Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients
Abstract
1. Introduction
2. Common Internal Disorders of Mango
- Soft nose is characterized by flesh softening and yellowing at the fruit apex due to mesocarp breakdown [23] (Figure 1 and Figure 2a). The ‘beak end’ of the fruit appears over-ripe, indicating rapid localised ripening [24]. Soft nose may be assessed by touching the beak end, it being softer than the rest of the fruit [25]. Soft nose incidence is both cultivar-dependent and less prevalent in alkaline soils than in acidic sandy soils [26].
- Stem-end cavity is characterized by cavity formation in the proximal area between the peduncle and the mesocarp as vascular tissue deterioration (Figure 1 and Figure 2b) [10]. In the early stages of development, tissues at the proximal end of affected fruit turn brown, followed by cavity formation and necrosis [25]. In advanced stages, necrosis around the cavity manifests in the internal mesocarp. Symptoms are like spongy tissue and jelly seed. Microscopic analysis reveals xylem deterioration, procambium damage, and calcium oxalate crystal accumulation in the cavity [10].
- Jelly seed appears as flesh breakdown in the mesocarp around the fruit seed/endocarp (Figure 2c). It manifests as a jelly-like mass around the fruit stone. Complete tissue disintegration leads to tissue browning and the appearance of watery translucent areas in extreme cases [27]. Jelly seed onset symptoms vary among cultivars. ‘Van Dyke’ and ‘Tommy Atkins’ showed jelly seed after 8 weeks of fruit set [10]. By contrast, symptoms in ‘Irwin’ appeared 12 weeks after fruit set [10]. Harvesting fruit early is recommended to mitigate jelly seed incidence [28].
- Internal flesh browning is an important physiological disorder of fleshy fruit (Figure 2d). It is typically associated with long-term storage-related physiological changes in fruit [29,30,31]. Flesh browning is considered a result of cell wall and membrane degradation. Concomitant release and mixing of enzymes are associated with browning (viz., polyphenol oxidase and phenylalanine ammonia-lyase) and phenolic substrate compounds. The enzymes and phenolics are usually localised in different cellular compartments to prevent their mixing. Upon cell wall weakening and degradation, phenolic compounds oxidise to coloured O-quinones [32]. Cell wall weakening is often associated with low Ca concentration in fruit mesocarp [33]. Lo’ay and Ameer [34] discerned positive effects of ascorbic acid-blended Ca nanoparticles for the alleviation of internal flesh browning in low-temperature stored ‘Hindi Be-Sennara’ mango by inhibiting cell wall degrading enzymes, such as pectinase and cellulase.
- Spongy tissue disorder is common in Indian mango cultivars, particularly ‘Alphonso’. Affected tissue displays symptoms like soft nose and jelly seed, with spongy mesocarp, off-odour, and pale-yellow or off-white flesh, with or without cavities (Figure 2e) [35,36]. Early studies implicated nutritional imbalance (e.g., high nitrogen and low calcium) and environmental factors (e.g., rain and field temperatures) in this disorder [36,37]. Dysfunction ultimately resulted in a shift from aerobic to anaerobic respiration, including an acid pH shift, cell wall degradation, and synthesis of free radicals [38].

3. Anatomy and Biochemistry of Internal Disorders
| Cultivar | Disorder | Enzyme | Activity | Reference |
|---|---|---|---|---|
| ‘Alphonso’ | Spongy tissue | Superoxide dismutase, catalase, peroxidase, polyphenol oxidase | Lower | [55] |
| ‘Tommy Atkins’ | Spongy tissue | Amylase | Lower | [56] |
| ‘Alphonso’ | Spongy tissue | α-amylase, invertase | Lower | [35] |
| ‘Tommy Atkins’ | Spongy tissue | Peroxidase, polyphenol oxidase | Higher | [43] |
| ‘Alphonso’ | Spongy tissue | Pectin methyl esterase, malic enzyme | Higher | [44] |
| ‘Chiin Hwang’ | Jelly seed, lumpy tissue, soft nose, | α-amylase activity | Lower | [57] |
| ‘Langra’ | Jelly seed | α-amylase, pectin methyl esterase, cellulase, polyphenol oxidase | Higher | [45] |
| ‘Dashehari’ | Jelly seed | α-amylase, pectin methyl esterase, Cellulase, polyphenol oxidase | Higher | [45] |
| ‘Amrapali’ | Jelly seed | Pectin methyl esterase, pectate lyase, polygalacturonase | Higher | [58] |
4. Factors Affecting Fruit Quality and Robustness
4.1. Role of Mineral Nutrients in Fruit Flesh
| Defect | N | Ca | K | P | Mg | B | N/Ca | Reference |
|---|---|---|---|---|---|---|---|---|
| Spongy tissue | ↑ | ↓ | ↑ | ↑ | [35,61,66,67] | |||
| Jelly seed | ↑ | ↓ | ↑ | [57,61,62] | ||||
| Internal flesh breakdown | ↑ | ↓ | ↑/↓ | ↑ | ↑/↓ | ↓ | ↑ | [7,8,18,44,64,68,69] |
| Soft nose | ↑ | ↓ | ↓ | ↑ | [24,57,70] |
| Cultivar | Disorder | Nutrient | Source | Method | Time of Application | Results | Reference |
|---|---|---|---|---|---|---|---|
| ‘Sensation’ | Spongy tissue, Jelly seed | N, Ca | NH4NO3, CaSO4·2H2O | Drip irrigation | Continuous throughout growing season | ↑N = ↑Disorder ↑Ca = ↓Disorder | [61] |
| ‘Van Dyke’ | Jelly seed | Ca | CaCl2, Ca(NO3)2 | Foliar | At fruit set | ↑Ca = ↓Disorder | [62] |
| ‘Tommy Atkins’ | Internal flesh breakdown | N, Ca | - | Drip irrigation | Continuous throughout growing season | ↑N = ↑Disorder ↑Ca = ↓Disorder | [68] |
| ‘Sensation’ | Internal flesh breakdown, Stem-end cavity | Ca, Mg, K, B | CaCl2, MgCl2, KCl, Solubor® (20% B) | Foliar | Weekly from 3 weeks after fruit set to 3 weeks before harvest | No effect | [69] |
| ‘Sensation’ | Internal flesh breakdown, Stem-end cavity, | Ca | Ca-EDTA | Stem injection | One month before harvest | ↑Ca = ↓Disorder | [69] |
| ‘Chaunsa’ | Soft nose | Ca, Mg | CaCl2, CaSO4, Ca(OH)2, MgCl2 | Foliar | 2 weeks before harvest | ↑Ca = ↓Disorder ↑Mg = ↓Disorder | [72] |
| ‘Keitt’ | Watery pulp breakdown | N, Ca | NH4NO2, CaSO4·H2O | Soil | Pre- and post-flowering | No effect | [64] |
4.1.1. Calcium
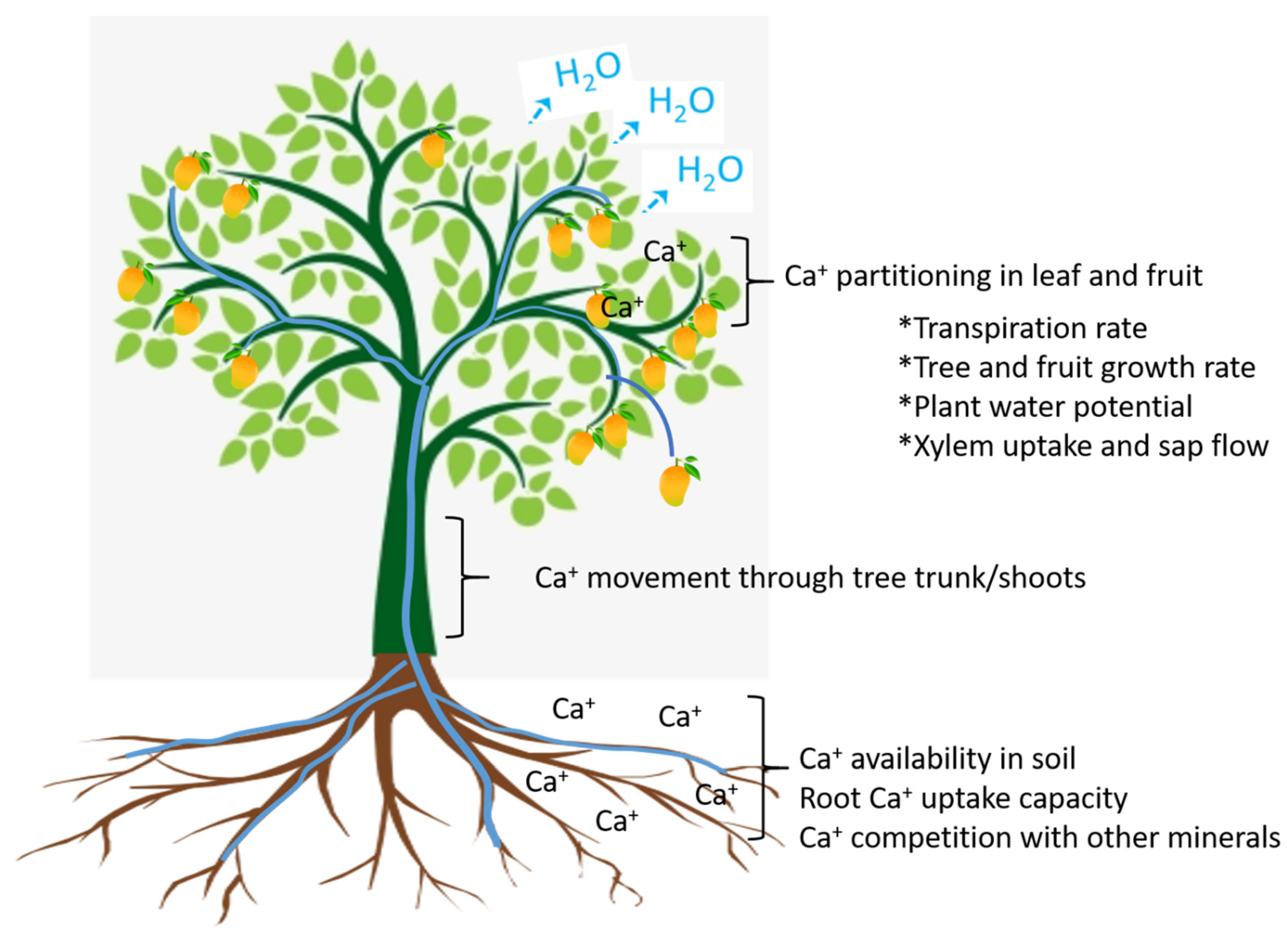
Calcium Translocation
Monitoring of Calcium in Mango Fruit
- Sampling
| Cultivars | Skin | Outer Flesh | Middle Flesh | Inner Flesh | Proximal Flesh | Distal Flesh | Reference |
|---|---|---|---|---|---|---|---|
| ‘Beverly’ | - | 7.21 mg/100 g FW | - | 4.51 mg/100 g FW | - | - | [70] |
| ‘Dashehari’ | 4570 mg/kg dw | - | - | 460 mg/kg dw | - | - | [118] |
| ‘Glen’ | 1900 mg/kg dw | - | - | 700 mg/kg dw | - | - | [119] |
| ‘Haden’ | 2300 mg/kg dw | - | - | 700 mg/kg dw | - | - | [119] |
| ’Irwin’ | 3600 mg/kg dw | - | - | 600 mg/kg dw | - | - | [119] |
| ‘Keitt’ | 3900 mg/kg dw | - | - | 1000 mg/kg dw | - | - | [64] |
| ‘Kent’ | - | 9.52 mg/100 g FW | - | 5.00 mg/100 g FW | - | - | [70] |
| ‘Kent’ | 3600 mg/kg dw | - | - | 900 mg/kg dw | - | - | [119] |
| ‘Kensington Pride’ | 523 mg/kg dw | 123 mg/kg dw | 55 mg/kg dw | 50 mg/kg dw | 94 mg/kg dw | 71 mg/kg dw | [82] |
| ‘Kensington Pride’ | 1670 mg/kg dw | - | - | 580 mg/kg dw | - | - | [120] |
| ‘Kensington Pride’ | 1740 mg/kg dw | 560 mg/kg dw | 400 mg/kg dw | 410 mg/kg dw | - | - | [117] |
| ‘Kensington Pride’ | 2400 mg/kg dw | - | - | 600 mg/kg dw | - | - | [119] |
Calcium Fractions in Fruit
4.1.2. Nitrogen
Sources and Translocation
Effect of Nitrogen on Fruit Quality
4.2. Irrigation and Fruit Quality
4.3. Temperature
4.4. Fruit Maturity and Postharvest Implications
5. Control Measures and Recommendations to Minimise Internal Disorders
6. Conclusions
- Nutrient imbalances, like high fruit N and low Ca, can negatively affect fruit quality and the incidence of postharvest disorders. Increasing Ca concentrations in fruit flesh is a challenge, as applying more Ca to the soil does not guarantee its accumulation in fruit.
- Controlling tree vigour by pruning, managing crop load by thinning, carefully timing irrigation, and small frequent nutrient applications can potentially improve Ca levels in fruit, especially during the early developmental stages when the Ca accumulation rate is high.
- While N manipulation is achievable due to its mobility, application strategies must limit fruit N without negatively affecting fruit development and yield.
- Careful monitoring of soil, leaf, and fruit nutrient status is recommended and desirable.
- When sampling and analysing fruit and leaf samples, adopting recommended strategies to minimise variation and avoid ambiguity in results is advised. Other factors, including temperature, humidity, light radiation, and vapour pressure deficit, are likely to differentially influence fruit development and physiology.
- Considering complex interplays of variables, the consistent production of robust, quality mango fruit requires a holistic approach. Mango fruit quality is an output of a dynamic system that integrates influences of the growing environment, genotype, and associated management practices at preharvest, harvest, and postharvest stages.
7. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiloes, A.M.; Azizan, F.A.; Checco, J.; Joyce, D.; Abdul Aziz, A. What do consumers want in fresh mangoes? A systematic literature review. Int. J. Food Sci. Technol. 2022, 57, 1473–1492. [Google Scholar] [CrossRef]
- Xu, X.; Lei, H.; Ma, X.; Lai, T.; Song, H.; Shi, X.; Li, J. Antifungal activity of 1-methylcyclopropene (1-MCP) against anthracnose (Colletotrichum gloeosporioides) in postharvest mango fruit and its possible mechanisms of action. Int. J. Food Microbiol. 2017, 241, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Tripathi, A.; Nimbolkar, P. Review on physiological disorders of tropical and subtropical fruits: Causes and management approach. Int. J. Agric. Environ. Biotechnol. 2016, 9, 925–935. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Kuang, Y. A study of the cause of the mango black tip disorder. Sci. Hortic. 1995, 64, 49–54. [Google Scholar] [CrossRef]
- Lim, T.K.; Khoo, K.C. Diseases and Disorders of Mango in Malaysia; Tropical Press: Singapore, 1985. [Google Scholar]
- Ram, S. Factors associated with black tip and internal necrosis in mango and their control. Acta Hortic. 1985, 231, 797–804. [Google Scholar] [CrossRef]
- Ma, X.; Yao, Q.; Ma, H.; Wu, H.; Zhou, Y.; Wang, S. Relationship between internal breakdown and mineral nutrition in the flesh of ‘Keitt’ mango. Acta Hortic. 2018, 1217, 351–355. [Google Scholar] [CrossRef]
- Torres, A.C.; Saúco, V.G. The study of the problem of mango (Mangifera indica L.) internal breakdown. Acta Hortic. 2004, 645, 167–174. [Google Scholar] [CrossRef]
- Oak, P.; Deshpande, A.; Giri, A.; Gupta, V. Metabolomic dynamics reveals oxidative stress in spongy tissue disorder during ripening of Mangifera indica L. fruit. Metabolites 2019, 9, 255. [Google Scholar] [CrossRef]
- Raymond, L.; Schaffer, B.; Brecht, J.K.; Crane, J.H. Internal breakdown in mango fruit: Symptomology and histology of jelly seed, soft nose and stem-end cavity. Postharvest Biol. Technol. 1998, 13, 59–70. [Google Scholar] [CrossRef]
- Njuguna, J.; Ambuko, J.; Hutchinson, M.; Owino, W. Incidence of jelly seed disorder in ‘Tommy Atkins’ and ‘Van Dyke’ mangoes as affected by agro-ecological conditions in Kenya. Int. J. Plant Soil Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Lo’ay, A. Ascorbic acid and tissue browning in mango CV Hindi Be-Sennara fruits (Mangifera indica L.) under cold storage. J. Agric. Sci. 2009, 34, 11301–11310. [Google Scholar]
- Bally, I.S.E.; Owens, L. Control of Internal Disorders in Mango; Horticultural Australia: Sydney, Australia, 2001. [Google Scholar]
- Cheema, G.; Dani, P. Report on the export of Mangoes to Europe in 1932 and 1933. Bull. Dep. Agric. Bombay 1935, 170, 1–31. [Google Scholar]
- Ullah, M.; Joyce, D.; Khanal, A.; Joyce, P.; White, N.; Macnish, A.; Webb, R. Mineral nutrition and internal defects in vapour heat treated mango fruit. Acta Hortic. 2022, 1375, 417–422. [Google Scholar] [CrossRef]
- Ullah, M.A. Preharvest Factors Affecting Internal Disorders in ‘B74’ Mango. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2023. [Google Scholar]
- Khanal, A. Relationship between Fruit Maturity and Internal Disorders of Vapour Heat Treated ‘B74’ Mango Fruit. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2024. [Google Scholar]
- Ma, X.; Wang, J.; Su, M.; Liu, B.; Du, B.; Zhang, Y.; He, L.; Wang, S.; Wu, H. The link between mineral elements variation and internal flesh breakdown of ‘Keitt’ Mango in a steep slope mountain area, Southwest China. Horticulturae 2022, 8, 533. [Google Scholar] [CrossRef]
- Kiran, P.R.; Jadhav, P.; Avinash, G.; Aradwad, P.; TV, A.; Bhardwaj, R.; Parray, R.A. Detection and classification of spongy tissue disorder in mango fruit during ripening by using visible-near infrared spectroscopy and multivariate analysis. J. Near Infrared Spectrosc. 2024, 09670335241269005. [Google Scholar] [CrossRef]
- Ma, X.; Liu, B.; Yao, J.; Zhang, Y.; Xu, W.; Yang, Y.; Xie, K.; Yu, D.; Wu, H.; Wang, S. Elucidating the underlying formation mechanism of spongy tissue in ‘Keitt’ mango via metabolomics and proteomics analysis. Postharvest Biol. Technol. 2024, 216, 113042. [Google Scholar] [CrossRef]
- Raymond, L.; Schaffer, B.; Brecht, J.K.; Hanlon, E.A. Internal breakdown, mineral element concentration, and weight of mango fruit. J. Plant Nutr. 1998, 21, 871–889. [Google Scholar] [CrossRef]
- Krishnamurthy, S. Internal breakdown during ripening of ‘Alphonso’ mango (Mangifera indica Linn.) in relation to specific gravity of the fruit. J. Food Sci. Technol. 1980, 17, 198–199. [Google Scholar]
- Young, T. “ Soft-nose”, a physiological disorder in mango fruits. Punjab Fruit J. 1960, 23, 259–261. [Google Scholar]
- Burdon, J.; Moore, K.; Wainwright, H. A preliminary examination of the physiological disorder ’soft-nose’ in mango fruit. Trop. Fruits XXIII IHC 1992, 296, 15–22. [Google Scholar] [CrossRef]
- Mead, A.; Winston, E. Description of the disorder ‘stem-end cavity’, possible causes and suggestions for reducing incidence in packing sheds. Acta Hortic. 1989, 291, 265–271. [Google Scholar] [CrossRef]
- De la Cruz Medina, J.; Garcia, H. Mango: Postharvest operations. In Compendium on Post-Harvest Operations; Food and Drug Administration of United States: Silver Spring, MD, USA, 2002; pp. 2–8. [Google Scholar]
- Van Lelyveld, L.; Smith, J. Physiological factors in the maturation and ripening of mango (Mangifera indica L.) fruit in relation to the jelly-seed physiological disorder. J. Hortic. Sci. 1979, 54, 283–287. [Google Scholar] [CrossRef]
- Singh, D.; Singh, V.; Ram, R.; Yadava, L. Relationship of heat units (degree days) with softening status of fruits in mango cv. ‘Dashehari’. Plant Arch. 2011, 11, 227–230. [Google Scholar]
- Selvarajah, S.; Bauchot, A.; John, P. Internal browning in cold-stored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol. Technol. 2001, 23, 167–170. [Google Scholar] [CrossRef]
- Pusittigul, I.; Kondo, S.; Siriphanich, J. Internal browning of pineapple (Ananas comosus L.) fruit and endogenous concentrations of abscisic acid and gibberellins during low temperature storage. Sci. Hortic. 2012, 146, 45–51. [Google Scholar] [CrossRef]
- Mogollon, M.; Jara, A.; Contreras, C.; Zoffoli, J.P. Quantitative and qualitative VIS-NIR models for early determination of internal browning in ‘Cripps Pink’ apples during cold storage. Postharvest Biol. Technol. 2020, 161, 111060. [Google Scholar] [CrossRef]
- Huang, S.-J.; Lin, S.-Y.; Wang, T.-T.; Hsu, F.-C. Combining acetic acid and ethanol as an anti-browning treatment for lettuce butt discoloration through repression of the activity and expression of phenylalanine ammonia lyase. Postharvest Biol. Technol. 2020, 164, 111151. [Google Scholar] [CrossRef]
- Chitarra, M.; Chitarra, A.; de Oliveira Lima, L. Internal breakdown in mango fruit: Changes in cell wall compounds. Acta Hortic. 1997, 485, 91–96. [Google Scholar] [CrossRef]
- Lo’ay, A.; Ameer, N. Performance of calcium nanoparticles blending with ascorbic acid and alleviation internal browning of ‘Hindi Be-Sennara’ mango fruit at a low temperature. Sci. Hortic. 2019, 254, 199–207. [Google Scholar] [CrossRef]
- Katrodia, J.S. The Study into the Casues of the Development of Spongy Tissue in Mango (Mangifera indica L.) Fruit of Cultivar ‘Alphonso’; Horticulture Department, NM College of Agriculture, Navsari Agriculture University: Gujarat, India, 1979. [Google Scholar]
- Katrodia, J. Spongy tissue in mango—Causes and control measures. Acta Hortic. 1989, 231, 814–826. [Google Scholar] [CrossRef]
- Rane, D.; Katrodia, J.; Kulkarni, D. Problem of spongy tissue development in mango (Mangifera indica L.) cv. Alphonso. A review. J. Maharashtra Agric. Univ. 1976, 1, 89–94. [Google Scholar]
- Ravindra, V.; Shivashankar, S. Spongy tissue in ‘Alphonso’ mango–significance of in situ seed germination events. Curr. Sci. 2004, 87, 1045–1049. [Google Scholar]
- Oosthuyse, S. Disorders of fibreless mangoes grown in South Africa for export. Yearb.–S. Afr. Mango Grow. Assoc. 1993, 13, 80–88. [Google Scholar]
- Hofman, P.; Holmes, R.; Barker, L. ‘B74’ Mango Quality Assessment Manual; Agri-Science Queensland, Department of Employment, Economic Development and Innovation: Brisbane, Australia, 2010. [Google Scholar]
- Ullah, M.A.; Kiloes, A.M.; Aziz, A.A.; Joyce, D.C. Impact of factors contributing to internal disorders of mango (Mangifera indica L.) fruit—A systematic literature review. Sci. Hortic. 2024, 331, 113150. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Lima, L.; Chitarra, A.; Chitarra, M.; Silva, E. Enzymatic activity changes in spongy tissue: A physiological ripening disorder of ‘Tommy Atkins’ mango. Acta Hortic. 1999, 485, 255–258. [Google Scholar] [CrossRef]
- Murthy, S.K. Chemical studies on internal breakdown in ‘Alphonso’ mango (Mangifera indica Linn.). J. Hortic. Sci. 1981, 56, 247–250. [Google Scholar] [CrossRef]
- Krishna, K.R.; Sharma, R.; Srivastav, M. Physiological and biochemical attributes associated with jelly-seed disorder in mango (Mangifera indica L.). Acta Physiol. Plant. 2020, 42, 90. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Lou, L.; Wang, Y.; Dai, B.; Wang, Y.; Kebbeh, M.; Huan, C.; Shen, S.; Liu, Y. Transcriptomic analysis reveals the role of abscisic acid and ethylene in regulating starch-source biosynthesis associated with soft nose disorder in ‘Keitt’mango fruit during postharvest. Postharvest Biol. Technol. 2024, 209, 112698. [Google Scholar] [CrossRef]
- Lammertyn, J.; Scheerlinck, N.; Verlinden, B.; Schotsmans, W.; Nicolaï, B.M. Simultaneous determination of oxygen diffusivity and respiration in pear skin and tissue. Postharvest Biol. Technol. 2001, 23, 93–104. [Google Scholar] [CrossRef]
- Mitcham, E.J.; McDonald, R.E. Respiration rate, internal atmosphere, and ethanol and acetaldehyde accumulation in heat-treated mango fruit. Postharvest Biol. Technol. 1993, 3, 77–86. [Google Scholar] [CrossRef]
- Jacobi, K.K.; MacRae, E.A.; Hetherington, S.E. Postharvest heat disinfestation treatments of mango fruit. Sci. Hortic. 2001, 89, 171–193. [Google Scholar] [CrossRef]
- Nishizawa, T.; Taira, S.; Nakanishi, M.; Ito, M.; Togashi, M.; Motomura, Y. Acetaldehyde, ethanol, and carbohydrate concentrations in developing muskmelon fruit (Cucumis melo L. cv. Andesu) are affected by short-term shading. HortScience 1998, 33, 992–994. [Google Scholar] [CrossRef]
- Deuchande, T.; Carvalho, S.M.P.; Giné-Bordonaba, J.; Vasconcelos, M.W.; Larrigaudière, C. Transcriptional and biochemical regulation of internal browning disorder in ‘Rocha’ pear as affected by O2 and CO2 concentrations. Postharvest Biol. Technol. 2017, 132, 15–22. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Barrera-Gavira, J.M.; Echeverria, G. New insights into early biochemical prediction of internal browning disorder in ‘Conference’ pears. Postharvest Biol. Technol. 2024, 207, 112595. [Google Scholar] [CrossRef]
- Pesis, E.; Dvir, O.; Feygenberg, O.; Arie, R.B.; Ackerman, M.; Lichter, A. Production of acetaldehyde and ethanol during maturation and modified atmosphere storage of litchi fruit. Postharvest Biol. Technol. 2002, 26, 157–165. [Google Scholar] [CrossRef]
- Pesis, E. The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Nagamani, J.E.; Shivashankara, K.S.; Roy, T.K. Role of oxidative stress and the activity of ethylene biosynthetic enzymes on the formation of spongy tissue in ‘Alphonso’ mango. J. Food Sci. Technol. 2010, 47, 295–299. [Google Scholar] [CrossRef]
- Lima, L.C.d.O.; Chitarra, A.B.; Chitarra, M.I.F. Changes in amylase activity starch and sugars contents in mango fruits pulp cv. Tommy Atkins with spongy tissue. Braz. Arch. Biol. Technol. 2001, 44, 59–62. [Google Scholar] [CrossRef]
- Lin, H.; Shiesh, C.; Chen, P. Physiological disorders in relation to compositional changes in mango (Mangifera indica L. ‘Chiin Hwang’) fruit. Acta Hortic. 2013, 984, 357–363. [Google Scholar] [CrossRef]
- Shivashankar, S.; Sumathi, M.; Singh, H.S. Premature seed germination induced by very-long-chain fatty acids causes jelly seed disorder in the mango (Mangifera indica L.) cultivar ‘Amrapali’ in India. J. Hortic. Sci. Biotechnol. 2016, 91, 138–147. [Google Scholar] [CrossRef]
- Rehman, A.; Malik, A.U.; Ali, H.; Alam, M.W.; Sarfraz, B. Preharvest factors influencing the postharvest disease development and fruit quality of mango. J. Environ. Agric. 2015, 3, 42–47. [Google Scholar]
- Hofman, P.; Marques, J.; Macnish, A.; Joyce, D. Interaction between production characteristics and postharvest performance and practices for fresh fruit. Acta Hortic. 2012, 1012, 55–69. [Google Scholar] [CrossRef]
- Cracknell Torres, A.; Cid Ballarin, M.; Socorro Monzon, A.; Fernandez Galvan, D.; Rosell Garcia, P. Incidence of internal fruit breakdown in various mango (Mangifera indica L.) cultivars. Acta Hortic. 2004, 645, 315–318. [Google Scholar] [CrossRef]
- Bitange, N.M.; Chemining’wa, G.N.; Ambuko, J.; Owino, W.O. Can calcium sprays alleviate jelly seed in mango fruits? J. Agric. Rural Dev. Trop. Subtrop. 2020, 121, 35–42. [Google Scholar]
- Joyce, D.C. Improved Fruit Robustness and Quality in Avocado Supply Chains (Mineral Nutrition). AV19004; Horticulture Australia Ltd.: Sydney, Australia, 2021. [Google Scholar]
- Bally, I.S.E. The Effect of Preharvest Nutrition and Crop Load on Fruit Quality and Postharvest Disease in Mango (Mangifera indica L.). Ph.D. Thesis, University of Queensland, Brisbane, Australia, 2006. [Google Scholar]
- Tarmizi, A.; Malik, T.T.A.; Pauziah, M.; Zahrah, T. Incidence of insidious fruit rot as related to mineral nutrients in ‘Harumanis’ mangoes. J. MARDI 1993, 21, 43–49. [Google Scholar]
- Selvaraj, Y.; Edward Raja, M.; Rawal, R. Biochemical studies on internal breakdown-a ripening disorder in mango fruits. Indian J. Hortic. 2000, 57, 183–188. [Google Scholar]
- Ma, X.; Liu, B.; Zhang, Y.; Su, M.; Zheng, B.; Wang, S.; Wu, H. Unraveling correlations between calcium deficiency and spongy tissue in mango fruit flesh. Sci. Hortic. 2023, 309, 111694. [Google Scholar] [CrossRef]
- Torres, A.; Cid, M.; Socorro, A.; Fernández, D.; Rosell, P.; Galán, V. Effects of nitrogen and calcium supply on the incidence of internal fruit breakdown in ‘Tommy Atkins’ mangoes (Mangifera indica L.) grown in a soilless system. Acta Hortic. 2004, 645, 387–393. [Google Scholar] [CrossRef]
- Hermoso, J.; Guirado, E.; Gómez, R.; Castilla, A.; Velasco, R.; Farré, J. Effects of nutrients and growth substances on internal breakdown of ‘Sensation’ mango fruits. Acta Hortic. 1997, 455, 92–99. [Google Scholar] [CrossRef]
- Burdon, J.; Moore, K.; Wainwright, H. Mineral distribution in mango fruit susceptible to the physiological disorder soft-nose. Sci. Hortic. 1991, 48, 329–336. [Google Scholar] [CrossRef]
- Silva, D.J.; Choudhury, M.M.; Mendes, A.M.S.; Dantas, B.F. Quality and nutrient level of mango cv. ‘Tommy Atkins’ as affected by calcium application before harvest. Rev. Bras. Farmacogn. 2008, 30, 74–78. [Google Scholar]
- Amin, M.; Malik, A.U.; Din, N.; Jabbar, A.; Ahmad, I. Mango soft nose disorder and fruit quality in relation to pre-and postharvest treatments. Life Sci. Int. J. 2007, 1, 455–462. [Google Scholar]
- Kamthan, A.; Kamthan, M.; Kumar, A.; Sharma, P.; Ansari, S.; Thakur, S.S.; Chaudhuri, A.; Datta, A. A calmodulin like EF hand protein positively regulates oxalate decarboxylase expression by interacting with E-box elements of the promoter. Sci. Rep. 2015, 5, 14578. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Effect of calcium chloride in combination with salicylic acid on post-harvest freshness of apples. Food Sci. Biotechnol. 2015, 24, 1139–1146. [Google Scholar] [CrossRef]
- Wainwright, H.; Burbage, M. Physiological disorders in mango (Mangifera indica L.) fruit. J. Hortic. Sci. 1989, 64, 125–135. [Google Scholar] [CrossRef]
- Kalcsits, L.; Mattheis, J.; Giordani, L.; Reid, M.; Mullin, K.; Beres, B. Fruit canopy positioning affects fruit calcium and potassium concentrations, disorder incidence, and fruit quality for ‘Honeycrisp’ apple. Can. J. Plant Sci. 2019, 99, 761–771. [Google Scholar] [CrossRef]
- Ho, L.; Belda, R.; Brown, M.; Andrews, J.; Adams, P. Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J. Exp. Bot. 1993, 44, 509–518. [Google Scholar] [CrossRef]
- Shear, C. Calcium-related disorders of fruits and vegetables. HortScience 1975, 10, 361–365. [Google Scholar] [CrossRef]
- Monselise, S.; Goren, R. Preharvest growing conditions and postharvest behavior of subtropical and temperate-zone fruits. HortScience 1987, 22, 1185–1189. [Google Scholar] [CrossRef]
- Ferguson, I.; Volz, R.; Harker, F.; Watkins, C.; Brookfield, P. Regulation of postharvest fruit physiology by calcium. Acta Hortic. 1994, 398, 23–30. [Google Scholar] [CrossRef]
- Gunjate, R.; Tare, S.; Rangwala, A.; Limaye, V. Calcium content in Alphonso mango fruits in relation to occurrence of spongy tissue [India]. J. Maharashtra Agric. Univ. 1979, 4, 159–161. [Google Scholar]
- Joyce, D.; Shorter, A.; Hockings, P. Mango fruit calcium levels and the effect of postharvest calcium infiltration at different maturities. Sci. Hortic. 2001, 91, 81–99. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2015, 181, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Konstankiewicz, K. Calcium effect on mechanical properties of model cell walls and apple tissue. J. Food Eng. 2011, 102, 217–223. [Google Scholar] [CrossRef]
- Tonetto de Freitas, S.; Mitcham, E.I. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- McLaughlin, S.; Wimmer, R. Tansley review no. 104: Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-end rot: A calcium deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hirons, A.D.; Thomas, P.A. Applied Tree Biology; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rogiers, S.; Keller, M.; Holzapfel, B.P.; Virgona, J.M. Accumulation of potassium and calcium by ripening berries on field vines of Vitis vinifera (L) cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 240–243. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Shackel, K.A.; Mitcham, E.J. Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom end rot development in tomato fruit. J. Exp. Bot. 2011, 62, 2645–2656. [Google Scholar] [CrossRef]
- Nordey, T.; Léchaudel, M.; Génard, M. The decline in xylem flow to mango fruit at the end of its development is related to the appearance of embolism in the fruit pedicel. Funct. Plant Biol. 2015, 42, 668–675. [Google Scholar] [CrossRef]
- Bally, I.S. Changes in the cuticular surface during the development of mango (Mangifera indica L.) cv. Kensington Pride’. Sci. Hortic. 1999, 79, 13–22. [Google Scholar] [CrossRef]
- Yu, X.-C.; Li, M.-J.; Gao, G.-F.; Feng, H.-Z.; Geng, X.-Q.; Peng, C.-C.; Zhu, S.-Y.; Wang, X.-J.; Shen, Y.-Y.; Zhang, D.-P. Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol. 2006, 140, 558–579. [Google Scholar] [CrossRef]
- Banuelos, G.S.; Bangerth, F.; Marschner, H. Relationship between polar basipetal auxin transport and acropetal Ca2+ transport into tomato fruits. Physiol. Plant. 1987, 71, 321–327. [Google Scholar] [CrossRef]
- Sorce, C.; Lombardi, L.; Remorini, D.; Montanaro, G. Occurrence of natural auxin and accumulation of calcium during early fruit development in kiwifruit. Aust. J. Crop Sci 2011, 5, 895. [Google Scholar]
- Else, M.A.; Stankiewicz-Davies, A.P.; Crisp, C.M.; Atkinson, C.J. The role of polar auxin transport through pedicels of Prunus avium L. in relation to fruit development and retention. J. Exp. Bot. 2004, 55, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Witney, G.; Hofman, P.; Wolstenholme, B. Effect of cultivar, tree vigour and fruit position on calcium accumulation in avocado fruits. Sci. Hortic. 1990, 44, 269–278. [Google Scholar] [CrossRef]
- Brown, M.M.; Ho, L. Factors affecting calcium transport and basipetal IAA movement in tomato fruit in relation to blossom-end rot. J. Exp. Bot. 1993, 44, 1111–1117. [Google Scholar] [CrossRef]
- Cutting, J.; Bower, J. The relationship between basipetal auxin transport and calcium allocation in vegetative and reproductive flushes in avocado. Sci. Hortic. 1989, 41, 27–34. [Google Scholar] [CrossRef]
- Montanaro, G.; Treutter, D.; Xiloyannis, C. Phenolic compounds in young developing kiwifruit in relation to light exposure: Implications for fruit calcium accumulation. J. Plant Interact. 2007, 2, 63–69. [Google Scholar] [CrossRef]
- Tonetto de Freitas, S.; McElrone, A.J.; Shackel, K.A.; Mitcham, E.J. Calcium partitioning and allocation and blossom-end rot development in tomato plants in response to whole-plant and fruit-specific abscisic acid treatments. J. Exp. Bot. 2014, 65, 235–247. [Google Scholar] [CrossRef]
- Falchi, R.; D’Agostin, E.; Mattiello, A.; Coronica, L.; Spinelli, F.; Costa, G.; Vizzotto, G. ABA regulation of calcium-related genes and bitter pit in apple. Postharvest Biol. Technol. 2017, 132, 1–6. [Google Scholar] [CrossRef]
- Ferguson, I.; Triggs, C. Sampling factors affecting the use of mineral analysis of apple fruit for the prediction of bitter pit. N. Z. J. Crop Hortic. Sci. 1990, 18, 147–152. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Ullah, M.A.; Joyce, D.C. Avocado (Persea americana cv.‘Hass’) fruit mineral composition at canopy level towards sustainable quality. Sustainability 2024, 16, 750. [Google Scholar] [CrossRef]
- Perring, M.A.; Jackson, C.H. The mineral composition of apples. Calcium concentration and bitter pit in relation to mean mass per apple. J. Sci. Food Agric. 1975, 26, 1493–1502. [Google Scholar] [CrossRef]
- Ullah, M.A.; Casey, L.; Webb, R.; Joyce, D. Mineral mapping for more robust avocados. Aust. Tree Crop Mag. 2023, 50–51. [Google Scholar]
- Woolf, A.B.; Bowen, J.H.; Ferguson, I.B. Preharvest exposure to the sun influences postharvest responses of ‘Hass’ avocado fruit. Postharvest Biol. Technol. 1999, 15, 143–153. [Google Scholar] [CrossRef][Green Version]
- Katrodia, J.; Sheth, I. Spongy tissue development in mango fruit of cultivar ‘Alphonso’ in relation to temperature and its control. Acta Hortic. 1988, 231, 827–834. [Google Scholar] [CrossRef]
- Gunjate, R.; Tare, S.; Rangawala, A.; Limaye, V. Changes in calcium content in Alphonso mango fruits and leaves from fruit-set to harvesting. Indian J. Hortic. 1979, 36, 383–386. [Google Scholar]
- Whangchai, K.; Gemma, H.; Uthaibutra, J.; Iwahori, S. Postharvest physiology and microanalysis of mineral elements of ‘Nam Dork Mai’ mango fruit grown under different soil composition. J. Jpn. Soc. Hortic. Sci. 2001, 70, 463–465. [Google Scholar] [CrossRef]
- Shorter, A.; Joyce, D. Effect of partial pressure infiltration of calcium into ‘Kensington’ mango fruit. Aust. J. Exp. Agric. 1998, 38, 287–294. [Google Scholar] [CrossRef]
- Singh, B.; Tandon, D.; Kalra, S. Changes in postharvest quality of mangoes affected by preharvest application of calcium salts. Sci. Hortic. 1993, 54, 211–219. [Google Scholar] [CrossRef]
- Singh, Z. Embryo abortion in relation to fruit size, quality, and concentrations of nutrients in skin and pulp of mango. J. Plant Nutr. 2005, 28, 1723–1737. [Google Scholar] [CrossRef]
- Hofman, P.; Joyce, D.; Beasley, D. Effect of preharvest bagging and of embryo abortion on calcium levels in ‘Kensington Pride’ mango fruit. Aust. J. Exp. Agric. 1999, 39, 345–349. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, N.; Jemric, T.; Kurtanjek, Z.; Cosic, T.; Pavlovic, I.; Blaskovic, D. Relationship between water-soluble Ca and other elements and bitter pit occurrence in ‘Idared’ apples: A multivariate approach. Ann. Appl. Biol. 2004, 145, 193–196. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.; Gao, F.; Huang, H. A comparative study of the pericarp of litchi cultivars susceptible and resistant to fruit cracking. J. Hortic. Sci. Biotechnol. 1999, 74, 351–354. [Google Scholar] [CrossRef]
- Yuan, W.; Kuang, X.; Huang, J.; Huang, X.; Li, J.; Wang, H. Comparative study of calcium level in the pericarp of different cracking resistant litchi cultivars and effects calcium application. Adv. Hortic. 2002, 5, 171–177. [Google Scholar]
- Huang, X.; Li, J.; Wang, H.; Huang, H.; Gao, F. Relationship between fruit cracking and calcium in litchi pericarp. Acta Hortic. 2001, 558, 209–215. [Google Scholar] [CrossRef]
- Tagliavini, M.; Quartieri, M.; Millard, P. Remobilised nitrogen and root uptake of nitrate for spring leaf growth, flowers and developing fruits of pear (Pyrus communis L.) trees. Plant Soil 1997, 195, 137–142. [Google Scholar] [CrossRef]
- Menino, M.R.; Carranca, C.; de Varennes, A. Distribution and remobilization of nitrogen in young non-bearing orange trees grown under Mediterranean conditions. J. Plant Nutr. 2007, 30, 1083–1096. [Google Scholar] [CrossRef]
- Neto, C.; Carranca, C.; Clemente, J.; de Varennes, A. Nitrogen distribution, remobilization and re-cycling in young orchard of non-bearing ‘Rocha’ pear trees. Sci. Hortic. 2008, 118, 299–307. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T. Nitrogen (N) for citrus trees. EDIS 2013, 2013. Available online: https://edis.ifas.ufl.edu/publication/SS580 (accessed on 11 September 2024). [CrossRef]
- Stellacci, A.M.; Cristiano, G.; Rubino, P.; De Lucia, B.; Cazzato, E. Nitrogen uptake, nitrogen partitioning and N-use efficiency of container-grown holm oak (Quercus ilex L.) under different nitrogen levels and fertilizer sources. J. Food Agric. Environ. 2013, 11, 990–994. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Nitrogen. In Principles of Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 2001; pp. 397–434. [Google Scholar]
- Tromp, J.; Ovaa, J. Uptake and distribution of nitrogen in young apple trees after application of nitrate or ammonium, with special reference to asparagine and arginine. Physiol. Plant. 1979, 45, 23–28. [Google Scholar] [CrossRef]
- Millard, P. Internal cycling of nitrogen in trees. Miner. Nutr. Deciduous Fruit Plants 1993, 383, 3–14. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Digman, M.A.; Gratton, E.; Treseder, K.K. Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest. Soil Biol. Biochem. 2012, 55, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Grassi, G.; Millard, P.; Gioacchini, P.; Tagliavini, M. Recycling of nitrogen in the xylem of Prunus avium trees starts when spring remobilization of internal reserves declines. Tree Physiol. 2003, 23, 1061–1068. [Google Scholar] [CrossRef]
- Niederholzer, F.; DeJong, T.; Saenz, J.-L.; Muraoka, T.; Weinbaum, S. Effectiveness of fall versus spring soil fertilization of field-grown peach trees. J. Am. Soc. Hortic. Sci. 2001, 126, 644–648. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Scandellari, F.; Allegra, M.; Torrisi, B.; Stagno, F.; Mimmo, T.; Zanotelli, D.; Gioacchini, P.; Millard, P.; Tagliavini, M. Seasonal dynamics of root uptake and spring remobilisation of nitrogen in field grown orange trees. Sci. Hortic. 2017, 226, 223–230. [Google Scholar] [CrossRef]
- Neilsen, G.; Hogue, E.; Meheriuk, M. Nitrogen fertilization and orchard-floor vegetation management affect growth, nutrition and fruit quality of Gala apple. Can. J. Plant Sci. 1999, 79, 379–385. [Google Scholar] [CrossRef]
- Fallahi, E.; Colt, W.M.; Fallahi, B. Optimum ranges of leaf nitrogen for yield, fruit quality, and photosynthesis in ‘BC-2 Fuji’ apple. J. Am. Pomol. Soc. 2001, 55, 68. [Google Scholar]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Bally, I.S.E. Crop Production: Mineral Nutrition. The Mango, Botany, Production and Uses, 2nd ed.; CAB International: Wallingford, UK, 2009; pp. 404–431. [Google Scholar]
- Simmons, S.L.; Hofman, P.J.; Whiley, A.W.; Hetherington, S.E. Effects of leaf: Fruit ratios on fruit growth, mineral concentration and quality of mango (Mangifera indica L. cv. ‘Kensington Pride’). J. Hortic. Sci. Biotechnol. 2015, 73, 367–374. [Google Scholar] [CrossRef]
- Crisostol, C.; Johnson, R.; DeJong, T.; Day, K. Orchard factors affecting postharvest stone fruit quality. HortScience 1997, 32, 820–823. [Google Scholar] [CrossRef]
- Torres, M.; Hermoso, J.; Farre, J. Influence of nitrogen and calcium fertilisation on productivity and fruit quality of the mango cv. ‘Sensation’. Acta Hortic. 2004, 645, 395–401. [Google Scholar] [CrossRef]
- Young, T.; Koo, R.C.; Miner, J. Effects of nitrogen, potassium and calcium fertilization on ‘Kent’ mangoes in deep, acid, and sandy soil. USA Fla. State Hortic. Soc. 1962, 75, 364–371. [Google Scholar]
- Marques, J.; Hofman, P.; Wearing, A. Rootstocks influence ‘Hass’ avocado fruit quality and fruit minerals. J. Hortic. Sci. Biotechnol. 2003, 78, 673–679. [Google Scholar] [CrossRef]
- Willingham, S.; Pegg, K.; Anderson, J.; Cooke, A.; Dean, J.; Giblin, F.; Coates, L. Effects of rootstock and nitrogen fertiliser on postharvest anthracnose development in ‘Hass’ avocado. Australas. Plant Pathol. 2006, 35, 619–629. [Google Scholar] [CrossRef]
- Cull, B. Mango crop management. Acta Hortic. 1991, 291, 154–173. [Google Scholar] [CrossRef]
- Ferguson, I. Calcium in plant senescence and fruit ripening. Plant Cell Environ. 1984, 7, 477–489. [Google Scholar] [CrossRef]
- Casero, T.; Benavides, A.; Puy, J.; Recasens, I. Relationships between leaf and fruit nutrients and fruit quality attributes in golden smoothee apples using multivariate regression techniques. J. Plant Nutr. 2004, 27, 313–324. [Google Scholar] [CrossRef]
- Wang, G.-y.; Zhang, X.-z.; Yi, W.; Xu, X.-f.; Han, Z.-h. Key minerals influencing apple quality in Chinese orchard identified by nutritional diagnosis of leaf and soil analysis. J. Integr. Agric. 2015, 14, 864–874. [Google Scholar] [CrossRef]
- Fallahi, E.; Righetti, T.L.; Raese, J.T. Ranking tissue mineral analysis to identify mineral limitations on quality in fruit. J. Am. Soc. Hortic. Sci. 1988, 113, 382–389. [Google Scholar] [CrossRef]
- Marcelle, R. Predicting storage quality from preharvest fruit mineral analyses a review. Acta Hortic. 1989, 274, 305–314. [Google Scholar] [CrossRef]
- Fallahi, E.; Simons, B.R. Interrelations among leaf and fruit mineral nutrients and fruit quality in ‘Delicious’ apples. J. Tree Fruit Prod. 1996, 1, 15–25. [Google Scholar] [CrossRef]
- Hamilton, D.; Martin, C.; Bennet, M.; Hearnden, M.; Asis, C.A. Effect of tree leaf N status and N application time on yield and fruit N partitioning of mango. Acta Hortic. 2017, 1183, 161–166. [Google Scholar] [CrossRef]
- Fallahi, E.; Conway, W.S.; Hickey, K.; Sams, C.E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. HortScience 1997, 32, 831–835. [Google Scholar] [CrossRef]
- Campisi-Pinto, S.; Zheng, Y.; Rolshausen, P.E.; Crowley, D.E.; Faber, B.; Bender, G.; Bianchi, M.; Khuong, T.; Lovatt, C.J. Optimal nutrient concentration ranges of ‘Hass’ avocado cauliflower stage inflorescences—Potential diagnostic tool to optimize tree nutrient status and increase yield. HortScience 2017, 52, 1707–1715. [Google Scholar] [CrossRef]
- Spreer, W.; Ongprasert, S.; Hegele, M.; Wünsche, J.N.; Müller, J. Yield and fruit development in mango (Mangifera indica L. cv. ‘Chok Anan’) under different irrigation regimes. Agric. Water Manag. 2009, 96, 574–584. [Google Scholar] [CrossRef]
- Schulze, K.; Spreer, W.; Keil, A.; Ongprasert, S.; Müller, J. Mango (Mangifera indica L. cv. Nam Dokmai) production in Northern Thailand—Costs and returns under extreme weather conditions and different irrigation treatments. Agric. Water Manag. 2013, 126, 46–55. [Google Scholar] [CrossRef]
- Simmons, S.; Hofman, P.; Hetherington, S. The effects of water stress on mango fruit quality. In Proceedings of the Mango 2000 Marketing Seminar and Production Workshop, Brisbane, Australia, 30 July–3 August; 1995; pp. 191–197. [Google Scholar]
- Simmons, S.; Hofman, P.; Whiley, A.; Hetherington, S. Effects of preharvest calcium sprays and fertilizers, leaf: Fruit ratios, and water stress on mango fruit quality. In ACIAR Proceedings; Australian Centre for International Agricultural Research: Bruce, Australia, 1998; pp. 19–26. [Google Scholar]
- Santos, M.R.d.; Martinez, M.A. Soil water distribution and extraction by ‘Tommy Atkins’ mango (Mangifera indica L.) trees under different irrigation regimes. Idesia 2013, 31, 7–16. [Google Scholar] [CrossRef]
- Shivashankara, K.; Mathai, C. Relationship of leaf and fruit transpiration rates to the incidence of spongy tissue disorder in two mango (Mangifera indica L.) cultivars. Sci. Hortic. 1999, 82, 317–323. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Van de Ende, B.; Jerie, P.; Chalmers, D. Responses of ‘Bartlett’ pear to withholding irrigation, regulated deficit irrigation, and tree spacing. J. Am. Soc. Hortic. Sci. 1989, 114, 15–19. [Google Scholar] [CrossRef]
- Ebel, R.C.; Proebsting, E.L.; Evans, R.G. Deficit irrigation to control vegetative growth in apple and monitoring fruit growth to schedule irrigation. HortScience 1995, 30, 1229–1232. [Google Scholar] [CrossRef]
- Caspari, H.W.; Behboudian, M.H.; Chalmers, D.J. Water use, growth, and fruit yield of Hosui’ Asian Pears under deficit irrigation. J. Am. Soc. Hortic. Sci. 1994, 119, 383–388. [Google Scholar] [CrossRef]
- Dos Santos, M.R.; Neves, B.R.; Da Silva, B.L.; Donato, S.L.R. Yield, water use efficiency and physiological characteristic of ‘Tommy Atkins’ Mango under partial rootzone drying irrigation system. Water Resour. Prot. 2015, 7, 1029–1037. [Google Scholar] [CrossRef]
- Adams, S.; Cockshull, K.; Cave, C. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Paull, R.E.; Chen, N.J. Heat treatment and fruit ripening. Postharvest Biol. Technol. 2000, 21, 21–37. [Google Scholar] [CrossRef]
- Siddiqui, M.W. Postharvest Biology and Technology of Horticultural Crops: Principles and Practices for Quality Maintenance; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chagne, D.; Dayatilake, D.; Diack, R.; Oliver, M.; Ireland, H.; Watson, A.; Gardiner, S.E.; Johnston, J.W.; Schaffer, R.J.; Tustin, S. Genetic and environmental control of fruit maturation, dry matter and firmness in apple (Malus x domestica Borkh.). Hortic. Res. 2014, 1, 14046. [Google Scholar] [CrossRef]
- Leonardi, C.; Baille, A.; Guichard, S. Predicting transpiration of shaded and non-shaded tomato fruits under greenhouse environments. Sci. Hortic. 2000, 84, 297–307. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Lang, A. Fruit transpiration in kiwifruit: Environmental drivers and predictive model. AoB Plants 2012, 2012, pls036. [Google Scholar] [CrossRef]
- Guichard, S.; Gary, C.; Leonardi, C.; Bertin, N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. J. Plant Growth Regul. 2005, 24, 201. [Google Scholar] [CrossRef]
- Génard, M.; Bruchou, C. Multivariate analysis of within-tree factors accounting for the variation of peach fruit quality. Sci. Hortic. 1992, 52, 37–51. [Google Scholar] [CrossRef]
- Hewett, E.W. An overview of preharvest factors influencing postharvest quality of horticultural products. Int. J. Postharvest Technol. 2006, 1, 4–15. [Google Scholar] [CrossRef]
- Lazare, S.; Vitoshkin, H.; Alchanatis, V.; Reshef, G.; Ziv, D.; Simenski, E.; Dag, A. Canopy-cooling systems applied on avocado trees to mitigate heatwaves damages. Sci. Rep. 2022, 12, 12563. [Google Scholar] [CrossRef] [PubMed]
- Tinyane, P.P.; Soundy, P.; Sivakumar, D. Growing ‘Hass’ avocado fruit under different coloured shade netting improves the marketable yield and affects fruit ripening. Sci. Hortic. 2018, 230, 43–49. [Google Scholar] [CrossRef]
- Lahak, M.; Alon, E.; Chen, A.; Rubinovich, L. Covering young avocado ‘Hass’ trees with high-density shading nets during the winter mitigates frost damage and improves tree performance. Trees 2024, 38, 327–338. [Google Scholar] [CrossRef]
- Cellier, P.; Ruget, F.; Chartier, M.; Bonhomme, R. Estimating the temperature of a maize apex during early growth stages. Agric. For. Meteorol. 1993, 63, 35–54. [Google Scholar] [CrossRef]
- Saudreau, M.; Sinoquet, H.; Santin, O.; Marquier, A.; Adam, B.; Longuenesse, J.-J.; Guilioni, L.; Chelle, M. A 3D model for simulating the spatial and temporal distribution of temperature within ellipsoidal fruit. Agric. For. Meteorol. 2007, 147, 1–15. [Google Scholar] [CrossRef]
- Durner, E.F. Principles of Horticultural Physiology; CABI: Wallingford, UK, 2013. [Google Scholar]
- Bautista, O.; Esguerra, E. Postharvest Technology for Southeast Asian Perishable Crops; Department of Agriculture, University of the Philippines Los Baños: Laguna, Philippines, 2007. [Google Scholar]
- Ferguson, I.; Volz, R.; Woolf, A. Preharvest factors affecting physiological disorders of fruit. Postharvest Biol. Technol. 1999, 15, 255–262. [Google Scholar] [CrossRef]
- Wills, R.B.; Golding, J. Advances in Postharvest Fruit and Vegetable Technology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wills, R.; Golding, J. Postharvest: An Introduction to the Physiology and Handling of Fruit and Vegetables; UNSW Press: Randwick, Australia, 2016. [Google Scholar]
- Bautista, O. Postharvest Technology for Southeast Asian Perishable Crops [Vegetables, Fruits, Plantation Crops, Ornamental Crops, Other Crops; University of the Philippines Los Banos: Laguna, Philippines, 1990. [Google Scholar]
- Hofman, P.J.; Whiley, A. Calypso™ Best Practice Guide—From Tree to Taste; Horticulture Australia Ltd.: North Sydney, Australia, 2010. [Google Scholar]
- Khanal, A.; Joyce, D.; Ullah, M.; Irving, D.; Macnish, A.; Joyce, P.; White, N.; Hoffman, E.; Webb, R. Fruit maturity and vapour heat treatment influence ‘flesh cavity with white patches’ disorder in ‘CalypsoTM’ mango. Acta Hortic. 2022, 1364, 241–248. [Google Scholar]
- Khanal, A.; Ullah, M.A.; Joyce, P.; White, N.; Macnish, A.; Hoffman, E.; Irving, D.; Webb, R.; Joyce, D. Impact of Fruit Maturity on Internal Disorders in Vapor Heat Treated Mango Cv.‘B74’. Sustainability 2024, 16, 5472. [Google Scholar] [CrossRef]
- Cutting, J.; Bower, J. Effect of harvest date and applied abscisic acid on browning potential of avocado fruit. S. Afr. Avocado Grow. Assoc. Yearb. 1987, 10, 130–132. [Google Scholar]
- Young, T.; Miner, J. Relation ship of nitrogen and calcium to” soft-nose” disorder in mangoes fruits. Proc. Am. Soc. Hortic. Sci. 1961, 78, 201–208. [Google Scholar]
- Shear, C. Calcium nutrition and quality in fruit crops. Commun. Soil Sci. Plant Anal. 1975, 6, 233–244. [Google Scholar] [CrossRef]
- Lewis, T.; Martin, D.; Cerny, J.; Ratkowsky, D. The effects of increasing the supply of nitrogen, phosphorus, calcium and potassium to the roots of Merton Worcester apple trees on leaf and fruit composition and on the incidence of bitter pit at harvest. J. Hortic. Sci. 1977, 52, 409–419. [Google Scholar] [CrossRef]
- Silva, J.; Hamasaki, R.; Paull, R.; Ogoshi, R.; Bartholomew, D.; Fukuda, S.; Hue, N.; Uehara, G.; Tsuji, G. Lime, gypsum, and basaltic dust effects on the calcium nutrition and fruit quality of pineapple. In Proceedings of the Vth International Pineapple Symposium, Port Alfred, South Africa, 11–16 April 2005; Volume 702, pp. 123–131. [Google Scholar]
- Subraman, H.; Krishnam, S.; Subhadra, N.; Dalal, V.; Randhawa, G.; Chacko, E. Studies on internal breakdown, A physiological ripening disorder in ‘Alphonso’ mangoes. Trop. Sci. 1971, 13, 203–210. [Google Scholar]
- Gunjate, R.; Walimbe, B.; Lad, B.; Limaye, V. Development of internal breakdown in Alphonso mango by post harvest exposure of fruits to sunlight. Sci. Cult. 1982, 48, 188–190. [Google Scholar]
- Katrodia, J.; Rane, D. Pattern of distribution of spongy tissue in the affected ‘Alphonso’ fruits at different locations. Acta Hortic. 1988, 231, 873–877. [Google Scholar] [CrossRef]
- Lad, B.; Gunjate, R.; Salvi, M. Causes and control measures of spongy tissue disorder in ‘Alphonso’ mango fruit: An integrated approach. Maharashtra J. Hortic. 1992, 6, 25–32. [Google Scholar]
- Baloch, M.; Bibi, F. Effect of harvesting and storage conditions on the post harvest quality and shelf life of mango (Mangifera indica L.) fruit. S. Afr. J. Bot. 2012, 83, 109–116. [Google Scholar] [CrossRef]
- Srivastav, M.; Singh, S.; Ajang, M. Evaluation of mango genotypes for jelly seed disorder. Indian J. Hortic. 2015, 72, 408–410. [Google Scholar] [CrossRef]
- Iyer, C.P.A.; Degani, C. Classical breeding and genetics. In The Mango, Botany, Production and Uses; Litz, R.E., Ed.; CAB International: Wallingford, UK, 1997; pp. 49–68. [Google Scholar]
- Iyer, C.; Subramanyam, M. Possibilities of overcoming physiological disorders in mango by breeding. Fruit Breed. Genet. 1992, 317, 241–244. [Google Scholar] [CrossRef]
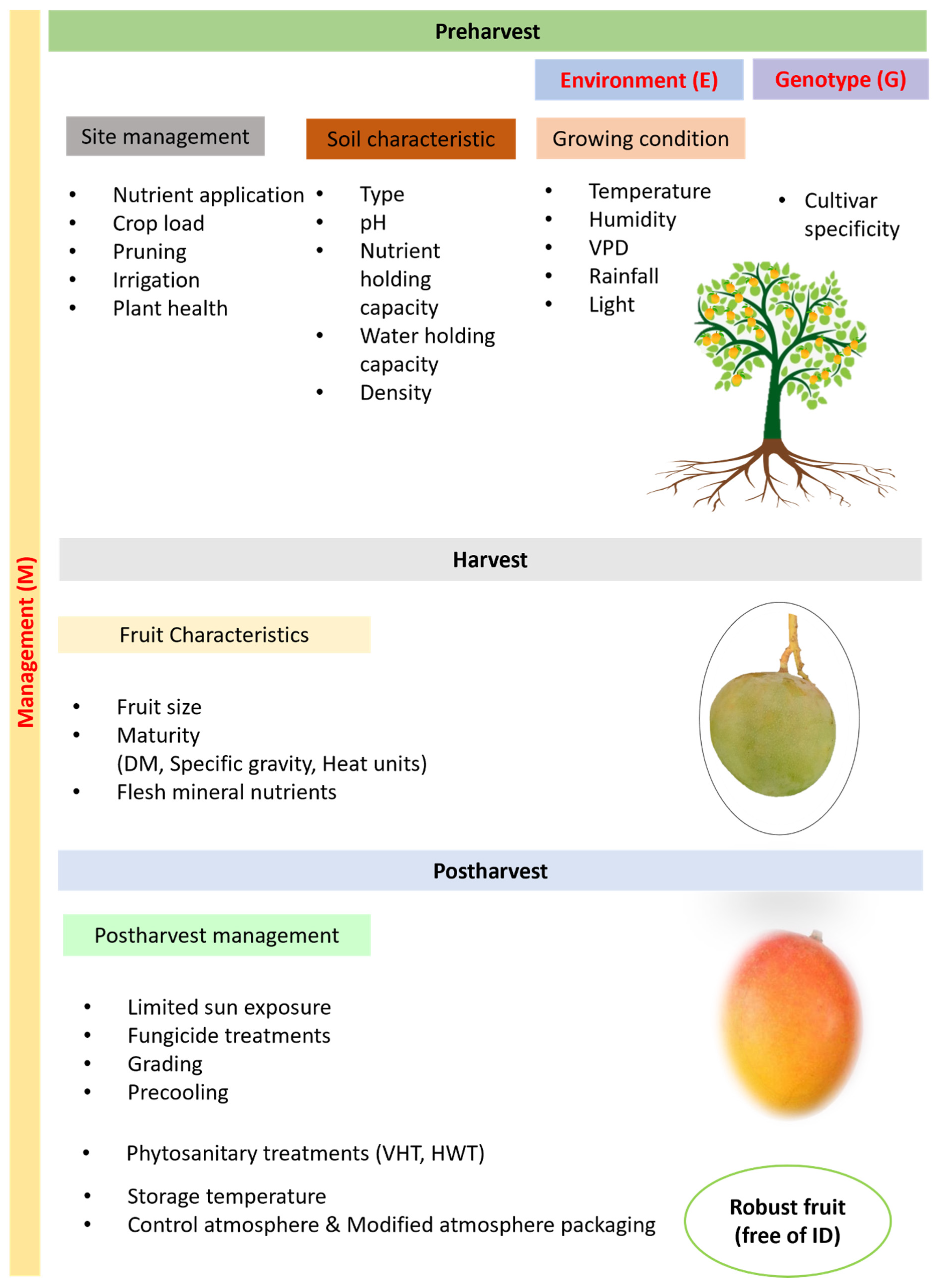
| Genotype | Cultivar (Rootstock, Scion) |
|---|---|
| Environment | Soil type (pH, cation exchange capacity, water holding capacity) Temperature (heat injury, chilling injury) Moisture (rain, relative humidity, precipitation) Radiation (day-length, wavelength, intensity) |
| Management | Canopy management (pruning, thinning, plant growth regulators) Nutrition (calcium, nitrogen, potassium, boron) Irrigation (time, frequency) Harvest (maturity, timing, method) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.A.; Khanal, A.; Joyce, P.; White, N.; Macnish, A.; Joyce, D. Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients. Plants 2024, 13, 2596. https://doi.org/10.3390/plants13182596
Ullah MA, Khanal A, Joyce P, White N, Macnish A, Joyce D. Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients. Plants. 2024; 13(18):2596. https://doi.org/10.3390/plants13182596
Chicago/Turabian StyleUllah, Muhammad Asad, Amit Khanal, Priya Joyce, Neil White, Andrew Macnish, and Daryl Joyce. 2024. "Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients" Plants 13, no. 18: 2596. https://doi.org/10.3390/plants13182596
APA StyleUllah, M. A., Khanal, A., Joyce, P., White, N., Macnish, A., & Joyce, D. (2024). Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients. Plants, 13(18), 2596. https://doi.org/10.3390/plants13182596







