A Mitogen-Activated Protein Kinase Pathway Is Required for Bacillus amyloliquefaciens PMB05 to Enhance Disease Resistance to Bacterial Soft Rot in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Plants and Bacteria
2.2. Preparation of HrpN Protein
2.3. Disease Control Assay
2.4. ROS Generation and Callose Deposition Assay
2.5. Stomatal Closure Assay
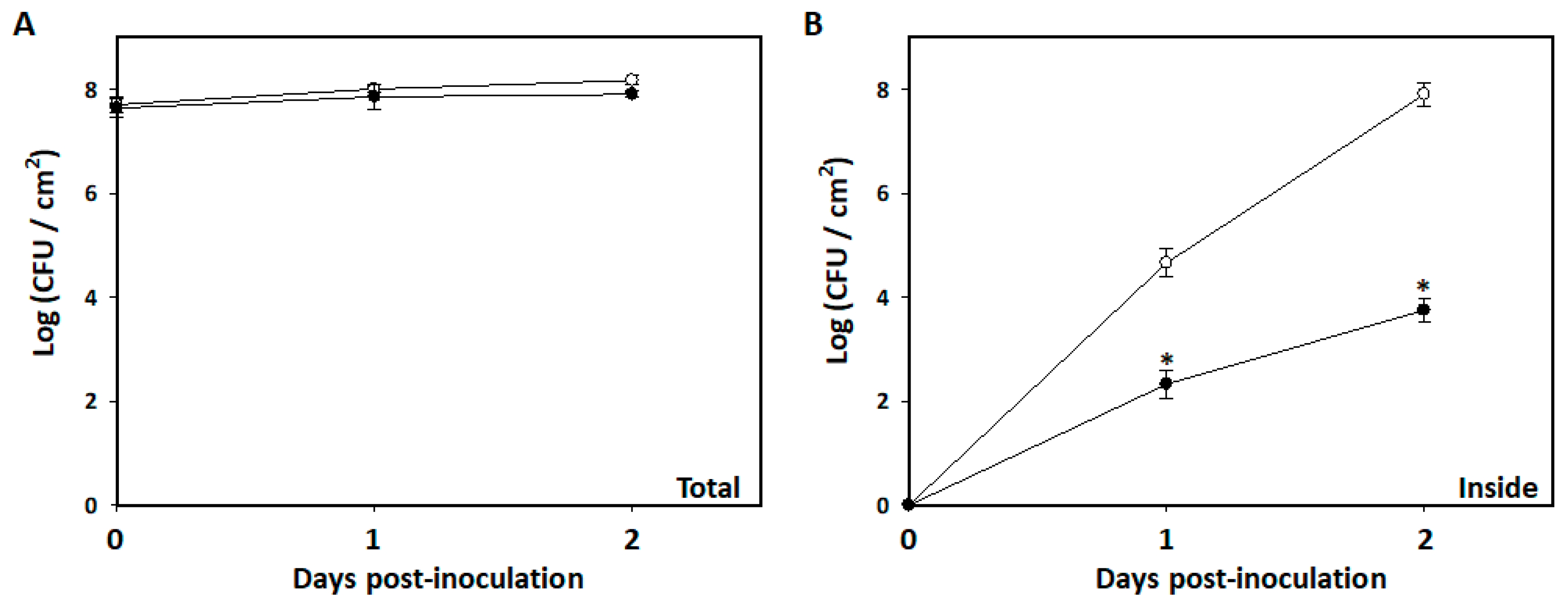
2.6. Population Assay of Soft Rot Bacteria Invading Leaf Tissues
2.7. Data Analysis
3. Results
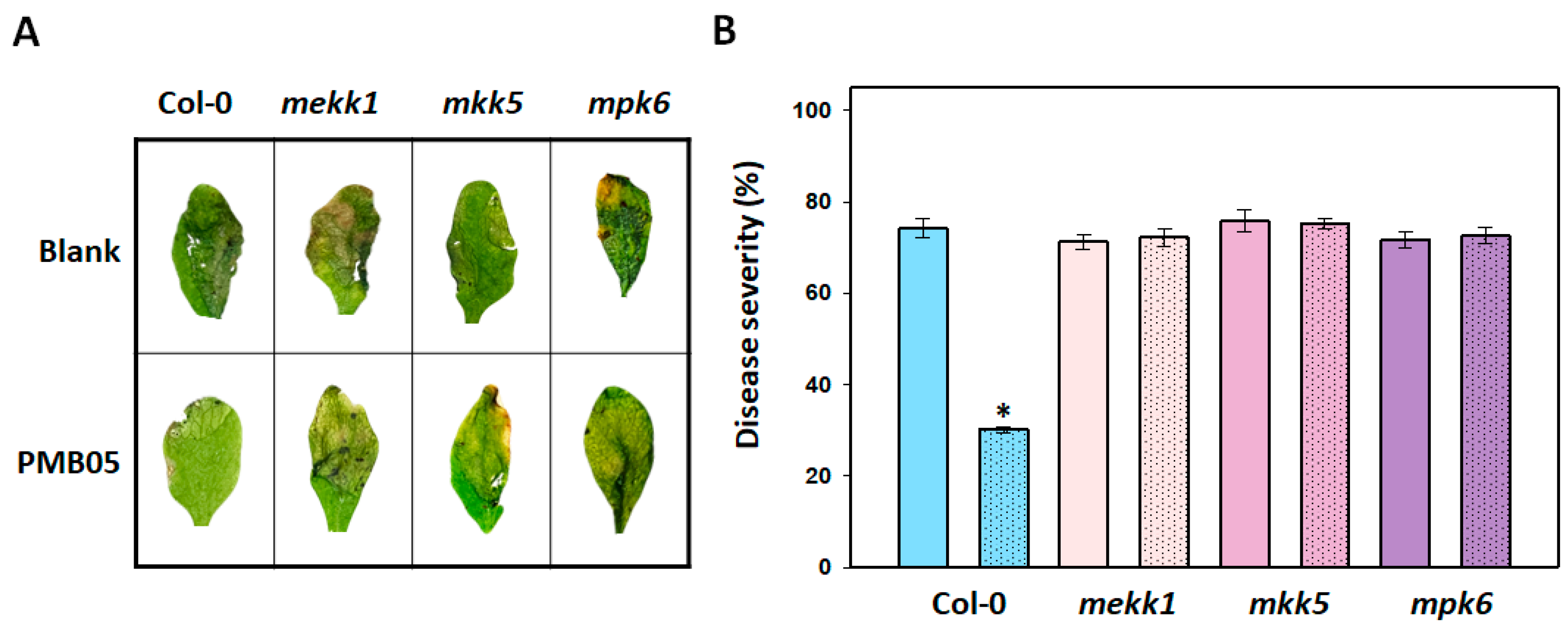
3.1. Effect of Bacillus amyloliquefaciens PMB05 on the Control of Soft Rot in the MAPK Mutants of Arabidopsis thaliana
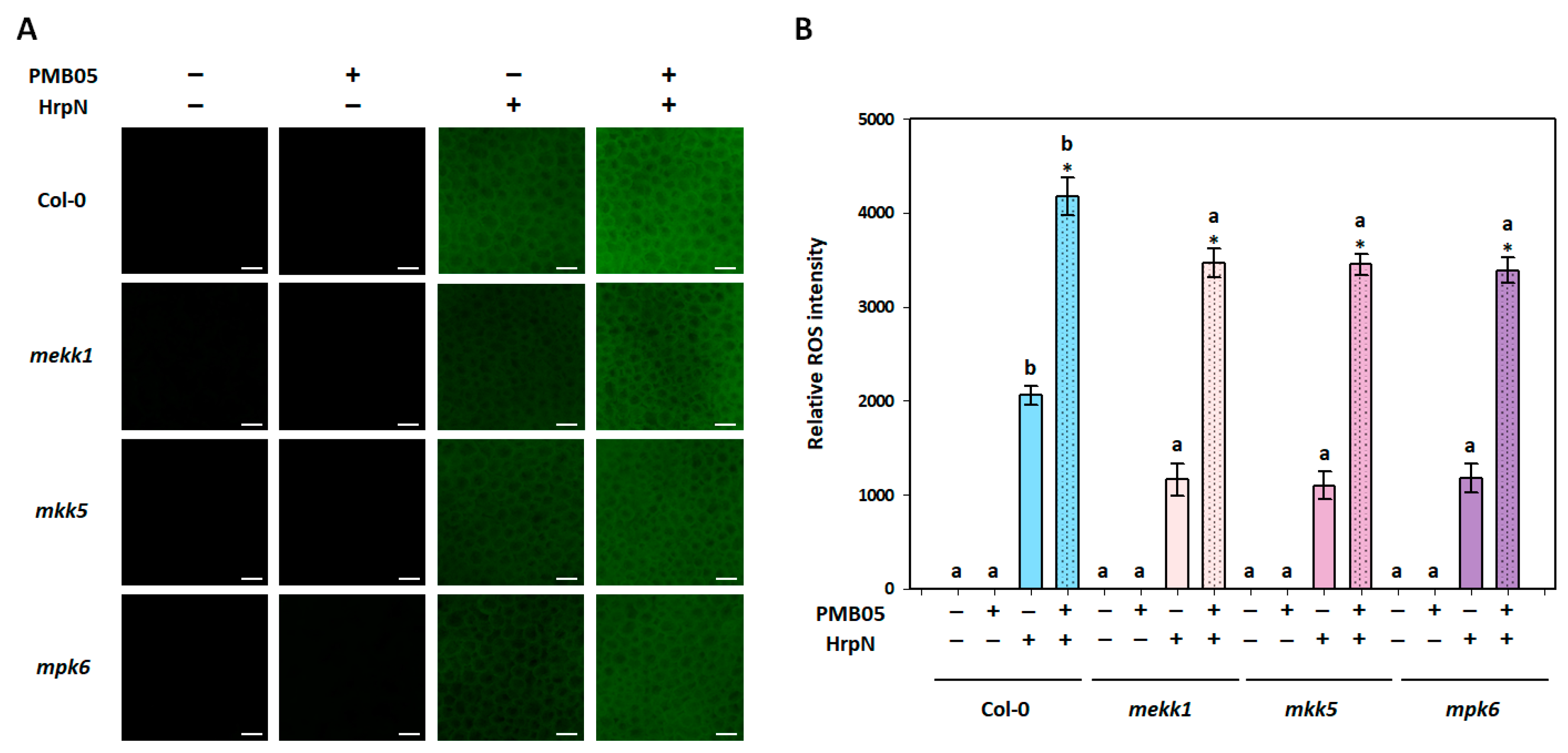
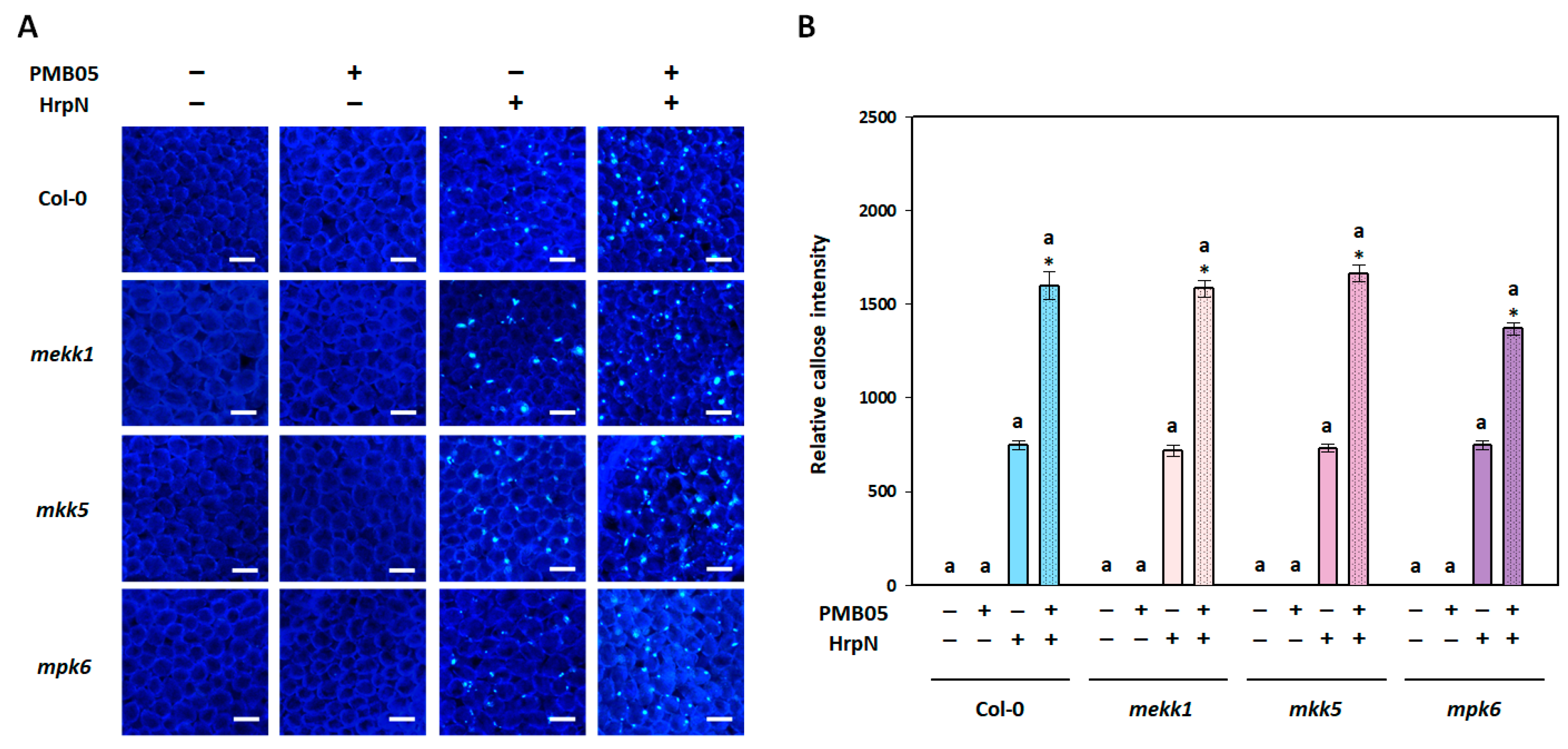
3.2. Intensification of Plant Immune Signals by Bacillus amyloliquefaciens PMB05 in MAPK-Deficient Mutants
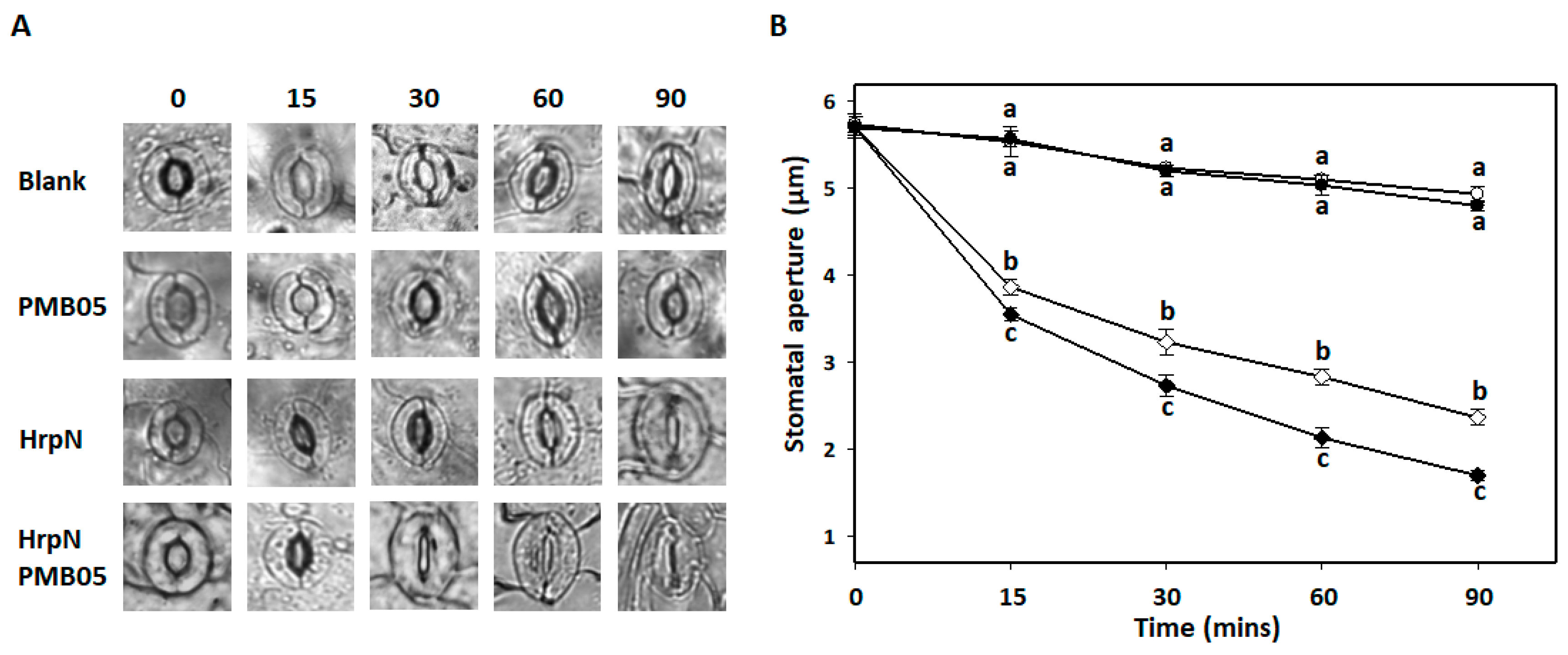
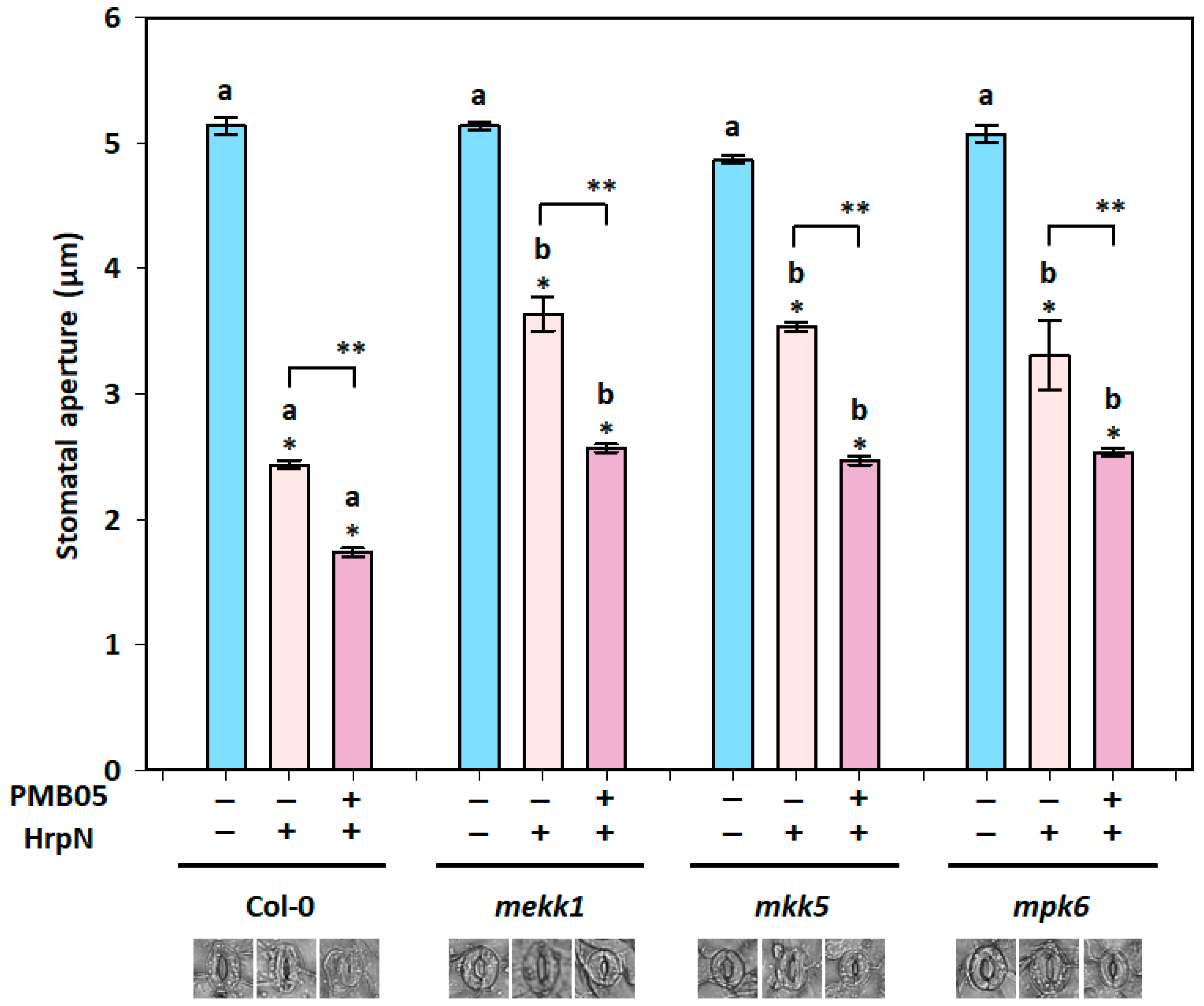
3.3. HrpN-Induced Stomatal Closure Affected by B. amyloliquefaciens PMB05
3.4. Restriction of Bacillus amyloliquefaciens PMB05 on the Invasion of Soft Rot Bacteria into Arabidopsis Leaves
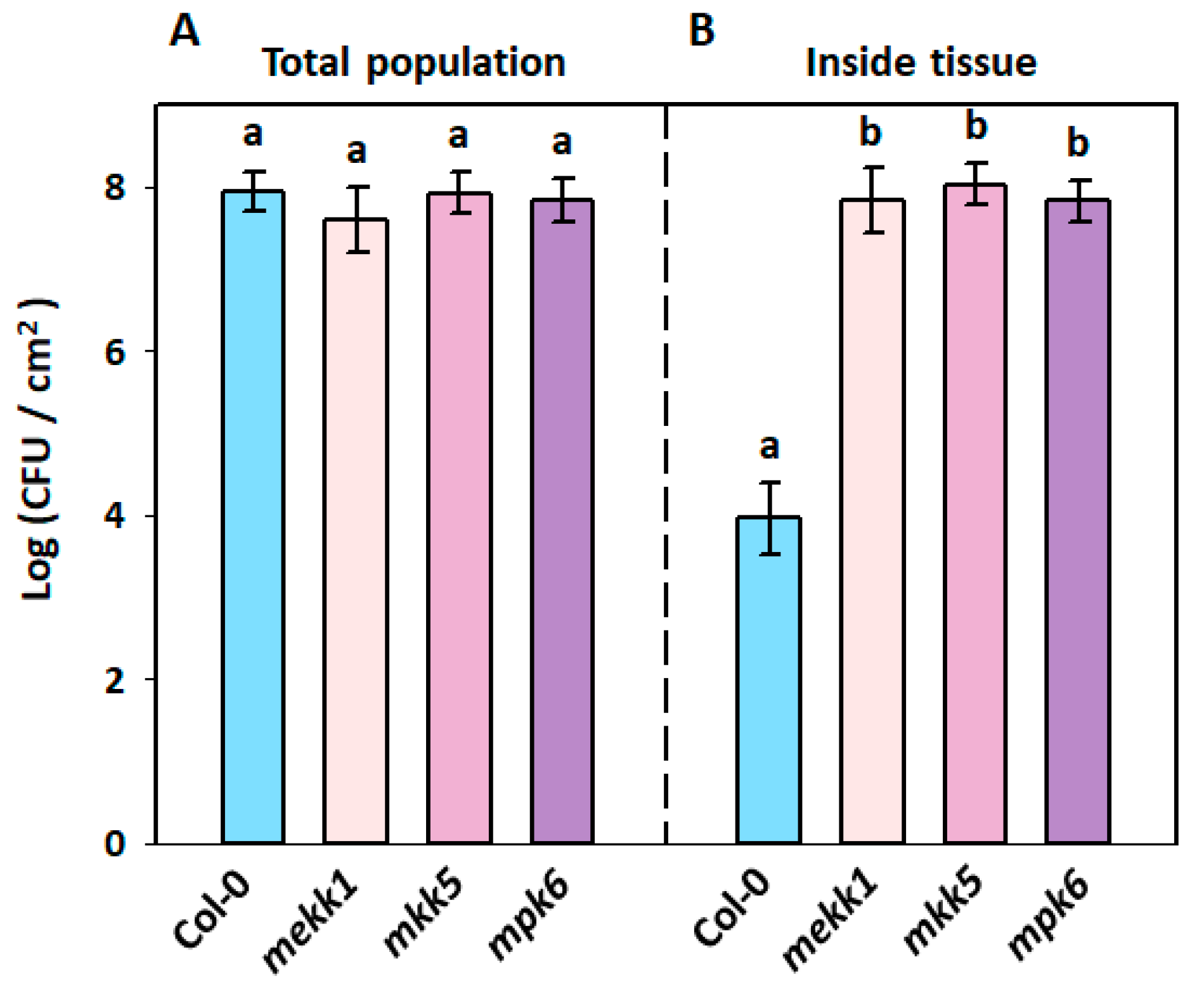
3.5. Restriction of Bacillus amyloliquefaciens PMB05 on Soft Rot Bacteria Invasion in Arabidopsis thaliana MAPK Mutants
3.6. Bacillus amyloliquefaciens PMB05-Accelerated Stomatal Closure upon HrpN Induction in MAPK Mutants
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Charkowski, A.O. The Changing Face of Bacterial Soft-Rot Diseases. Annu. Rev. Phytopathol. 2018, 56, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Hauben, L.; Moore, E.R.B.; Vauterin, L.; Steenacker, M.; Mergaert, J.; Verdonck, L.; Swings, J. Phylogenetic position of phytopathogens within Enterobacteriaceae. Syst. Appl. Microbial. 1998, 21, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, P.J.; Shie, W.T.; Chern, L.L.; Chao, Y.C. Pectobacterium chrysanthemi as the dominant casual agent of bacterial soft rot in Oncidium “Grower Ramsey”. Eur. J. Plant Pathol. 2015, 142, 331–343. [Google Scholar] [CrossRef]
- Azaiez, S.; Ben, S.I.; Karkouch, I.; Essid, R.; Jallouli, S.; Djebali, N.; Elkahoui, S.; Limam, F.; Tabbene, O. Biological control of the soft rot bacterium Pectobacterium carotovorum by Bacillus amyloliquefaciens strain Ar10 producing glycolipid-like compounds. Microbiol. Res. 2018, 217, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Htwe Maung, C.E.; Choub, V.; Cho, J.Y.; Kim, K.Y. Control of the bacterial soft rot pathogen, Pectobacterium carotovorum by Bacillus velezensis CE 100 in cucumber. Microb. Pathog. 2022, 173 Pt A, 105807. [Google Scholar] [CrossRef]
- Zeriouh, H.; Romero, D.; Garcia-Gutierrez, L.; Cazorla, F.M.; de Vicente, A.; Perez-Garcia, A. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef]
- Molina, L.; Constantinescu, F.; Michel, L.; Reimmann, C.; Duffy, B.; Défago, G. Degradation of pathogen quorum-sensing molecules by soil bacteria: A preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 2003, 45, 71–81. [Google Scholar] [CrossRef]
- Pan, J.; Huang, T.; Yao, F.; Huang, Z.; Powell, C.A.; Qiu, S.; Guan, X. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 2008, 163, 711–716. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Lai, I.-L.; Zheng, J.-L.; Lin, Y.-H. Using dynamic changes of chlorophyll fluorescence in Arabidopsis thaliana to evaluate plant immunity-intensifying Bacillus spp. strains. Phytopathology 2019, 109, 1566–1576. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Lin, S.-T.; Li, A.-T.; Li, S.-H.; Hsiao, C.-Y.; Lin, Y.-H. Bacillus amyloliquefaciens PMB05 increases resistance to bacterial wilt by activating MAPK and ROS pathway crosstalk in Arabidopsis thaliana. Phytopathology 2022, 112, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-H.; Chuang, C.-Y.; Zheng, J.-L.; Chen, H.-H.; Liang, Y.-S.; Huang, T.-P.; Lin, Y.-H. Bacillus amyloliquefaciens strain PMB05 intensifies plant immune responses to confer resistance against bacterial wilt of tomato. Phytopathology 2020, 110, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-Y.; Blanco, S.D.; Peng, A.-L.; Fu, J.-Y.; Chen, B.-W.; Luo, M.-C.; Xie, X.-Y.; Lin, Y.-H. Seed Treatment with Calcium Carbonate Containing Bacillus amyloliquefaciens PMB05 Powder Is an Efficient Way to Control Black Rot Disease of Cabbage. Agriculture 2023, 13, 926. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Chen, X.; Wang, F.; Hsiao, C.-Y.; Yang, C.-Y.; Lin, S.-T.; Wu, L.-H.; Chen, Y.-K.; Liang, Y.-S.; Lin, Y.-H. Bacillus amyloliquefaciens strains control strawberry anthracnose through antagonistic activity and plant immune response intensification. Biol. Control 2021, 157, 104592. [Google Scholar] [CrossRef]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.-P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kikuchi, S.; Ohsugi, R. CDPK-mediated abiotic stress signaling. Plant Signal. Behav. 2012, 7, 817–821. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef] [PubMed]
- de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.-M.; Li, Y.-Z.; Zhang, Z.-C.; Wang, Q.-L.; Liang, Y. Silencing the SpMPK1, SpMPK2, and SpMPK3 Genes in Tomato Reduces Abscisic Acid-Mediated Drought Tolerance. Int. J. Mol. Sci. 2013, 14, 21983–21996. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, Z.; Ma, W.; Zhang, S.; Hou, S.; Wei, J.; Dong, S.; Yu, X.; Song, Y.; Gao, W.; et al. Melatonin functions in priming of stomatal immunity in Panax notoginseng and Arabidopsis thaliana. Plant Physiol. 2021, 187, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yang, F.; Li, L.; Chen, H.; Chen, S.; Zhang, J. The Arabidopsis Transcription Factor BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1 Is a Direct Substrate of MITOGEN-ACTIVATED PROTEIN KINASE6 and Regulates Immunity. Plant Physiol. 2015, 167, 1076–1086. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.-M. Plant Immunity Triggered by Microbial Molecular Signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Staskawicz, B.J.; Dangl, J.L. The plant immune system: From discovery to deployment. Cell 2024, 187, 2095–2116. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Frei Dit Frey, N.; Garcia, A.; Bigeard, J.; Zaag, R.; Bueso, E.; Garmier, M.; Pateyron, S.; De Tauzia-Moreau, M.-L.; Brunaud, V.; Balzergue, S.; et al. Functional analysis of Arabidopsis immune-related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome Biol. 2014, 15, R87. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rodriguez, M.C.; Adams-Phillips, L.; Liu, Y.; Wang, H.; Su, S.-H.; Jester, P.J.; Zhang, S.; Bent, A.F.; Krysan, P.J. MEKK1 Is Required for flg22-Induced MPK4 Activation in Arabidopsis Plants. Plant Physiol. 2007, 143, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose Deposition: A Multifaceted Plant Defense Response. Mol. Plant-Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-K.; Liu, Y.-B.; Zhang, M.-Y.; Li, D.-Q. Stomatal development and movement. Plant Signal. Behav. 2010, 5, 1176–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.; Kim, Y.J.; Kim, M.-H.; Kwak, J.M. MAPK Cascades in Guard Cell Signal Transduction. Front. Plant Sci. 2016, 7, 80. [Google Scholar] [CrossRef]
- Sopeña-Torres, S.; Jordá, L.; Sánchez-Rodríguez, C.; Miedes, E.; Escudero, V.; Swami, S.; López, G.; Piślewska-Bednarek, M.; Lassowskat, I.; Lee, J.; et al. YODA MAP3K kinase regulates plant immune responses conferring broad-spectrum disease resistance. New Phytol. 2018, 218, 661–680. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Plant Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef]
- Baker, C.M.; Chitrakar, R.; Obulareddy, N.; Panchal, S.; Williams, P.; Melotto, M. Molecular battles between plant and pathogenic bacteria in the phyllosphere. Braz. J. Med. Biol. Res. 2010, 43, 698–704. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.C.; Desikan, R.; Moser, R.C.; Neill, S.J. ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J. Exp. Bot. 2000, 51, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, M.; Zhang, L.; Sun, T.; Liu, Y.; Lukowitz, W.; Xu, J.; Zhang, S. Regulation of Stomatal Immunity by Interdependent Functions of a Pathogen-Responsive MPK3/MPK6 Cascade and Abscisic Acid. Plant Cell 2017, 29, 526–542. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.-T.; Liu, S.-K.; Li, J.-R.; Blanco, S.D.; Tsai, H.-W.; Xie, J.-X.; Tsai, Y.-C.; Tzean, Y.; Lin, Y.-H. A Mitogen-Activated Protein Kinase Pathway Is Required for Bacillus amyloliquefaciens PMB05 to Enhance Disease Resistance to Bacterial Soft Rot in Arabidopsis thaliana. Plants 2024, 13, 2591. https://doi.org/10.3390/plants13182591
Li A-T, Liu S-K, Li J-R, Blanco SD, Tsai H-W, Xie J-X, Tsai Y-C, Tzean Y, Lin Y-H. A Mitogen-Activated Protein Kinase Pathway Is Required for Bacillus amyloliquefaciens PMB05 to Enhance Disease Resistance to Bacterial Soft Rot in Arabidopsis thaliana. Plants. 2024; 13(18):2591. https://doi.org/10.3390/plants13182591
Chicago/Turabian StyleLi, Ai-Ting, Shang-Kai Liu, Jia-Rong Li, Sabrina Diana Blanco, Hsin-Wei Tsai, Jia-Xin Xie, Yun-Chen Tsai, Yuh Tzean, and Yi-Hsien Lin. 2024. "A Mitogen-Activated Protein Kinase Pathway Is Required for Bacillus amyloliquefaciens PMB05 to Enhance Disease Resistance to Bacterial Soft Rot in Arabidopsis thaliana" Plants 13, no. 18: 2591. https://doi.org/10.3390/plants13182591
APA StyleLi, A.-T., Liu, S.-K., Li, J.-R., Blanco, S. D., Tsai, H.-W., Xie, J.-X., Tsai, Y.-C., Tzean, Y., & Lin, Y.-H. (2024). A Mitogen-Activated Protein Kinase Pathway Is Required for Bacillus amyloliquefaciens PMB05 to Enhance Disease Resistance to Bacterial Soft Rot in Arabidopsis thaliana. Plants, 13(18), 2591. https://doi.org/10.3390/plants13182591






