosa Ait.) seedling seed orchard. Theor. Appl. Genet. 2003, 107, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Filho, A.; Orellan, E.; Nascimento, F.; Dias, A.N.; Inoue, M.T. Produção de sementes de Araucaria angustifolia em plantio e em floresta natural no centro-sul do Estado do Paraná. Floresta 2011, 41, 155–162. [Google Scholar] [CrossRef]
- SEAB/DERAL—Secretaria de Estado da Agricultura e Abastecimento/Departamento de Economia Rural. Boletim do Pinhão. 2017. Available online: http://www.agricultura.pr.gov.br (accessed on 28 February 2020).
- Zhang, Y.; Majumdar, S. Land expectation value to profit maximization: Re-examination of the Faustmann Formula. In Post-Faustmann Forest Resource Economics; Springer: Dordrecht, The Netherlands, 2013; pp. 277–287. [Google Scholar]
- Machado Neto, A.D.S.; Jasmim, J.M.; Ponciano, N.J. Economic indicators of tropical flower production in Rio de Janeiro State. Rev. Ceres 2013, 60, 173–184. [Google Scholar] [CrossRef]
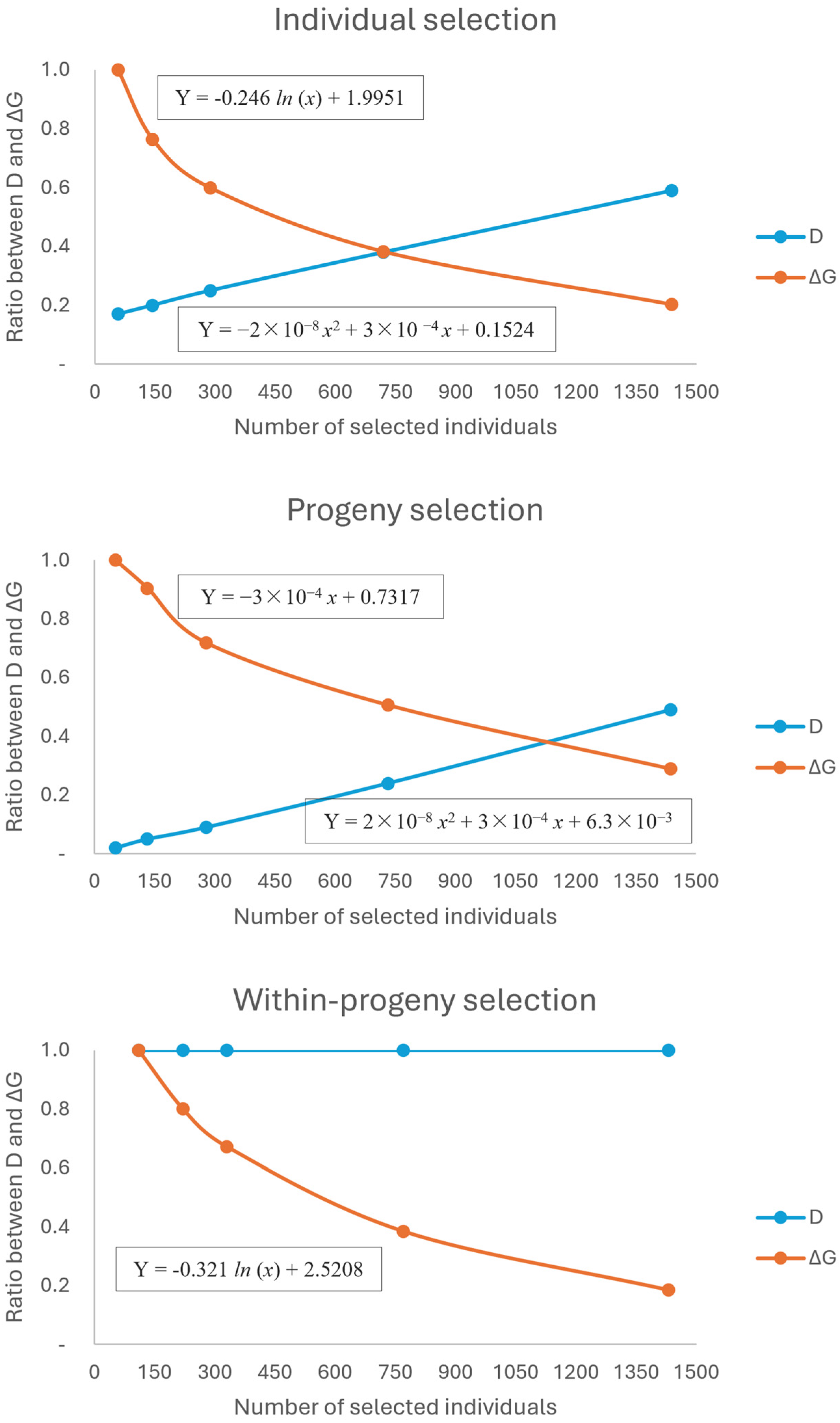
| Selection Method | Number of Individuals | Genetic Gain (%) | Diversity | WV33 [MAI] | FWV40 [MAI] | TWV |
|---|---|---|---|---|---|---|
| Individual | 750 | 45.6 | 0.37 | 58.3 [3.1] | 89.5 [3.7] | 147.8 |
| progeny | 1164 | 28.6 | 0.38 | 42.2 [3.1] | 113.4 [3.9] | 155.6 |
| Within-progeny | 110 | 54.9 | 1.00 | 85.6 [3.1] | 49.9 [3.4] | 135.5 |
| Parameter | Production System by Optimization Scenario | |||||
|---|---|---|---|---|---|---|
| Wood | Wood + Nuts | |||||
| Individual | Progeny | Within-Progeny | Individual | Progeny | Within-Progeny | |
| Nis | 375 | 582 | 110 | 375 | 582 | 110 |
| ΔG | 45.6 | 28.6 | 44.0 | 45.6 | 28.6 | 44.0 |
| D | 0.37 | 0.38 | 1.00 | 0.37 | 0.38 | 1.00 |
| TR | 2545.63 | 3206.75 | 2106.57 | 6554.18 | 7215.31 | 6115.12 |
| TC | 1399.82 | 1807.58 | 738.57 | 2151.70 | 2559.46 | 1490.44 |
| TNR | 1145.81 | 1399.17 | 1368.00 | 4402.48 | 4655.84 | 4624.67 |
| NPV | 169.66 | 147.65 | 574.72 | 2678.86 | 2656.85 | 3083.93 |
| ANPV | 32.59 | 28.36 | 110.39 | 514.54 | 510.31 | 592.34 |
| B/C | 1.13 | 1.09 | 1.88 | 2.41 | 2.15 | 3.50 |
| LEV | 407.34 | 354.50 | 1379.86 | 6431.69 | 6378.86 | 7404.22 |
| IRR | 10.09 | 9.38 | 20.44 | 49.74 | 37.46 | 130.75 |
| EPB | 7 | 7 | 7 | 3 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, J.A.R.; Freitas, M.L.M.; Paiva, D.I.; Souza, B.M.d.; Sousa, V.A.D.; Martins, K.; Oliveira, E.B.; Aguiar, A.V.D. Economic Evaluation of Conservation through Use of an Araucaria angustifolia Provenance and Progeny Test. Plants 2024, 13, 2580. https://doi.org/10.3390/plants13182580
Machado JAR, Freitas MLM, Paiva DI, Souza BMd, Sousa VAD, Martins K, Oliveira EB, Aguiar AVD. Economic Evaluation of Conservation through Use of an Araucaria angustifolia Provenance and Progeny Test. Plants. 2024; 13(18):2580. https://doi.org/10.3390/plants13182580
Chicago/Turabian StyleMachado, José Arimatéia Rabelo, Miguel Luiz Menezes Freitas, Daniela Ivana Paiva, Bruno Marchetti de Souza, Valderês Aparecida De Sousa, Karina Martins, Edilson Batista Oliveira, and Ananda Virginia De Aguiar. 2024. "Economic Evaluation of Conservation through Use of an Araucaria angustifolia Provenance and Progeny Test" Plants 13, no. 18: 2580. https://doi.org/10.3390/plants13182580
APA StyleMachado, J. A. R., Freitas, M. L. M., Paiva, D. I., Souza, B. M. d., Sousa, V. A. D., Martins, K., Oliveira, E. B., & Aguiar, A. V. D. (2024). Economic Evaluation of Conservation through Use of an Araucaria angustifolia Provenance and Progeny Test. Plants, 13(18), 2580. https://doi.org/10.3390/plants13182580






