Comprehensive Identification and Expression Analysis of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Brachypodium distachyon
Abstract
1. Introduction
2. Results
2.1. Identification of MATE Genes in the Brachypodium distachyono Genomes
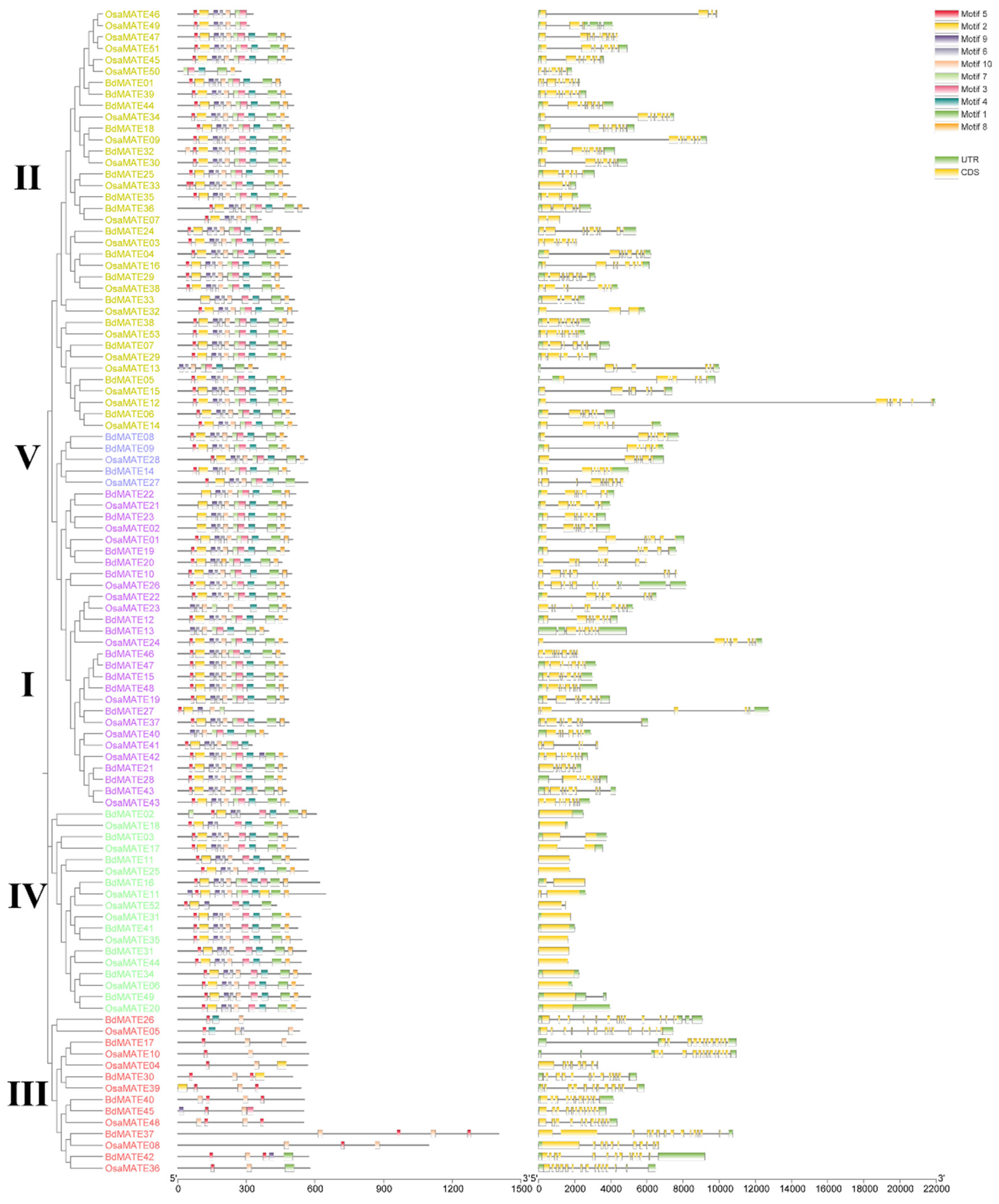
2.2. Phylogenetic Analysis and Structural Characterization of BdMATE Genes
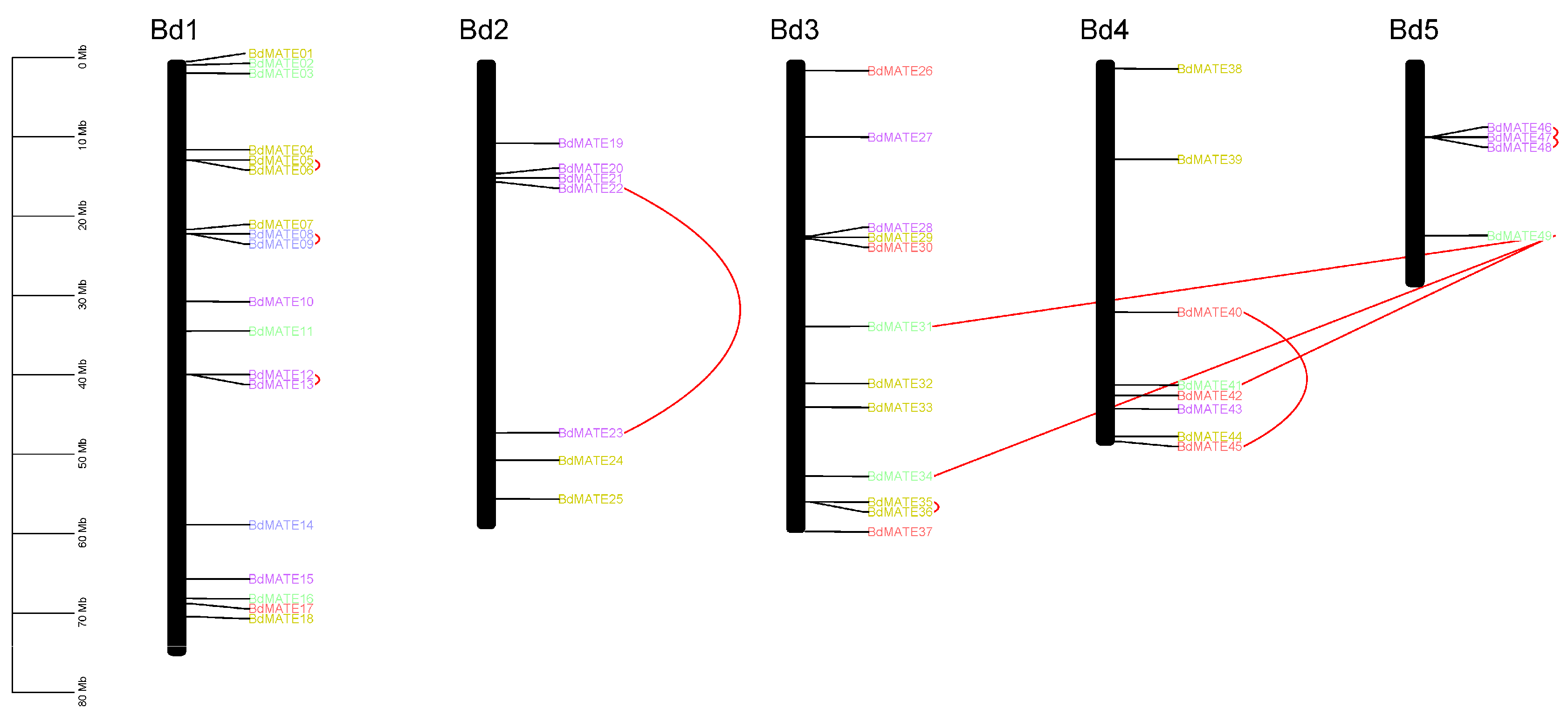
2.3. Chromosomal Distribution and Duplication of Brachypodium distachyon MATE Genes
2.4. Three-Dimensional Structure Prediction of BdMATEs
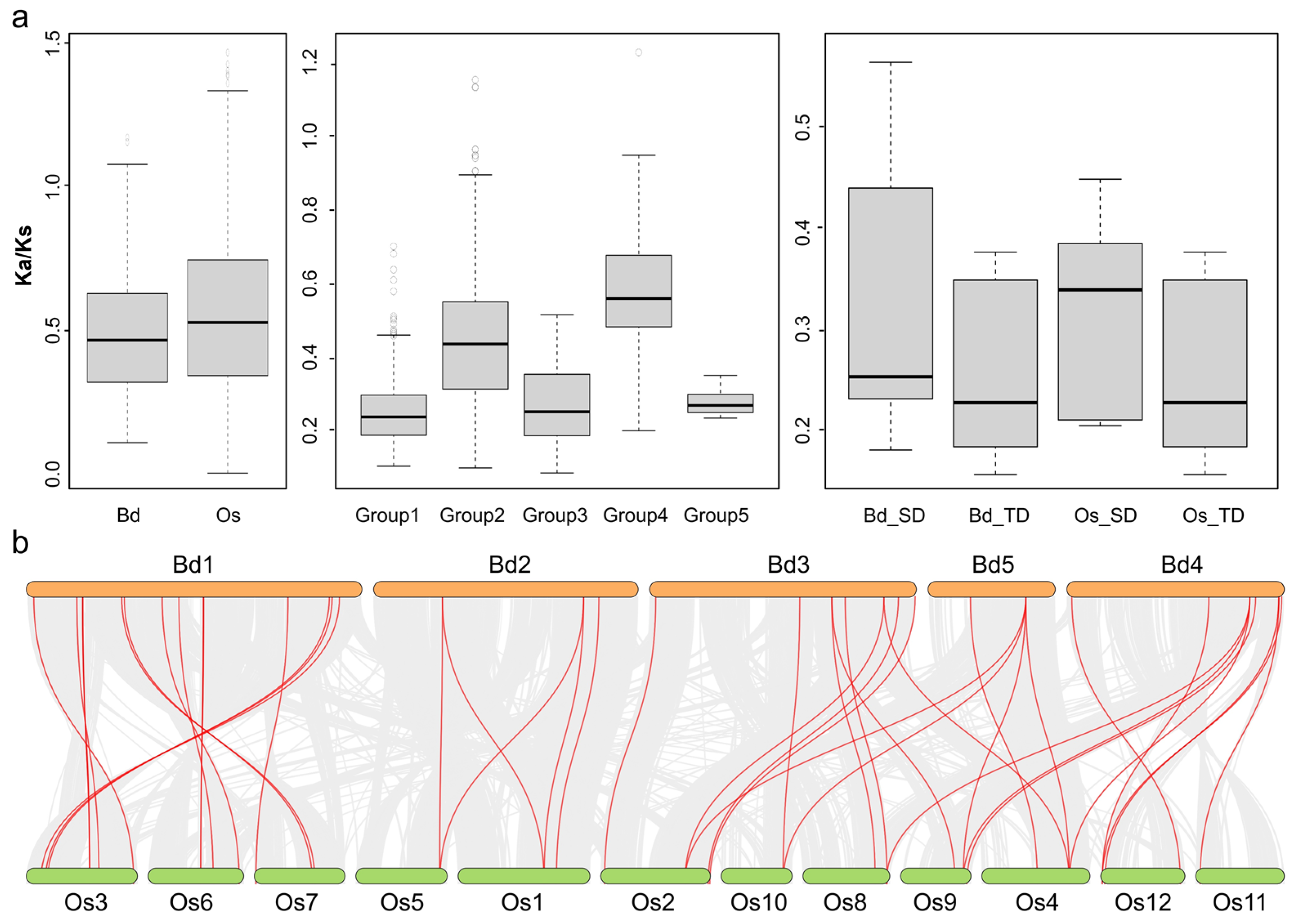
2.5. Collinearity Analysis of MATE Genes between Brachypodium distachyon and Oryza sativa
2.6. Analysis of cis-Regulatory Elements in MATE Promoters
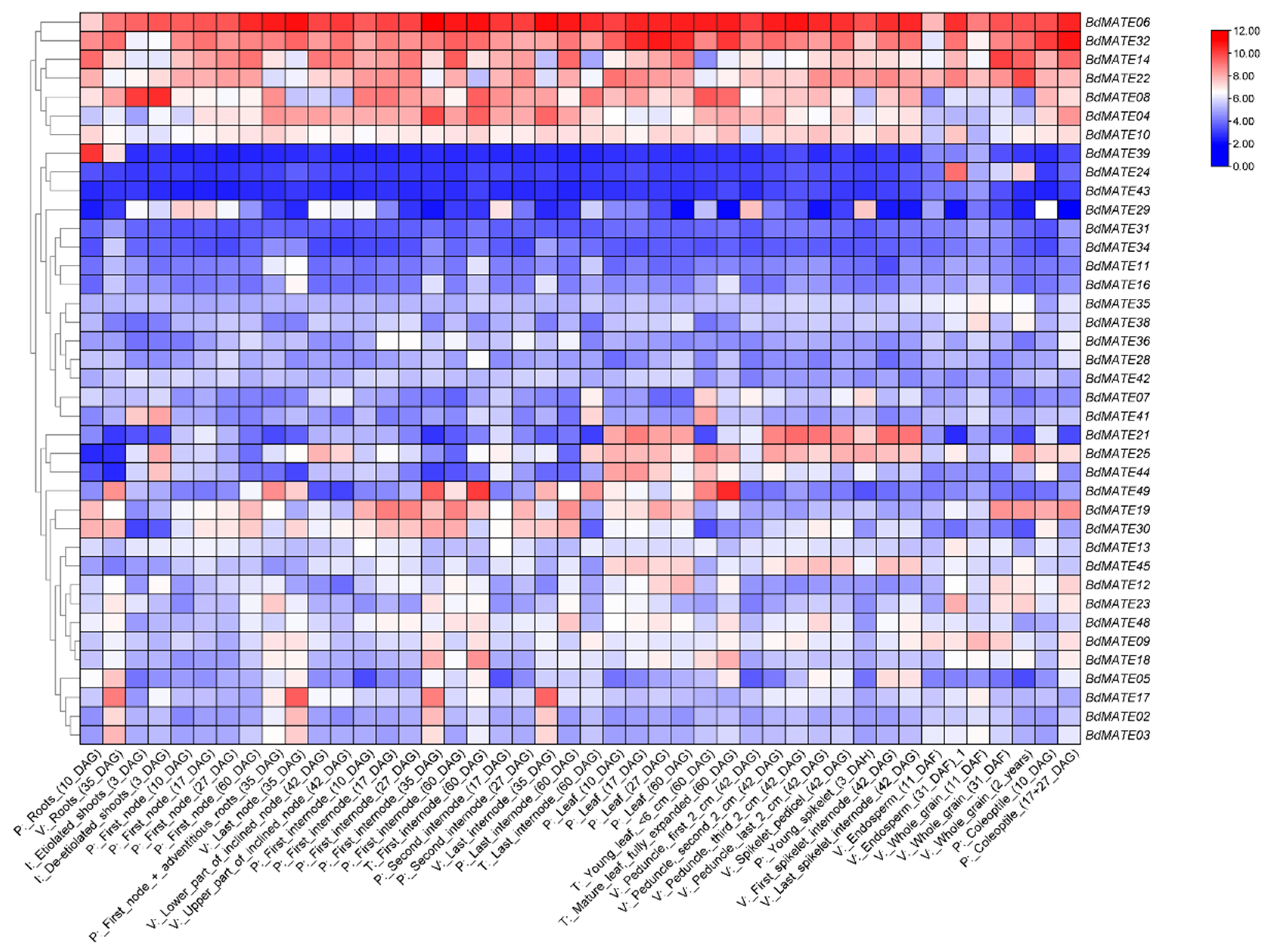
2.7. Analysis of BdMATE Gene Expression Patterns
2.8. Expression Analysis of Phytohormone-Treated BdMATE Genes
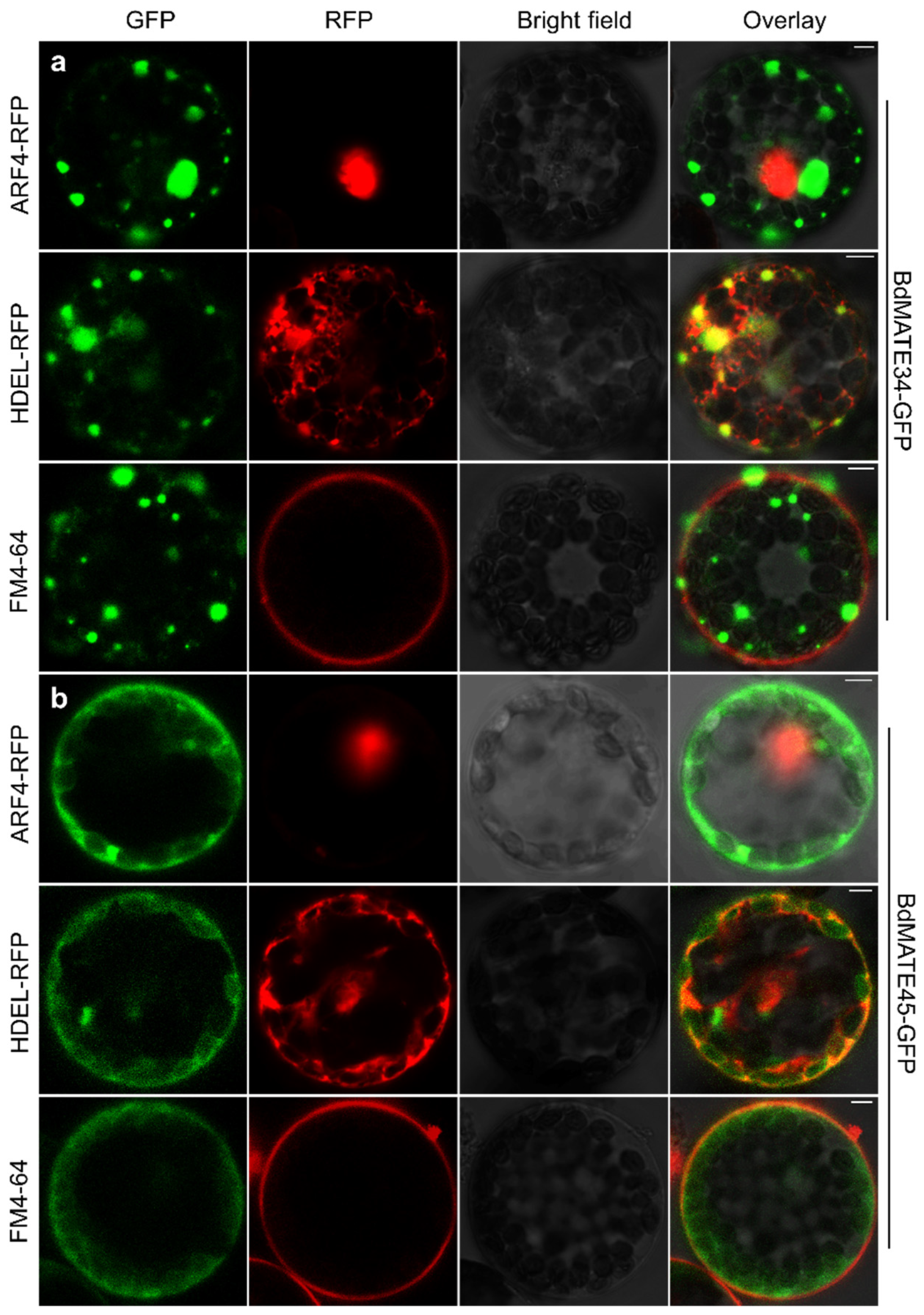
2.9. BdMATE Genes Subcellular Location
3. Discussion
3.1. MATE Gene Family Conservation in the Poaceae
3.2. Tandem Duplications Contributed to MATE Gene Expansion in Brachypodium distachyon
3.3. MATE Genes Function and Gene Expression
4. Materials and Methods
4.1. MATE Gene Identification
4.2. Analysis of Gene Structure and Domain Architecture
4.3. Prediction of cis-Regulatory Elements in Gene Promoters and Subcellular Localization of Proteins
4.4. Phylogenetic Analysis of MATE Genes
4.5. Analysis of Gene Duplication and Synteny
4.6. Gene Expression Profiling of MATE Genes in Brachypodium distachyon
4.7. Quantitative RT-PCR Analysis
4.8. Subcellular Localization of the BdMATE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| Bd | Brachypodium distachyon |
| CRE | cis-regulatory element |
| GA | gibberellin |
| GSDS | Gene Structure Display Server |
| HMM | hidden Markov model |
| IAA | indole-3-acetic acid |
| Ka | non-synonymous distance |
| Ks | synonymous distance |
| MATE | Multidrug and Toxic Compound Extrusion |
| MeJA | methyl jasmonate acid |
| MEME | multiple EM for motif elicitation |
| MUSCLE | Multiple Sequence Comparison by Log Expectation |
| MW | molecular weight |
| Os | Oryza sativa |
| pI | isoelectric point |
| qRT-PCR | quantitative reverse transcription PCR |
| SA | salicylic acid |
References
- Kar, D.; Pradhan, A.A.; Dutta, A.; Bhagavatula, L.; Datta, S. The Multidrug and Toxic Compound Extrusion (MATE) Family in Plants and Their Significance in Metal Transport. In Plant Metal and Metalloid Transporters; Kumar, K., Srivastava, S., Eds.; Springer Nature: Singapore, 2022; pp. 151–177. [Google Scholar]
- Santos, A.L.D.; Chaves-Silva, S.; Yang, L.; Maia, L.G.S.; Chalfun-Junior, A.; Sinharoy, S.; Zhao, J.; Benedito, V.A. Global analysis of the MATE gene family of metabolite transporters in tomato. BMC Plant Biol. 2017, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007, 446, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006, 27, 587–593. [Google Scholar] [CrossRef]
- Takanashi, K.; Shitan, N.; Yazaki, K. The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 2002, 31, 394–395. [Google Scholar] [CrossRef]
- Miyauchi, H.; Moriyama, S.; Kusakizako, T.; Kumazaki, K.; Nakane, T.; Yamashita, K.; Hirata, K.; Dohmae, N.; Nishizawa, T.; Ito, K.; et al. Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, L.; Liu, D.; Ma, S.; Dai, Y.; Zhang, X.; Wang, Y.; Hu, T.; Xiao, M.; Zhou, Y.; et al. Identification and Expression of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Capsicum annuum and Solanum tuberosum. Plants 2020, 9, 1448. [Google Scholar] [CrossRef]
- Su, Q.; Rong, W.; Zhang, Z. The Pathogen-Induced MATE Gene TaPIMA1 Is Required for Defense Responses to Rhizoctonia cerealis in Wheat. Int. J. Mol. Sci. 2022, 23, 3377. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Sun, M.; Ji, Z.; Zhang, H.; Qiu, D.; Cai, Q.; Xia, Y.; Yuan, X.; Chen, X.; et al. MINI BODY1, encoding a MATE/DTX family transporter, affects plant architecture in mungbean (Vigna radiata L.). Front. Plant Sci. 2022, 13, 1064685. [Google Scholar] [CrossRef]
- Ma, Y.; Li, D.; Zhong, Y.; Wang, X.; Li, L.; Osbourn, A.; Lucas, W.J.; Huang, S.; Shang, Y. Vacuolar MATE/DTX protein-mediated cucurbitacin C transport is co-regulated with bitterness biosynthesis in cucumber. New Phytol. 2023, 238, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Gani, U.; Nautiyal, A.K.; Kundan, M.; Rout, B.; Pandey, A.; Misra, P. Two homeologous MATE transporter genes, NtMATE21 and NtMATE22, are involved in the modulation of plant growth and flavonol transport in Nicotiana tabacum. J. Exp. Bot. 2022, 73, 6186–6206. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Yang, X.; Tang, L.; Wan, C.; Liu, J.; Chen, C.; Zhang, H.; He, C.; Liu, C.; et al. MATE transporter GFD1 cooperates with sugar transporters, mediates carbohydrate partitioning and controls grain-filling duration, grain size and number in rice. Plant Biotechnol. J. 2023, 21, 621–634. [Google Scholar] [CrossRef]
- Dong, B.; Meng, D.; Song, Z.; Cao, H.; Du, T.; Qi, M.; Wang, S.; Xue, J.; Yang, Q.; Fu, Y. CcNFYB3-CcMATE35 and LncRNA CcLTCS-CcCS modules jointly regulate the efflux and synthesis of citrate to enhance aluminium tolerance in pigeon pea. Plant Biotechnol. J. 2023, 22, 181–199. [Google Scholar] [CrossRef]
- Duan, W.; Lu, F.; Cui, Y.; Zhang, J.; Du, X.; Hu, Y.; Yan, Y. Genome-Wide Identification and Characterisation of Wheat MATE Genes Reveals Their Roles in Aluminium Tolerance. Int. J. Mol. Sci. 2022, 23, 4418. [Google Scholar] [CrossRef]
- Singh, D.; Tripathi, A.; Mitra, R.; Bhati, J.; Rani, V.; Taunk, J.; Singh, D.; Yadav, R.K.; Siddiqui, M.H.; Pal, M. Genome-wide identification of MATE and ALMT genes and their expression profiling in mungbean (Vigna radiata L.) under aluminium stress. Ecotoxicol. Environ. Saf. 2024, 280, 116558. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Chen, X.; Song, B.; Liu, H.; Li, J.; Wang, R.; Wu, J. Genome-wide identification of the MATE gene family and functional characterization of PbrMATE9 related to anthocyanin in pear. Hortic. Plant J. 2023, 9, 1079–1094. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, L.; Xu, J.; Han, Y.; Li, L. Genome-wide identification, characterization and expression analysis of MATE family genes in apple (Malus × domestica Borkh). BMC Genom. 2021, 22, 632. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Haq, F.; Arif, S.; Abdullah, M.; Niu, Q.; Zhang, Y. Genome-wide identification and comparative analysis of MATE gene family in Cucurbitaceae species and their regulatory role in melon (Cucumis melo) under salt stress. Hortic. Environ. Biotechnol. 2022, 63, 595–612. [Google Scholar] [CrossRef]
- Khan, D.; Hui, L.; Khokhar, A.A.; Hussain, M.A.; Lv, W.; Zaman, Q.U.; Wang, H.-F. Functional characterization of MATE gene family under abiotic stresses and melatonin-mediated tolerance in dragon fruit (Selenicereus undatus L.). Plant Stress 2024, 11, 100300. [Google Scholar] [CrossRef]
- Shijili, M.; Valsalan, R.; Mathew, D. Genome wide identification and characterization of MATE family genes in mangrove plants. Genetica 2023, 151, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, G.; Tian, W.; Li, D.; Meng, L.; Wu, D.; He, T. Genome-Wide Identification of MATE Gene Family in Potato (Solanum tuberosum L.) and Expression Analysis in Heavy Metal Stress. Front. Genet. 2021, 12, 650500. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hou, Y.; Wang, X.; Li, Y.; Wu, J.; Lou, H. Genome-Wide Identification, Expression Analysis under Abiotic Stress and Co-Expression Analysis of MATE Gene Family in Torreya grandis. Int. J. Mol. Sci. 2024, 25, 3859. [Google Scholar] [CrossRef]
- International Brachypodium, I. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 2010, 463, 763–768. [Google Scholar] [CrossRef]
- Garvin, D.; Gu, Y.Q.; Hasterok, R.; Hazen, S.; Jenkins, G.; Mockler, T.; Mur, L.; Vogel, J.P. Development of Genetic and Genomic Research Resources for Brachypodium distachyon, a New Model System for Grass Crop Research. Crop Sci. 2008, 48 (Suppl. S1), S-69–S-84. [Google Scholar] [CrossRef]
- Hasterok, R.; Catalan, P.; Hazen, S.P.; Roulin, A.C.; Vogel, J.P.; Wang, K.; Mur, L.A.J. Brachypodium: 20 years as a grass biology model system; the way forward? (vol 27, pg 1002, 2022). Trends Plant Sci. 2023, 28, 252–253. [Google Scholar] [CrossRef]
- Wang, L.; Bei, X.; Gao, J.; Li, Y.; Yan, Y.; Hu, Y. The similar and different evolutionary trends of MATE family occurred between rice and Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Juliao, M.H.M.; Silva, S.R.; Ferro, J.A.; Varani, A.M. A Genomic and Transcriptomic Overview of MATE, ABC, and MFS Transporters in Citrus sinensis Interaction with Xanthomonas citri subsp. citri. Plants 2020, 9, 794. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef]
- Nei, M. The new mutation theory of phenotypic evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 12235–12242. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Szewczyk, P.; Karyakin, A.; Evin, M.; Hong, W.X.; Zhang, Q.; Chang, G. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 2010, 467, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Kusakizako, T.; Miyauchi, H.; Ishitani, R.; Nureki, O. Structural biology of the multidrug and toxic compound extrusion superfamily transporters. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools—An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Sibout, R.; Proost, S.; Hansen, B.O.; Vaid, N.; Giorgi, F.M.; Ho-Yue-Kuang, S.; Legée, F.; Cézart, L.; Bouchabké-Coussa, O.; Soulhat, C.; et al. Expression atlas and comparative coexpression network analyses reveal important genes involved in the formation of lignified cell wall in Brachypodium distachyon. New Phytol. 2017, 215, 1009–1025. [Google Scholar] [CrossRef]
- Yu, L.; Liu, D.; Chen, S.; Dai, Y.; Guo, W.; Zhang, X.; Wang, L.; Ma, S.; Xiao, M.; Qi, H.; et al. Evolution and Expression of the Membrane Attack Complex and Perforin Gene Family in the Poaceae. Int. J. Mol. Sci. 2020, 21, 5736. [Google Scholar] [CrossRef]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Metraux, J.P. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef]
- Yazaki, K. Transporters of secondary metabolites. Curr. Opin. Plant Biol. 2005, 8, 301–307. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, J.; Jiang, Y.; Jin, J.; Zhou, W.; Wang, Y.; Han, G.; Zhao, Y.; Cheng, B. Genomewide analysis of MATE-type gene family in maize reveals microsynteny and their expression patterns under aluminum treatment. J. Genet. 2016, 95, 691–704. [Google Scholar] [CrossRef]
- Li, N.; Meng, H.; Xing, H.; Liang, L.; Zhao, X.; Luo, K. Genome-wide analysis of MATE transporters and molecular characterization of aluminum resistance in Populus. J. Exp. Bot. 2017, 68, 5669–5683. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Wang, W.; Gai, J.; Li, Y. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genom. 2016, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, Q.; Li, P.; Yang, L.; Sun, X.; Benedito, V.A.; Wen, J.; Chen, B.; Mysore, K.S.; Zhao, J. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J. 2017, 90, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Yang, J.; Wan, Y.; Xiang, S.; Guan, M.; Du, H.; Tang, Z.; Lu, K.; Li, J.; Qu, C. A Genome-Wide Survey of MATE Transporters in Brassicaceae and Unveiling Their Expression Profiles under Abiotic Stress in Rapeseed. Plants 2020, 9, 1072. [Google Scholar] [CrossRef]
- Dong, B.Y.; Niu, L.L.; Meng, D.; Song, Z.H.; Wang, L.T.; Jian, Y.; Fan, X.H.; Dong, M.Z.; Yang, Q.; Fu, Y.J. Genome-wide analysis of MATE transporters and response to metal stress in Cajanus cajan. J. Plant Interact. 2019, 14, 265–275. [Google Scholar] [CrossRef]
- Ali, E.; Saand, M.A.; Khan, A.R.; Shah, J.M.; Feng, S.M.; Ming, C.; Sun, P.L. Genome-wide identification and expression analysis of detoxification efflux carriers (DTX) genes family under abiotic stresses in flax. Physiol. Plant. 2020, 171, 483–501. [Google Scholar] [CrossRef]
- Huang, J.J.; An, W.J.; Wang, K.J.; Jiang, T.H.; Ren, Q.; Liang, W.H.; Wang, H.H. Expression profile analysis of MATE gene family in rice. Biol. Plant. 2019, 63, 556–564. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, J.; Wang, C.; Peng, H.; Zeng, J.; Chen, F. Genome-wide identification and expression pattern analysis of the MATE gene family in carmine radish (Raphanus sativus L.). Gene 2023, 887, 147734. [Google Scholar] [CrossRef]
- Contreras, R.; Figueiras, A.M.; Gallego, F.J.; Benito, C. Brachypodium distachyon: A model species for aluminium tolerance in Poaceae. Funct. Plant Biol. 2014, 41, 1270–1283. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef]
- Masuda, S.; Terada, T.; Yonezawa, A.; Tanihara, Y.; Kishimoto, K.; Katsura, T.; Ogawa, O.; Inui, K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 2006, 17, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Nimmy, M.S.; Kumar, V.; Suthanthiram, B.; Subbaraya, U.; Nagar, R.; Bharadwaj, C.; Jain, P.K.; Krishnamurthy, P. A Systematic Phylogenomic Classification of the Multidrug and Toxic Compound Extrusion Transporter Gene Family in Plants. Front. Plant Sci. 2022, 13, 774885. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yu, C.S.; Shen, Y.O.; Fang, X.D.; Chen, L.; Min, J.M.; Cheng, J.W.; Zhao, S.C.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M.K. Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol. Biol. 2018, 98, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.; Inze, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+ -antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Peeters, A.J.; Leon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.P.; Wilkins, C.; Demidchik, V.; Davies, J.M.; Glover, B.J. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J. Exp. Bot. 2010, 61, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.R.; Lei, B.; Huang, H.L.; Li, J.N.; Yin, J.M.; Tang, Z.L.; Wang, R.; Chen, L. TRANSPARENT TESTA 12 genes from Brassica napus and parental species: Cloning, evolution, and differential involvement in yellow seed trait. Mol. Genet. Genom. 2009, 281, 109–123. [Google Scholar] [CrossRef]
- Gomez, C.; Conejero, G.; Torregrosa, L.; Cheynier, V.; Terrier, N.; Ageorges, A. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 2011, 67, 960–970. [Google Scholar] [CrossRef]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Fournier-Level, A.; Verries, C.; Souquet, J.M.; Mazauric, J.P.; Klein, M.; Cheynier, V.; et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef]
- Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. A rice FRD3-like (OsFRDL1) gene is expressed in the cells involved in long-distance transport. Soil. Sci. Plant Nutr. 2004, 50, 1133–1140. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J. Exp. Bot. 2016, 67, 5485–5494. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009, 149, 297–305. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011, 68, 1061–1069. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Z.Q.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.Q.; Li, G.X.; Wu, Y.R.; Yang, J.L.; Ma, J.F.; et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Shaff, J.; Liang, C.; Jia, X.; Li, Z.; Magalhaes, J.; Kochian, L.V. A promoter-swap strategy between the AtALMT and AtMATE genes increased Arabidopsis aluminum resistance and improved carbon-use efficiency for aluminum resistance. Plant J. 2012, 71, 327–337. [Google Scholar] [CrossRef]
- Rogers, E.E.; Guerinot, M.L. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 2002, 14, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Burko, Y.; Geva, Y.; Refael-Cohen, A.; Shleizer-Burko, S.; Shani, E.; Berger, Y.; Halon, E.; Chuck, G.; Moshelion, M.; Ori, N. From organelle to organ: ZRIZI MATE-Type transporter is an organelle transporter that enhances organ initiation. Plant Cell Physiol. 2011, 52, 518–527. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, J.; Park, M.J.; Kim, Y.S.; Kim, S.G.; Jung, J.H.; Park, C.M. A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem. J. 2012, 442, 551–561. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Liang, S.; Ge, Q.; Li, Y.; Shao, J.; Qi, Y.; An, L.; Yu, F. A subgroup of MATE transporter genes regulates hypocotyl cell elongation in Arabidopsis. J. Exp. Bot. 2015, 66, 6327–6343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qian, C.; Guo, X.; Liu, E.; Mao, K.; Mu, C.; Chen, N.; Zhang, W.; Liu, H. ELS1, a novel MATE transporter related to leaf senescence and iron homeostasis in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 476, 319–325. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef]
- Sun, X.; Gilroy, E.M.; Chini, A.; Nurmberg, P.L.; Hein, I.; Lacomme, C.; Birch, P.R.; Hussain, A.; Yun, B.W.; Loake, G.J. ADS1 encodes a MATE-transporter that negatively regulates plant disease resistance. New Phytol. 2011, 192, 471–482. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Li, S.; Qin, G.; Novak, O.; Pencik, A.; Ljung, K.; Aoyama, T.; Liu, J.; Murphy, A.; et al. ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet. 2014, 10, e1003954. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Jiang, Z.; Gu, H.; Qu, L.J. ADP1 affects abundance and endocytosis of PIN-FORMED proteins in Arabidopsis. Plant Signal. Behav. 2015, 10, e973811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, M.; Liu, X.; Xue, H.; Wu, Y.; Shi, L.; Wang, R.; Chen, Y.; Xu, N.; Zhao, J.; Shao, J.; et al. Noncanonical ATG8-ABS3 interaction controls senescence in plants. Nat. Plants 2019, 5, 212–224. [Google Scholar] [CrossRef]
- Dobritzsch, M.; Lubken, T.; Eschen-Lippold, L.; Gorzolka, K.; Blum, E.; Matern, A.; Marillonnet, S.; Bottcher, C.; Drager, B.; Rosahl, S. MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides. Plant Cell 2016, 28, 583–596. [Google Scholar] [CrossRef]
- Diener, A.C.; Gaxiola, R.A.; Fink, G.R. Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 2001, 13, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Finn, R.D.; Mistry, J.; Schuster-Bockler, B.; Griffiths-Jones, S.; Hollich, V.; Lassmann, T.; Moxon, S.; Marshall, M.; Khanna, A.; Durbin, R.; et al. Pfam: Clans, web tools and services. Nucleic Acids Res. 2006, 34, D247–D251. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Feng, D.R.; Li, W.Y.; Wang, H.B.; Wang, J.F.; Liu, B. Comprehensive and evolutionary analysis of protein tyrosine phosphatases (PTP) in the green plants. Plant Omics 2013, 6, 215–223. [Google Scholar]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Liu, D.; Zhang, X.; Guo, J.; Zhang, T.; Lai, L.; Li, Y.; Chen, Q.; Yu, L. Genome-Wide Analysis of the Membrane Attack Complex and Perforin Genes and Their Expression Pattern under Stress in the Solanaceae. Int. J. Mol. Sci. 2023, 24, 13193. [Google Scholar] [CrossRef]
- Yu, L.J.; Chen, F.; Peng, Y.J.; Xie, L.J.; Liu, D.; Han, M.Q.; Chen, F.; Xiao, S.; Huang, J.C.; Li, J. Arabidopsis thaliana Plants Engineered To Produce Astaxanthin Show Enhanced Oxidative Stress Tolerance and Bacterial Pathogen Resistance. J. Agr. Food Chem. 2019, 67, 12590–12598. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, R.; Lin, Y.; Yang, Y.; Chen, Q.; Li, M.; Zhang, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Genome-Wide Analysis of Polygalacturonase Gene Family Reveals Its Role in Strawberry Softening. Plants 2024, 13, 1838. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhou, P.; Li, C. Genome-Wide Identification and Characterization of RdHSP Genes Related to High Temperature in Rhododendron delavayi. Plants 2024, 13, 1878. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.S.; Liu, D.; Guo, W.; Liu, Z.X.; Zhang, X.; Shi, L.L.; Zhou, D.M.; Wang, L.N.; Kang, K.; Wang, F.Z.; et al. Poaceae-specific β-1,3;1,4-d-glucans link jasmonate signalling to OsLecRK1-mediated defence response during rice-brown planthopper interactions. Plant Biotechnol. J. 2023, 21, 1286–1300. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.; Fan, Y.; Ma, J.; Sun, W.; et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, D1229–D1236. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, Y.; Bao, Y.; Bassham, D.C.; Chen, L.; Chen, Q.-F.; Hou, S.; Hwang, I.; Huang, L.; Lai, Z.; et al. Studying plant autophagy: Challenges and recommended methodologies. Adv. Biotechnol. 2023, 1, 2. [Google Scholar] [CrossRef]
- Yu, W.W.; Chen, Q.F.; Liao, K.; Zhou, D.M.; Yang, Y.C.; He, M.; Yu, L.J.; Guo, D.Y.; Xiao, S.; Xie, R.H.; et al. The calcium-dependent protein kinase CPK16 regulates hypoxia-induced ROS production by phosphorylating the NADPH oxidase RBOHD in Arabidopsis. Plant Cell 2024, 36, 3451–3466. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Guo, Y.; Zhang, T.; Liu, D.; Wang, L.; Hu, R.; Zhou, D.; Zhou, Y.; Chen, Q.; Yu, L. Comprehensive Identification and Expression Analysis of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Brachypodium distachyon. Plants 2024, 13, 2586. https://doi.org/10.3390/plants13182586
Ma S, Guo Y, Zhang T, Liu D, Wang L, Hu R, Zhou D, Zhou Y, Chen Q, Yu L. Comprehensive Identification and Expression Analysis of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Brachypodium distachyon. Plants. 2024; 13(18):2586. https://doi.org/10.3390/plants13182586
Chicago/Turabian StyleMa, Sirui, Yixian Guo, Tianyi Zhang, Di Liu, Linna Wang, Ruiwen Hu, Demian Zhou, Ying Zhou, Qinfang Chen, and Lujun Yu. 2024. "Comprehensive Identification and Expression Analysis of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Brachypodium distachyon" Plants 13, no. 18: 2586. https://doi.org/10.3390/plants13182586
APA StyleMa, S., Guo, Y., Zhang, T., Liu, D., Wang, L., Hu, R., Zhou, D., Zhou, Y., Chen, Q., & Yu, L. (2024). Comprehensive Identification and Expression Analysis of the Multidrug and Toxic Compound Extrusion (MATE) Gene Family in Brachypodium distachyon. Plants, 13(18), 2586. https://doi.org/10.3390/plants13182586






