Abstract
Passiflora edulis Sims (2n = 18) is a perennial plant with high utilization values, but its spontaneous polyploidy in nature has yet to be seen. Thus, this study aims to enhance our understanding of polyploidy P. edulis and provide rudimentary knowledge for breeding new cultivars. In this study, colchicine-induced tetraploid P. edulis (2n = 36) was used as experimental material (T1, T2, and T3) to explore the variances between it and its diploid counterpart in morphology, physiology, and biochemical characteristics, and a comparison of their performance under cold stress was conducted. We measured and collected data on phenotype parameters, chlorophyll contents, chlorophyll fluorescence, photosynthesis, osmotic substances, and antioxidant enzymes. The results showed that tetraploid P. edulis exhibited a shorter phenotype, more giant leaves, darker leaf color, and longer and fewer roots. Moreover, the physiological and biochemical analysis indicated that the tetraploid P. edulis had better photosynthesis systems and higher chlorophyll fluorescence parameters than the diploid P. edulis. Additionally, the tetraploid P. edulis had higher activity of antioxidant enzymes (SOD, POD, CAT) and lower MDA content to maintain better resistance in low temperatures. Overall, we conclude that there were apparent differences in the morphological, physiological, and biochemical traits of the tetraploid and diploid P. edulis. The tetraploid plants showed better photosynthesis systems, higher osmotic substance content, and antioxidant enzyme activity than the diploid, even under cold stress. Our results suggest that tetraploids with more abundant phenotype variation and better physiological and biochemical traits may be used as a new genetic germplasm resource for producing new P. edulis cultivars.
1. Introduction
Passiflora edulis Sims is a perennial vine plant belonging to the Passifloraceae family. It is widely distributed in tropical and subtropical areas, boasting edible, medicinal, and ornamental values [1,2]. Its fruit is popular in the food processing industry and has broad market prospects owing to its rich nutrient elements and unique flavors [3]. Thus, cultivating commercial P. edulis varieties with high quality is of great importance.
Polyploidization refers to increases in genome DNA content, is a dominant feature of extant plant diversity [4], and is the main driving force in the evolution of plants [5]. After multiplying their chromosomes, different plant species’ morphological characteristics change to various degrees. From the perspective of most reports about polyploidy, it can be seen that larger stoma [6], thicker leaves [7], darker leaf color [8], increased flower diameter [9,10], stem diameter [11], and fruit sizes [12] presented in plants with higher ploidy. A higher organic nutrient content and better environmental stress resistance were observed in polyploids regarding physiological and biochemical traits. For example, tetraploid Eucalyptus urophylla reported higher net photosynthetic rates and a higher content of specific secondary metabolites [13], and the tetraploid line of Centella asiatica (L.) exhibited higher total triterpenoids than its diploid counterpart [14]. In addition, tetraploid bermudagrass “CX” showed significantly greater shade tolerance compared to its triploid bermudagrass, for the former can maintain higher photosynthetic performance, photosynthetic pigments, and a lower efficient use of light energy under shade stress [15]. Diploid Populus ussuriensis Kom. suffered more severe salt injury than polyploid [16]. What is more, tetraploid plants could be used as breeding material and bred with diploid ones to produce triploid plants [17], while triploids have the characteristics of few seeds [18]. For the fruit utilization of P. edulis, the seed is an important influence factor; thus, the study of tetraploid P. edulis helps establish the triploid breeding system and reduces the cost and difficulty of processing. Based on the importance of using polyploidy technology in promoting plant breeding, scientists are trying to achieve the goal of improving target plants quickly through artificial induction methods [19,20]. Colchicine induction is one of the most commonly used polyploid induction methods. It is applied to a wide range of crops, including Actinidia chinensis [21], Cerasus humilis [22], and Chinese jujube (Ziziphus jujuba Mill.) [23].
In nature, the wild polyploid P. edulis (2n = 18) is still absent. Zhangqin once used three kinds of passionfruit (P. edulis, P. edulis var. flavicarpa Deg., and Tai-Nung No. 1) to induce artificial triploids and tetraploids by culturing the endosperm of mature seeds and treating the adventitious buds with colchicine, respectively. She conducted a preliminary morphologic evaluation of the polyploidies, and the results showed that noticeable differences in leaf morphology and stoma were observed in the polyploid passionfruit, compared to the diploid [24]. However, the emphasis of Zhang’s research was more on the in vitro induction of polyploid passionfruit, and there is a lack of specific and further analysis in terms of the physiological and biochemical traits of tetraploid P. edulis. In recent years, the differences in morphology, chlorophyll content, and chlorophyll fluorescence parameters and the photosynthetic capacity variation rules of triploid P. edulis “Mantianxing” seedling leaves were revealed; as these findings demonstrated, the triploid plant was different from the diploid one in terms of its phenotype and superior to the diploid one in physiological indexes [25]. However, it is unknown whether tetraploid P. edulis, like most plants, has a decided polyploidization advantage in morphology, physiology, and biochemistry compared with its diploid counterpart. Thus, this research used tetraploid P. edulis seedlings as materials, conducted a relatively complete study on the above indexes, and further evaluated the performance of diploids and tetraploids under cold stress. It makes up for the gaps in related fields to a certain extent.
Temperature is one of the most vital factors which regulate plant development. Low temperatures can cause severe and irreversible damage to plant cells and induce physiological and metabolic changes affecting plant growth [26]. As a tropical and subtropical plant [27], low temperature is the main barrier for P. edulis to expand its habitat. Thus, to explore the adaptability of P. edulis to low temperatures, we analyzed the performance of photosynthetic characteristics and the activity of antioxidant enzymes, Malondialdehyde, and proline in diploid and tetraploid P. edulis under cold stress. This research aims to provide insights into the variations of P. edulis with different ploidies and offer a reference for expanding the variety of breeding in cold areas.
2. Results
2.1. Ploidy Levels Identification
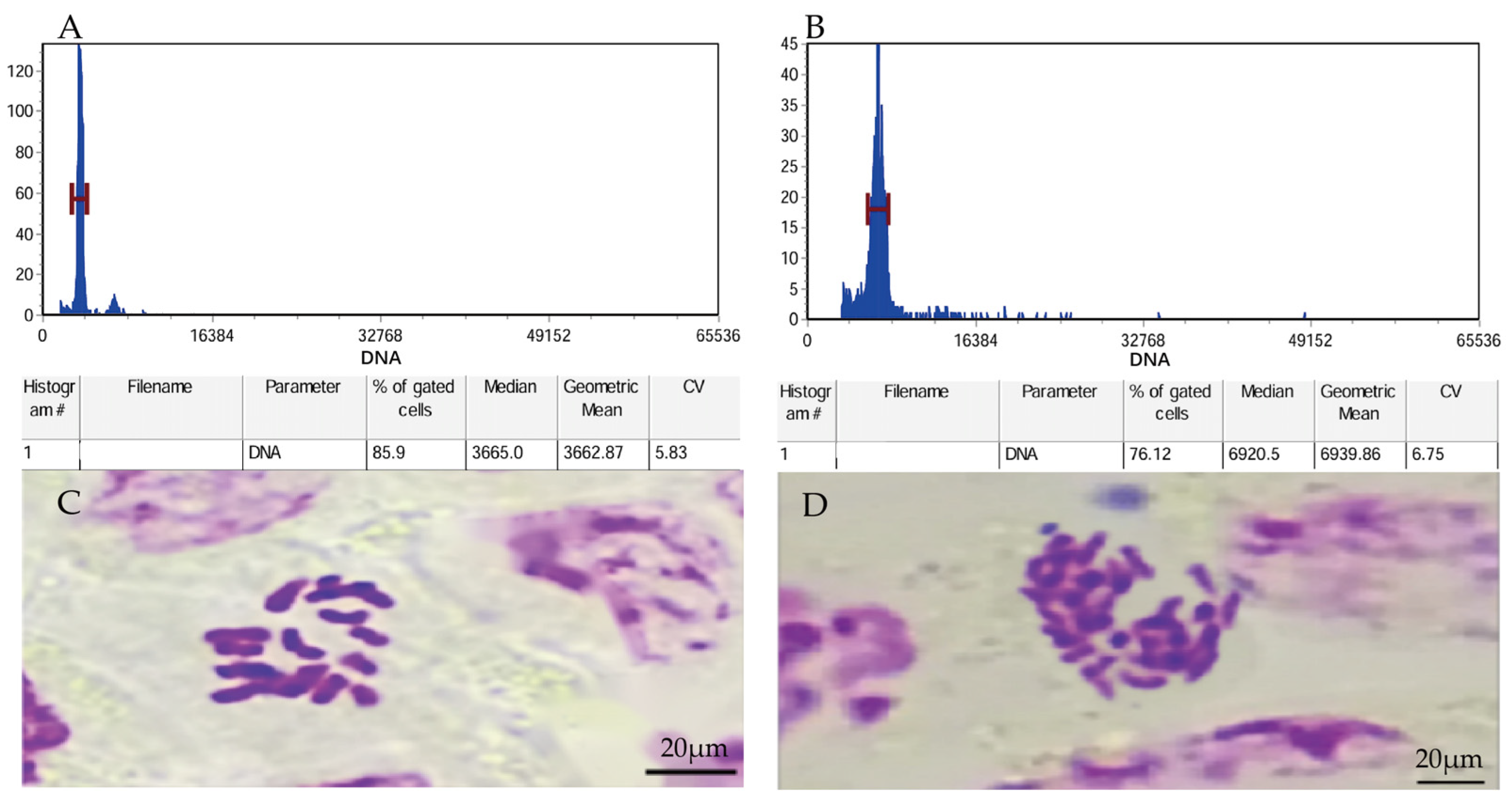
Flow cytometry and chromosome counting were used to verify the ploidy levels. The results showed that, in flow cytometry, the fluorescence intensity in diploid leaves was 3665.0 while that in leaves treated with colchicine was 6920.5, presenting a nearly doubled 2C-DNA compared with the former. The diploid plants contained 18 chromosomes, while the variant plants had 36 chromosomes. These results suggested that P. edulis plants were tetraploid plants after being treated with colchicine (Figure 1).
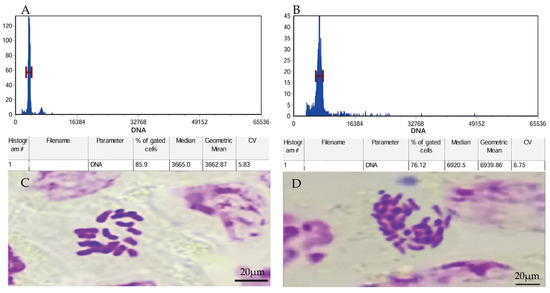
Figure 1.
Flow cytometric profiles of diploid and P. edulis treated by colchicine (tetraploid). (A) The value corresponding to the diploid flow cytometer fluorescence peak on the abscissa. (B) The value corresponding to the tetraploid flow cytometer fluorescence peak on the abscissa. (C) Chromosomes prepared from diploid (2n = 2x = 18) and (D) chromosomes prepared from tetraploid (2n = 4x = 36) samples of P. edulis.
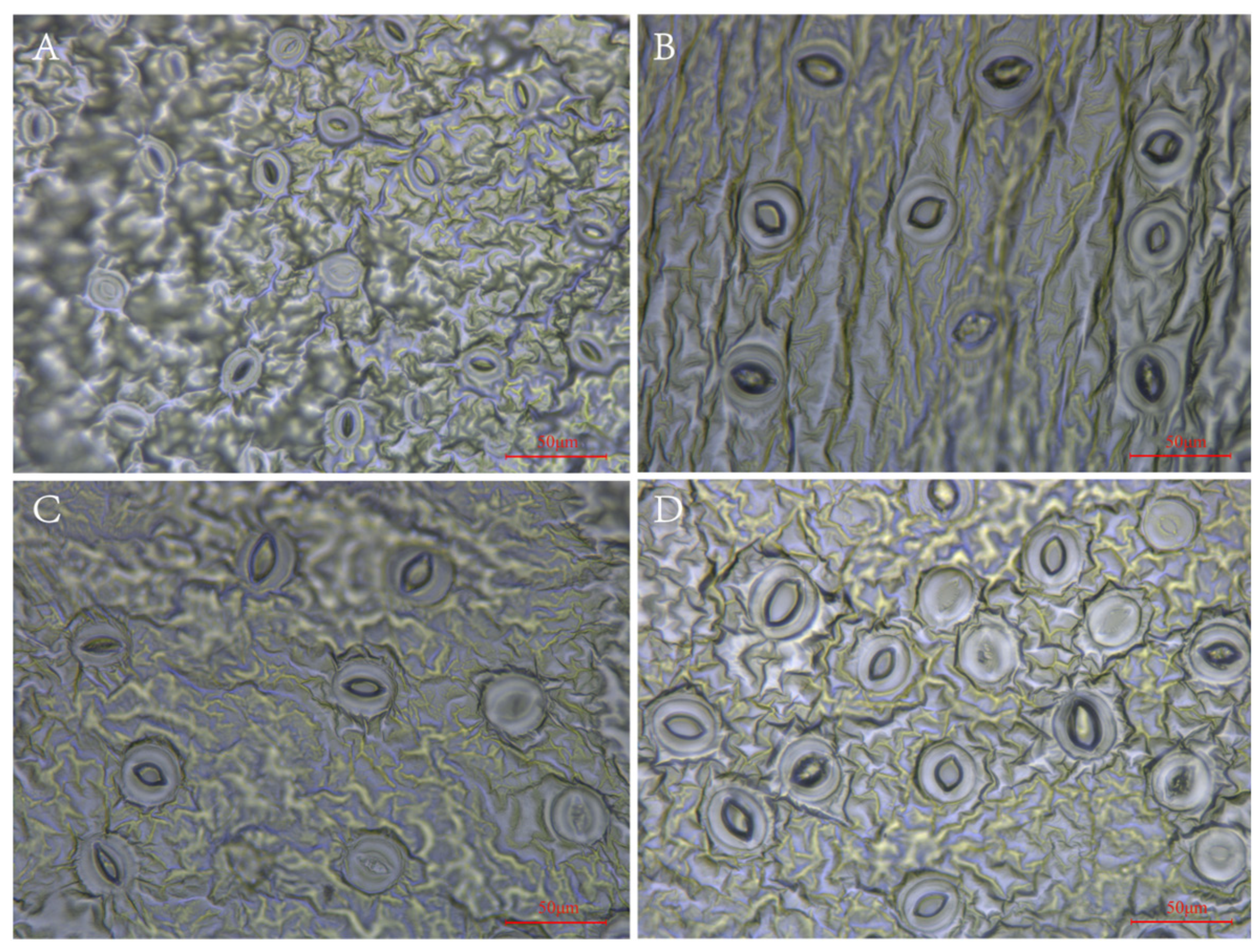
From Figure 2, it can be observed that the tetraploids indeed had larger but fewer stomas than the diploid. The means of stoma length and width of the tetraploids significantly (p < 0.05) increased by 44.78% and 65.72%, respectively, as compared to those of the diploid plants. In contrast, the diploid had higher stoma numbers and density (Figure 2, Supplementary Table S1).
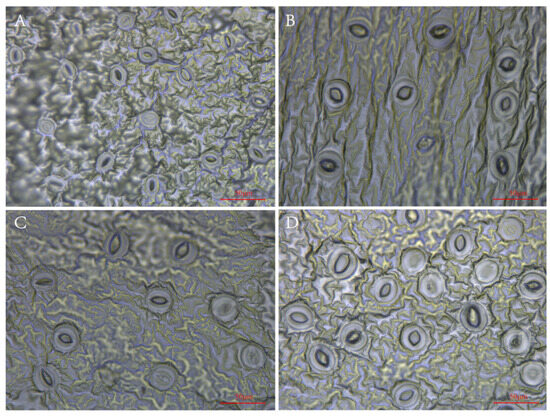
Figure 2.
Stoma morphology of diploid and tetraploid P. edulis. (A) Stoma morphology of diploid. (B) Stoma morphology of tetraploid T1. (C) Stoma morphology of tetraploid T2. (D) Stoma morphology of tetraploid T3.
2.2. Morphological Characteristics Comparison of Diploid and Tetraploid P. edulis
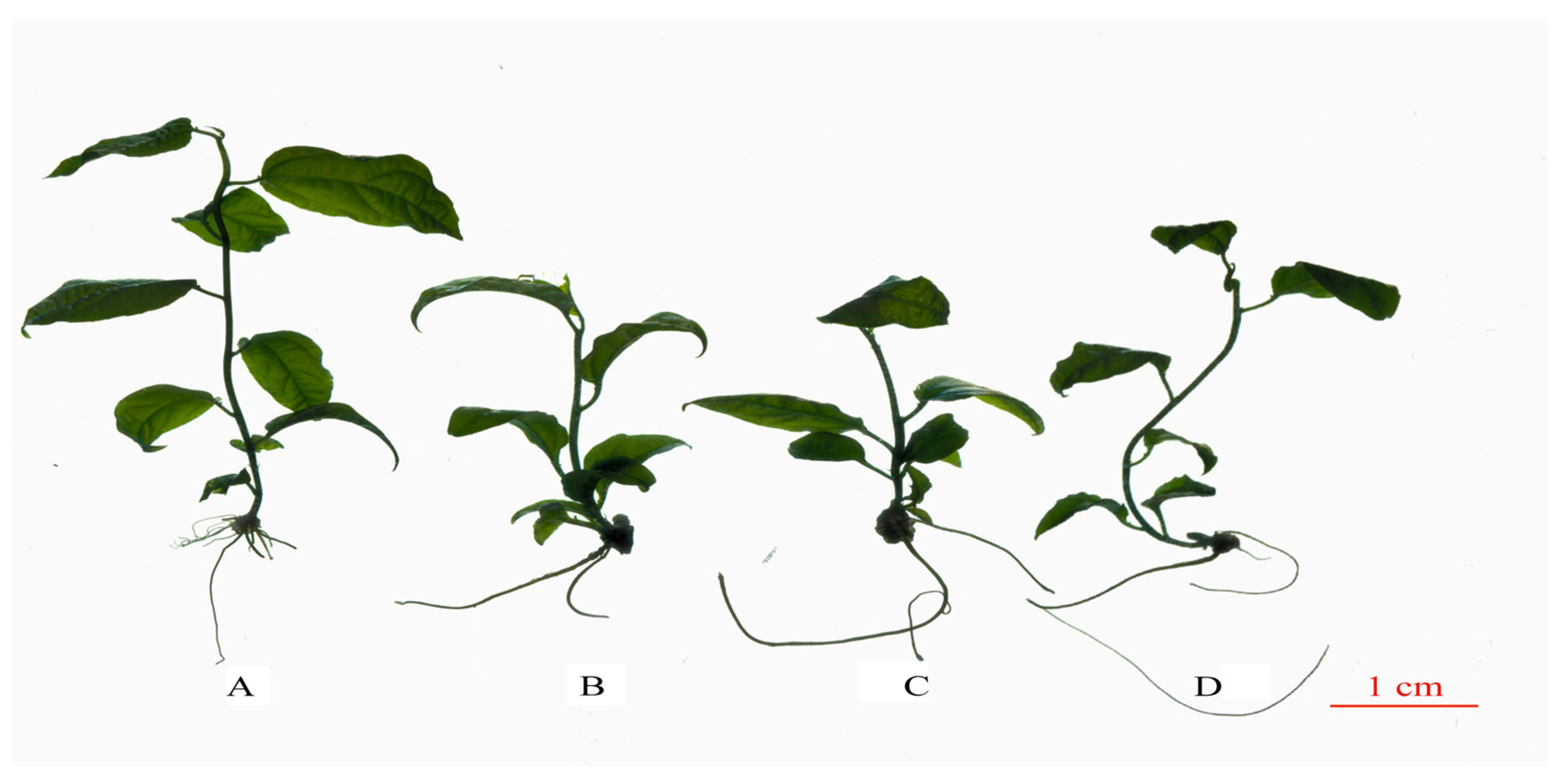
Primary observation found significant morphological differences between the diploid and tetraploid plants (Figure 3). Overall, compared with the diploid, the tetraploid plants had the dwarfing phenotype, thicker ground diameter, darker leaf color, more leaf curves, and increased root length but fewer root numbers. Results indicated that polyploidization led to plant dwarfing but facilitated the development of the root system. Additionally, the increase in genome DNA content significantly influenced the leaf morphology. Moreover, the ground diameter of the tetraploids was 23.58% thicker on average than that of the diploid, and the highest value of ground diameter was tetraploid T3 (Supplementary Table S2).

Figure 3.
Morphological diagram of diploid and tetraploid P. edulis. (A) Morphological diagram of diploid. (B) Morphological diagram of tetraploid P. edulis T1. (C) Morphological diagram of tetraploid P. edulis T2. (D) Morphological diagram of tetraploid P. edulis T3.
2.2.1. Root System Comparison of Diploid and Tetraploids
The differences between the underground parts of different ploidy levels were observed. The measurement of the root system showed that the means of the three tetraploid lines were 31.39% longer than that of the diploid, with T1 presenting a significant (p < 0.05) difference. However, the average root numbers for the diploid plant significantly (p < 0.05) increased by 183.64%, compared to the tetraploids. In a word, tetraploids had longer but fewer roots (Supplementary Table S2).
2.2.2. Leaf Morphology
Leaf morphology comparison with more details suggested that the tetraploid plants had significant differences with the diploid in leaf thickness, leaf width, petiole length, leaf area, leaf serration numbers, and leaf color. Compared with the diploid, the means of leaf thickness, leaf width, petiole length, and leaf area in the tetraploids were increased by 19.22%, 18.90%, 51.22%, and 14.72% more than the diploid, respectively. There was no significant difference in leaf length between the two ploidy levels. With each ploidy level, the leaf color tends to be darker; tetraploid plants are all dark green, while diploid plants are dark yellow-green (Supplementary Tables S3 and Figure 4).
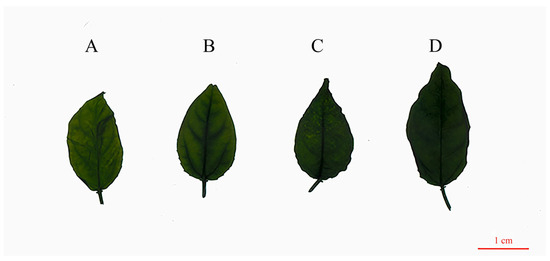
Figure 4.
Leaf morphology of diploid and tetraploid plants of diploid and tetraploid P. edulis. (A) Diploid. (B) Tetraploid T1. (C) Tetraploid T2. (D) Tetraploid T3.
2.2.3. Variation Analysis of Diploid and Tetraploid Morphological Indices
The variation analysis of the morphological indices of diploid and tetraploid P. edulis (Table 1) indicated that the most significant coefficient of variation was RL and RN in the diploid and tetraploid plants, respectively. In contrast, the smallest coefficient of variation was LA. However, except GD, RL, and PL, the coefficient of variation of RN, PH, LA, LW, IN, LSN, LL, and LT in tetraploid was higher than that of diploid, respectively.

Table 1.
Variation analysis of morphological indices of diploid and tetraploid P. edulis.
2.3. Variation of Physiological Indices
2.3.1. Soluble Sugar and Soluble Protein Contents
We examined the changes in soluble sugar and soluble protein content between the diploid and tetraploid plants. The mean soluble sugar contents in tetraploid T1, T2, and T3 were significantly (p < 0.05) raised by 65.01%, 62.79%, and 63.60%, respectively, as compared to the diploid. Results also showed a similar trend in the changes in soluble protein content among the two ploidy levels; for example, the soluble protein content in the diploid measured as 35.33 ± 4.87 mg·g−1, while the soluble protein contents in tetraploid T1, T2, and T3 were 30.46%, 27.69%, and 33.75% higher than the diploid, respectively (Table 2). These results indicated that the soluble sugar and protein content showed an absolute advantage after P. edulis polyploidization.

Table 2.
Variation analysis of physiological index in diploid and tetraploid plants of P. edulis.
2.3.2. Variation Analysis of Chlorophyll Content
Genome multiplication also affects chlorophyll content, which is essential for the promotion of plant growth and development. Compared with diploid, tetraploid significantly (p < 0.05) had more abundant chlorophyll content. The mean content of chlorophyll a in the tetraploids increased by 20.12% over the diploid. The mean content of chlorophyll b increased by 21.53% over the diploid. The mean of total chlorophyll content in the tetraploids was 20.48% higher than in the diploid. The average carotenoid content of the tetraploid lines was 28.97% higher than that of the diploid lines. These results can be summarized by saying that the tetraploids had richer chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content than the diploid (Table 2). This indicates that tetraploid is equipped with a better light capture capacity.
2.4. Difference in Antioxidant Enzyme and MDA, Pro Content in Diploid and Tetraploid
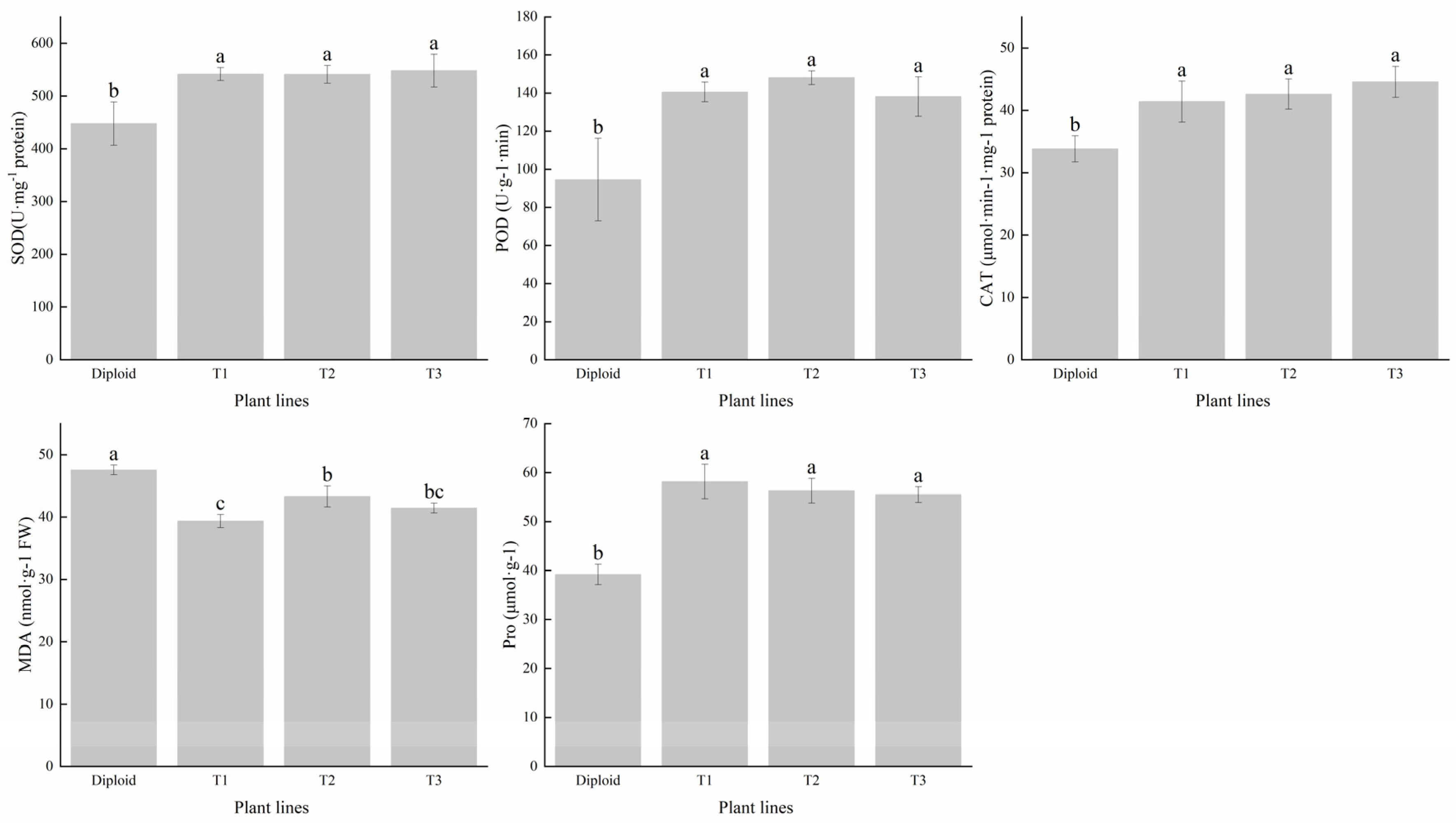
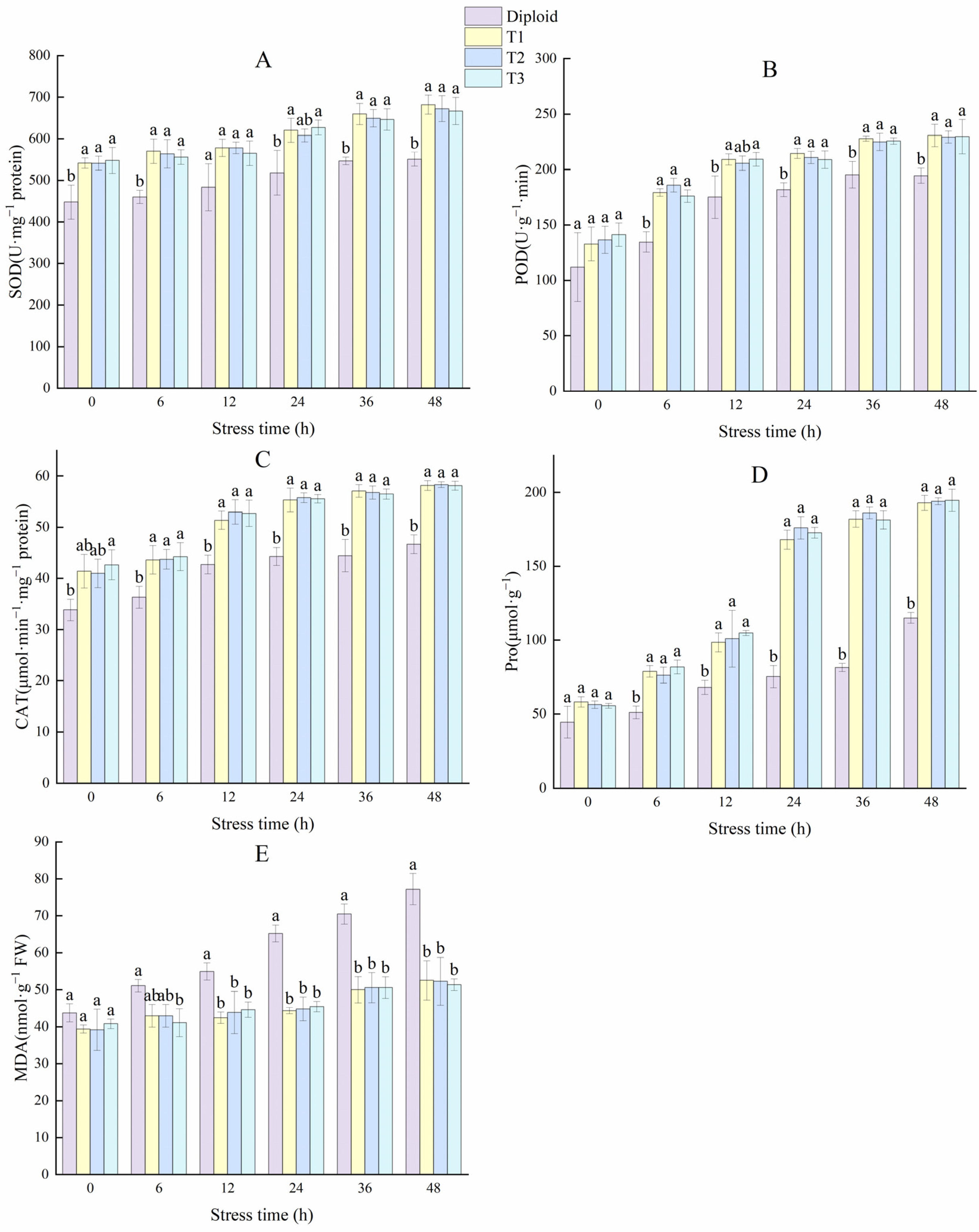
We tested the contents of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) in the two ploidies. The average SOD content of tetraploid was 21.45% higher than that of diploid. The activity of POD had significantly (p < 0.05) increased after tetraploidization. Its average content in the three tetraploid lines was 50.49% higher than in the diploid. The mean CAT content of the tetraploids was 26.73% higher than that of the diploid, especially the T3 line, which had a conspicuous increase in advantage; the increased amplitude was 31.81% higher than that of the diploid (Figure 5). In general, the activity of the antioxidant enzyme of tetraploid was superior to that of diploid, and we can make a forecast that tetraploids of P. edulis may have a better performance than diploids under abiotic stress, according to the critical function of these enzymes in plant growth and development.
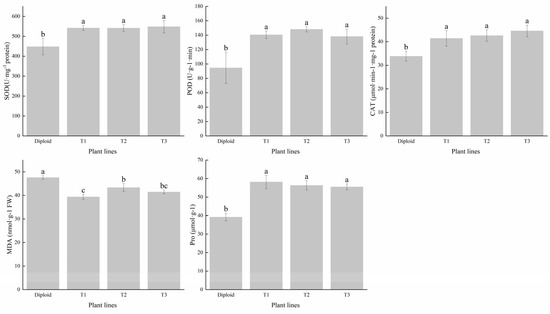
Figure 5.
Difference analysis of antioxidant enzyme (SOD, POD, and CAT) and contents of MDA and Pro in diploid and tetraploid of P. edulis. Note: Different letters indicate significant differences, and the same letters mean insignificant differences (p < 0.05). Duncan test.
To reveal the stress resistance of the two ploidy levels of P. edulis, we tested the content of MDA and Pro. The results indicated that the diploid had a higher MDA content than all three tetraploids, which was 14.99% higher than the mean of the tetraploids, indicating that the damage degree of the diploid was more severe than the tetraploid in some stress conditions. The change trend in Pro content was different from MDA, compared with diploid; the tetraploids’ average Pro content was 44.56% higher than the diploid, which can be used to adjust the stability of the membrane (Figure 5). These results indicated that tetraploid P. edulis may have better plant stress resistance.
2.5. Comparison of Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters between Diploid and Tetraploid P. edulis
2.5.1. Comparison of Photosynthetic Characteristics
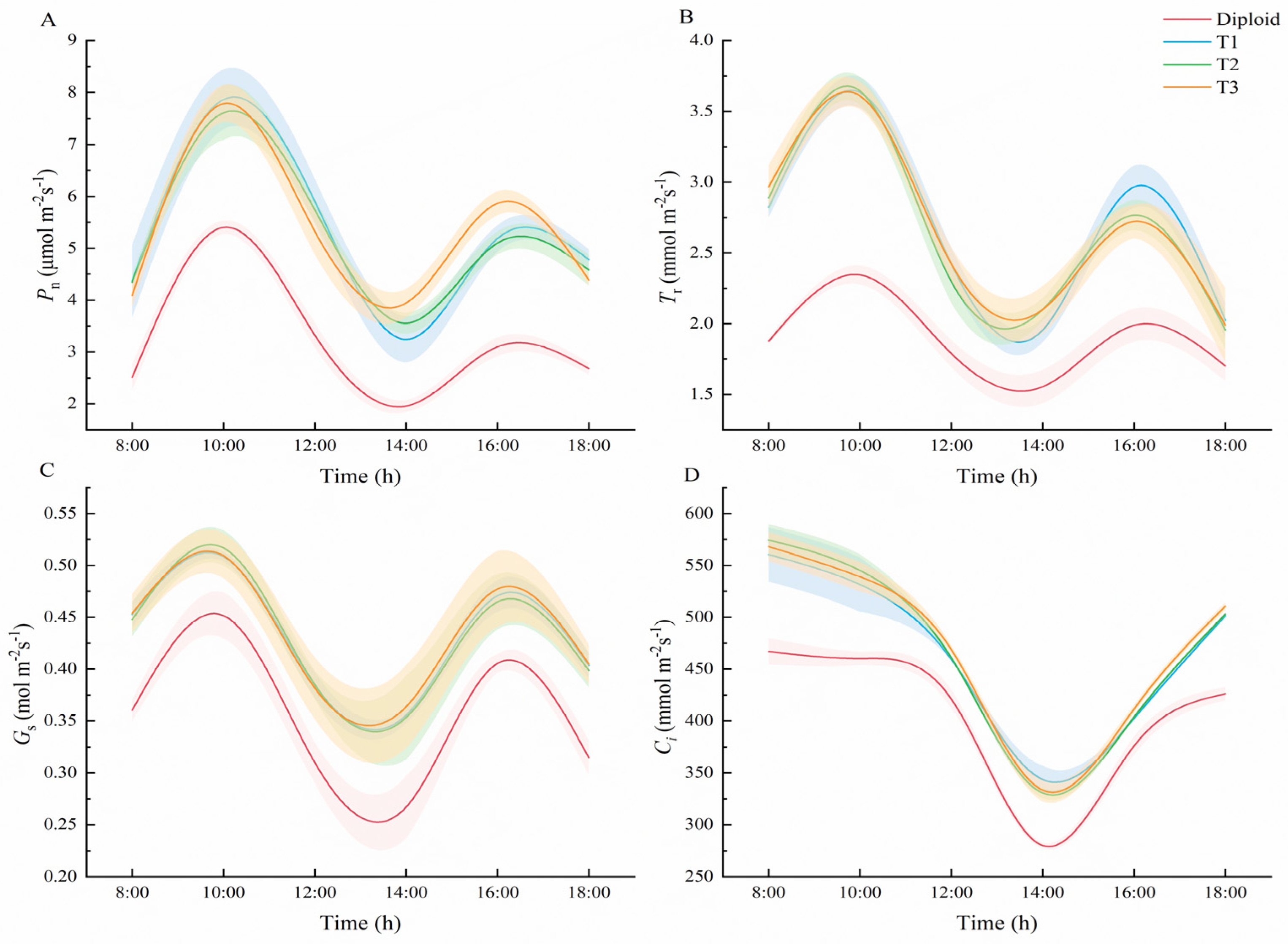
According to Figure 6, the trends in diurnal variation of Pn, daily variation of Tr, Gs, and daily variation of Ci in diploid and tetraploid P. edulis were similar. As went on, the trends in Pn, Tr, and Gs in both the diploid and tetraploids presented as bimodal curves, and all increased to the peak at 10:00, and to the valley at 14:00, when the average Pn of tetraploid was 43.62%, and 83.14% higher than that of diploid, respectively (Supplementary Table S4). Overall, the Pn of the three tetraploids was significantly (p < 0.05) higher than the diploid at each time, which means an increase in ploidy level may cause the improvement of the net photosynthetic rate in the plant. At 10:00 and 16:00, the average Tr of tetraploid was 54.82% and 41.71% higher than that of diploid, and the average Gs of tetraploid increased by 13.25% and 16.10%, respectively (Supplementary Tables S5 and S6). The Tr of the three tetraploids was significantly (p < 0.05) higher than the diploid at each time, except at 18:00, which may relate to the size of the plant’s stoma. The daily variation trend of Ci in diploid and tetraploid were very different from the other three trends, shown as capital letter “U”. From 8:00 to 14:00, the value of Ci in the two ploidy species declined continually and reached the minimum value at 14:00. From 14:00 to 18:00, the concentration of Ci in both diploid and tetraploid increased continually. On the whole trend, the Ci values of the tetraploids were all significantly (p < 0.05) greater than that of the diploid (Supplementary Table S7).
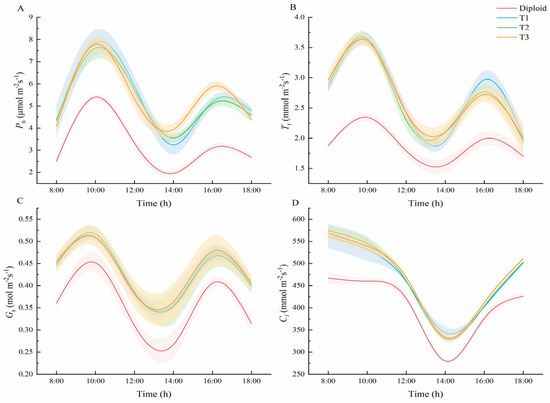
Figure 6.
Comparison of photosynthetic characteristics between diploid and tetraploid. ((A) Diurnal variation trend in net photosynthetic rate of diploid and tetraploid P. edulis. (B) Diurnal variation trend in transpiration rate of diploid and tetraploid P. edulis. (C) Diurnal variation of stomatal conductance degree days of diploid and tetraploid P. edulis. (D) Diurnal variation trend in intercellular CO2 concentration in diploid and tetraploid P. edulis.)
2.5.2. Comparison of Chlorophyll Fluorescence Parameters between Diploid and Tetraploid P. edulis
As shown in Table 3, a significant difference existed in the initial photochemical efficiency and initial fluorescence parameters between diploid and tetraploid. Compared with diploid, the average value of Fo in tetraploid was 27.85% higher than diploid, especially the T1 line, which was significantly (p < 0.05) higher than the latter. Similar results were observed in other parameters, the average values of Fm, Fv, Fm/Fo, Fv/Fm, and Fv/Fo in tetraploid were 17.40%, 19.10%, 46.62%, 16.63%, and 14.96% higher than those of diploid, respectively.

Table 3.
Analysis of the difference in initial photochemical efficiency and initial fluorescence parameters between diploid and tetraploid PS II of P. edulis.
2.5.3. Kinetic Curve of Chlorophyll Fluorescence Induction
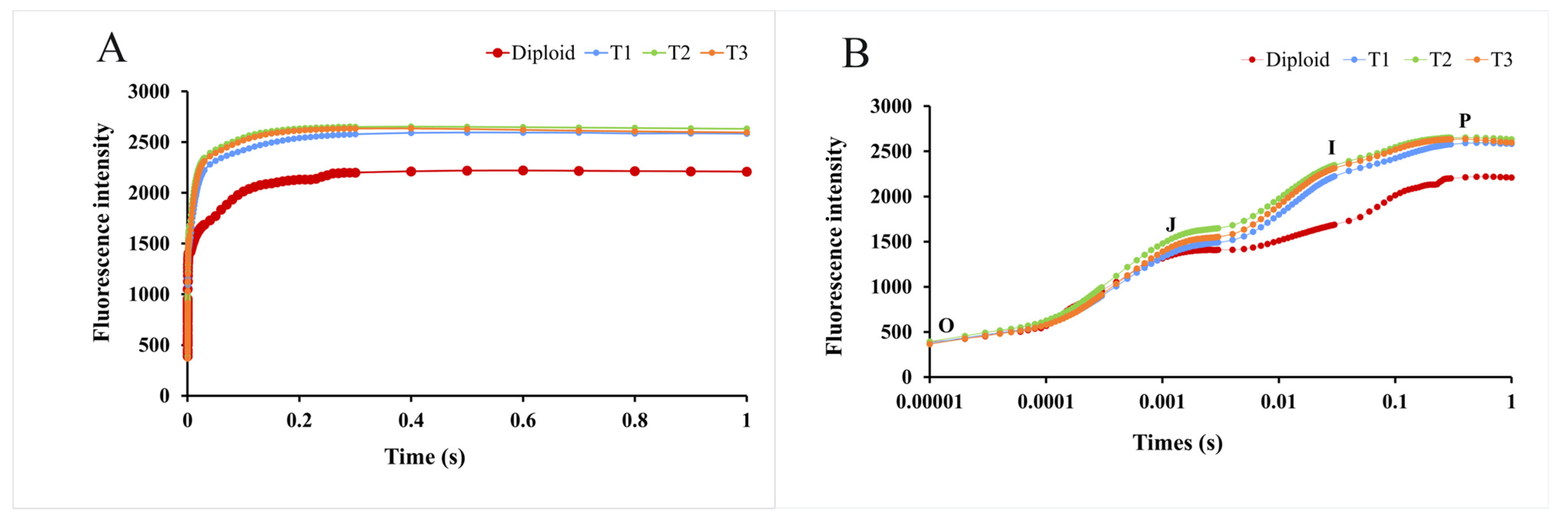
Green plants contain many chloroplast stromata, which can produce red and changing fluorescence under light. With time exposure, the fluorescence changes and is presented as a dynamic curve, called the kinetic curve of chlorophyll fluorescence induction (OJIP curve). As Figure 7 shows, as time went on, the fluorescence intensity of diploid and tetraploid P. edulis increased continually and presented differences at the “J” point. During the whole process, the fluorescence intensity of the tetraploid was higher than that of the diploid, especially at the “I” and “P” points. Based on this analysis, we could conclude that tetraploid P. edulis had a larger fluorescence yield and better photosynthetic efficiency than diploid.
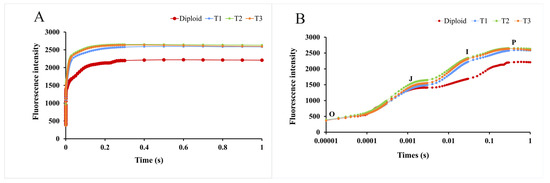
Figure 7.
Kinetic curves of chlorophyll fluorescence induction of diploid and tetraploid P. edulis. (A) represents the difference in fluorescence intensity between diploid and tetraploid P. edulis on a linear time coordinate, and (B) represents the difference in fluorescence intensity between diploid and tetraploid P. edulis on a logarithmic time coordinate.
2.6. Correlation Analysis and Principal Component Analysis of Various Indices between Tetraploid and Diploid P. edulis
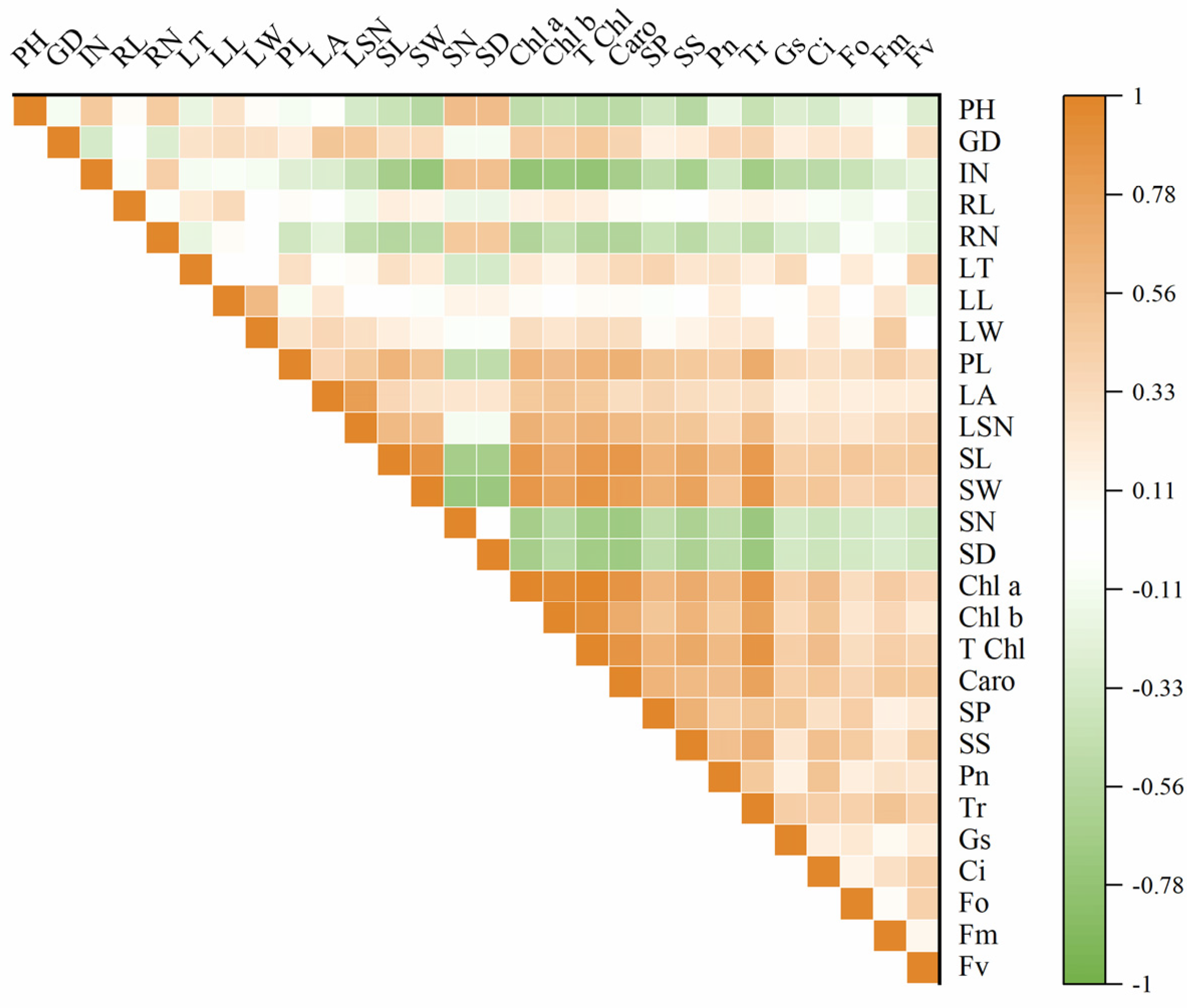
Significant differences were found between diploid and tetraploid when their morphological and physiological indices were compared. To explore the correlations and rules in these indices of diploid and tetraploid, correlation analysis was performed on the 28 indexes of the diploid and tetraploid of P. edulis. The results showed that plant height and internode numbers were significantly positively correlated with stoma number and density; on the contrary, they were negatively correlated with stoma length and width, chlorophyll content, soluble sugar, soluble protein, and photosynthetic characteristics. Leaf area positively correlated with chlorophyll content. Stoma size was very significantly positively correlated with chlorophyll content and carotenoids. This suggested that the larger the stoma size, the higher the chlorophyll content, and the better the photosynthetic characteristics. Stoma number and stoma density were very significantly negatively correlated with chlorophyll a, chlorophyll b, total chlorophyll content, and carotenoids. Chlorophyll a, chlorophyll b, total chlorophyll content, and carotenoid content were positively correlated with soluble sugar, soluble protein, Pn, Tr, Gs, and Ci. The content of chlorophyll a was positively correlated with Fm and Fv. An extremely significant positive correlation existed between the total chlorophyll content, carotenoids, and Fo, Fm, and Fv. The results of soluble sugar and soluble protein analysis showed that soluble sugar content was positively correlated with Pn, Tr, Gs, Ci, and Fo. The same correlation was observed in the soluble protein and Pn, Tr, Ci, Fo, and Fv (Figure 8). To further analyze the relationship between each index, PCA (principal component analysis) was carried out (Supplementary Figure S1). According to the result, there were seven components with eigenvalues greater than one, and the cumulative contribution rate reached 77.98%, which contained almost the information of the original data and could be used to evaluate and judge the germplasm resources of P. edulis. The first principal component was highly correlated with chlorophyll content, stoma width, carotenoid, and stoma length. These traits were mainly involved in the process of photosynthesis and respiration. The second principal component was highly related to leaf area, length, width, and stoma density. From Supplementary Figure S1, there was a clear separation between diploid and tetraploid T1, T2, and T3 in the coordinate system, and three tetraploid lines showed higher overlap ratio, indicating a significant difference between diploid and tetraploid, and no significant difference between tetraploid lines.
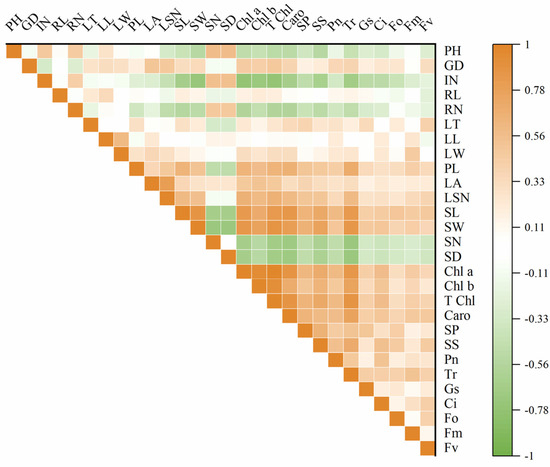
Figure 8.
Correlogram displaying correlations among different studied characteristics of two P. edulis cultivars. Positive and negative correlations are represented in orange and green colors, respectively. The correlation coefficient based on the color index is shown on the right side of the correlogram. PH: plant height; GD: ground diameter; IN: internode number; RL: root length; RN: root number; LT: leaf thickness; LL: leaf length; LW: leaf width; PL: petiole length; LA: leaf area; LSN: leaf serration number; SL: stomata length; SW: stomata width; SN: stomata number; SD: stomata density; Chl a: chlorophyll a; Chl b: chlorophyll b; T Chl: total chlorophyll content; Caro: carotenoid, SP: soluble protein; SS: soluble sugar. Pn: net photosynthetic rate; Tr: transpiration rate; Gs: stomatal conductance; Ci: intercellular CO2 concentration; Fo: minimum fluorescence yield in the absence of photosynthetic light; Fm: maximum fluorescence yield in the absence of photosynthetic light; Fv: variable fluorescence.
2.7. The Analysis of Diploid and Tetraploid P. edulis under Cold Stress
2.7.1. The Photosynthetic Characteristics Variation of Different Ploidy P. edulis under Cold Stress
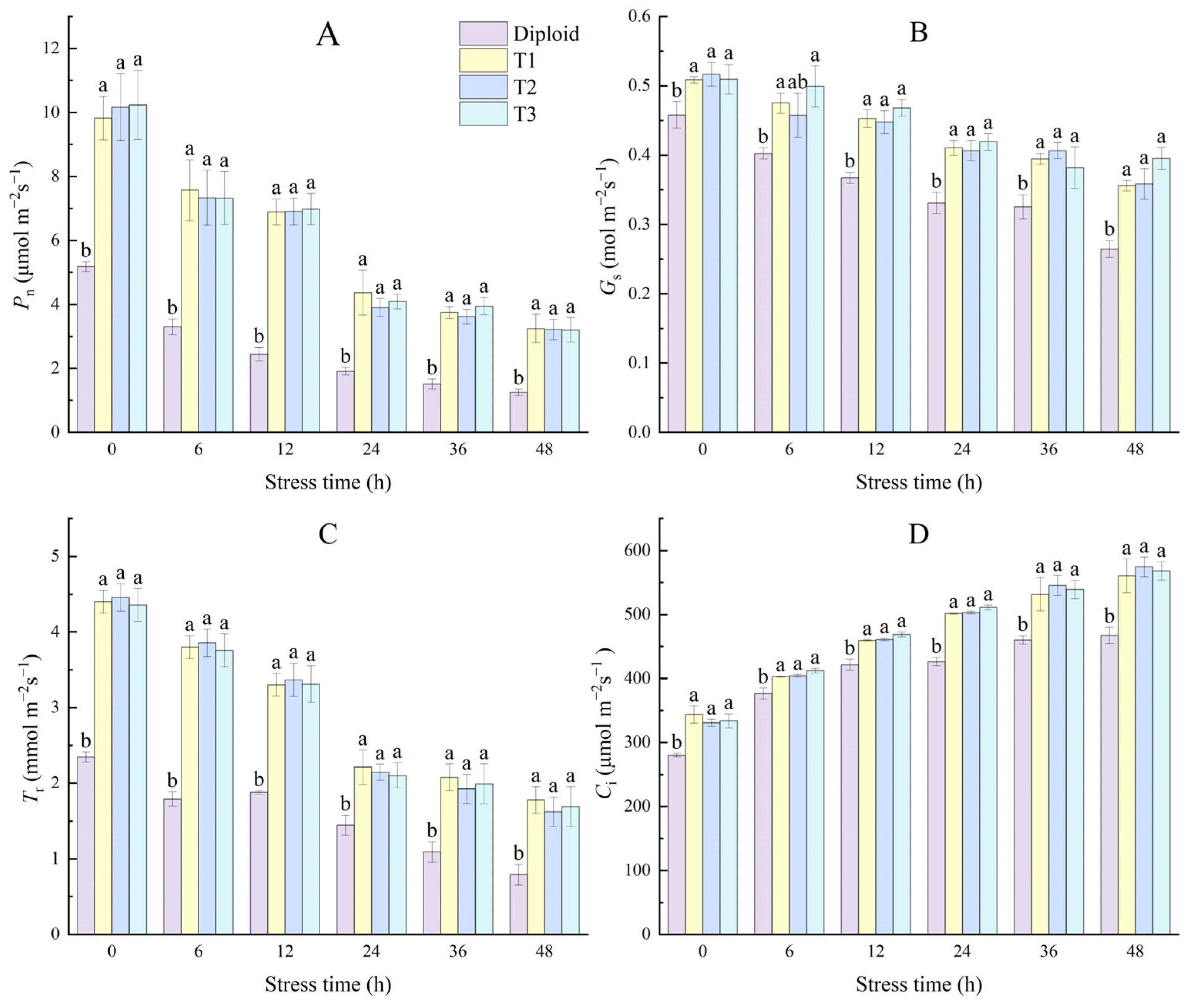
Figure 9 shows that cold stress specifically affected diploid and tetraploid P. edulis. With the stress time prolonged, the damage degree increased, and the net photosynthetic rate, stoma conductance, and transpiration rate in both diploid and tetraploid leaves showed a downward trend and hit its lowest point at 48 h. During the whole stress treatment, the net photosynthetic rate in both diploid and tetraploid rapidly decreased in the 0~24 h, then slowing the decline, and all the Pn of the three tetraploid lines were significantly (p < 0.05) higher than the diploid. However, there was no significance between tetraploid plants. At 48 h, the values of Pn in T1, T2, and T3 were almost three times those of diploid. The trend in variation of Tr was similar to Pn; at 48 h, the values of Tr in tetraploid T1, T2, and T3 were 125.35%, 105.63%, and 114.08% higher than diploid, respectively. Stoma is the channel through which water vapor and CO2 exchange between plants and the atmosphere, which also affects the photosynthesis and transpiration of plants. From Figure 9, the values of Gs in diploid and tetraploid decreased with the stress times. However, there was significant variation in both ploidies, indicating that the stress effect of the photosynthetic efficiency under low temperatures was primarily related to stoma inhibition. Unlike the former three indices, the Ci of the two ploidy plants increased with the stress time; the Ci of diploid and tetraploid T1 lines rose significantly in the early stage of treatment but not significantly in the late stage. The intercellular CO2 concentration of tetraploid T2 and T3 lines increased significantly each time.
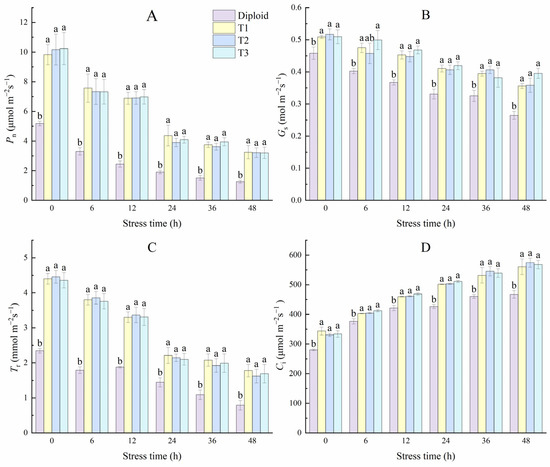
Figure 9.
Effect of low-temperature stress on photosynthesis characteristics of diploid and tetraploid P. edulis. (A) Net photosynthetic rate (Pn). (B) Stomatal conductance (Gs). (C) Transpiration rate (Tr). (D) Intercellular CO2 concentration (Ci). Different letters indicate significant differences in the different plant lines at the same stress stage, and the same letters mean insignificant differences (p < 0.05).
2.7.2. The Changes in Enzyme Activity (SOD, POD, and Pro) and MDA Content of Diploid and Tetraploid P. edulis under Low-Temperature Stress
The variations of enzyme activity in the diploid and tetraploid plants are shown in Figure 10. The enhancement of SOD activity can improve oxidation resistance and stabilize membrane permeability in plants. Compared to the diploid, the SOD activities of the tetraploid plants were significantly (p < 0.05) higher. The SOD activity in the T1, T2, and T3 plants increased by 23.88%, 22.08%, and 21.07% more than the diploid at 48 h, respectively. The POD activity in the diploid and tetraploids rose rapidly in the first 12 h, and tetraploid plants maintained a significantly (p < 0.05) higher activity than the diploid from the 6 h to the end. At 48 h, the POD activity of tetraploid T1, T2, and T3 plants was 18.67%, 17.94%, and 18.12% higher than the diploid plant, respectively. That means the tetraploids performed a better active oxygen scavenging capacity under stress. The changing trend in the activity of CAT was similar to POD; the CAT activity of the tetraploids T1, T2, and T3 was 24.66%, 25.00%, and 24.57% higher than the diploid at 48 h, respectively. From these results, it can be concluded that tetraploids have more advantages in terms of antioxidant balance under cold stress (Figure 10). In general, plants with strong stress resistance will produce more Pro to reduce water potential and regulate the osmotic balance of plants. Pro activity is crucial for plants to improve cold resistance. Tetraploid plants showed a significantly (p < 0.05) higher Pro activity than diploid, especially during 12~48 h. At 48 h, the Pro activity of tetraploid T1, T2, and T3 plants were 67.65%, 68.59%, and 69.20% higher than diploid.
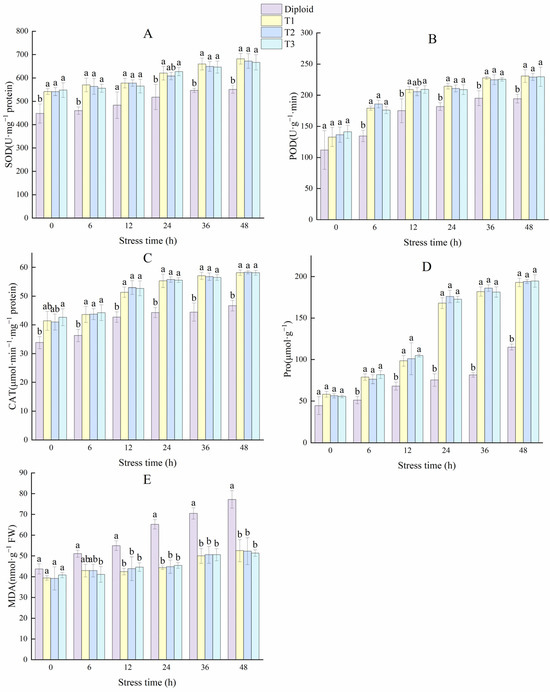
Figure 10.
Effect of low-temperature stress on antioxidant enzymes (SOD, POD, CAT), Pro, and MDA content of diploid and tetraploids. (A) SOD (superoxide); (B) POD (peroxidase); (C) CAT (catalase); (D) Pro (proline); (E) MDA (malondialdehyde). Different letters indicate significant differences in the different plant lines at the same stress stage, and the same letters mean insignificant differences (p < 0.05).
Malondialdehyde (MDA) is one of the commonly used indices to investigate the aging and resistance of plants. Cold stress increased MDA in the two ploidy levels, and there was no significant variation in the tetraploid plants during each stress period. In the diploid plant, the amount of MDA increased by 76.59% at 48 h compared to 0 h, and its increase amplitude was significantly higher than the tetraploid plants, indicating diploid plants are more vulnerable to cold stress than tetraploids (Figure 10).
3. Discussion
3.1. Morphological Characteristics Analysis
Multiple researchers have revealed that polyploidization changes plants’ morphology, physiology, and biochemistry [28,29] and also contributes to plant diversification and evolution [30]. For example, the tetraploid birch (Betula pendula) showed a significantly more extensive leaf area than its diploid counterpart [31]. According to previous studies, the so-called “gigas effect” may explain the more giant leaves in tetraploids; increased polyploid cell size could result in more giant leaf organs [32]. According to Zhang’s results, polyploid passionfruit (P. edulis, P. edulis var. flavicarpa Deg., and Tai-Nung No. 1) exhibited gigantism, with characteristics such as fewer and larger stomata, thicker and darker leaves, and a curved shape observed in tetraploids. Meanwhile, diploid plants presented flat and smooth leaves [24]. These results were consistent with the findings of this study. However, the difference between triploid and tetraploid P. edulis still needs further study. Moreover, we further analyzed the coefficient of variation of most of the morphological indexes (plant height, leaf area, root number) in tetraploid and diploid plants. The results verified tetraploids had more morphological variations than diploids (Table 1). Novel phenotypic variation may provide a selectional basis for crop improvement [33]. It is suggested that tetraploid plants can provide more plentiful choices for breeders to promote the breeding programs of new cultivars of P. edulis. It has been reported that tetraploid Zizyphus jujuba Mill. cv. Zhanhua had significantly larger but fewer stomas, and the grafted tetraploid plants onto mature trees of Z. jujuba Mill. cv. Zhanhua resulted in thicker stems, rounder, and succulent leaves compared to their diploid counterparts [34], suggesting tetraploid P. edulis could be used as a novel grafting stock material in the breeding program of P. edulis. This provides new research ideas for breeders.
Many mechanisms may cause plant phenotype changes. According to previous studies, DNA sequence, cis- and trans-acting effects, chromatin modifications, RNA-mediated pathways, and regulatory networks modulate the differential expression of homoeologous genes and phenotypic variation [35], as well as the phenotype variation related to the allele-dosage effects [36]. For example, the changes in (fw2.2) alleles influenced the fruit size in tomatoes [37]. Additionally, according to Wang’s results, differentially abundant proteins that are related to cell division were found to be more abundant in tetraploid Paulownia australis than in diploid. These proteins play a fundamental role in plant organ growth and development and could contribute to the variability in leaf traits. [38]. However, the regulation mechanism behind the phenotypic variation of tetraploid P. edulis still needs more in-depth study.
3.2. Physiological Traits
Multiplying the number of chromosomes in P. edulis affects physiological traits. Soluble sugar and protein are essential substances in promoting plant growth and development. Soluble sugar can provide enough nutrients, which further contributes to building macromolecules and energy for specific and coordinated development [39]. Soluble protein is related to osmotic regulation in plant cells and plays a critical role in plant abiotic stress response [40]. Supported by previous studies, the increase in soluble protein content was highly associated with the cold resistance of plants [41]. According to our results, the content of soluble sugar and protein in P. edulis increased with the increase in ploidy level. The average soluble sugar and soluble protein in tetraploid increased by 63.80% and 30.63%, respectively, compared with diploid (Table 2). It helps provide more energy for tetraploid P. edulis’ growth and development. Similar results also had been obtained in the tetraploid Fig tree (Ficus carica L.); its total soluble sugar content increased by 17.5% and 22.8% in 4× plants of “Sabz” and “Torsh”, respectively, compared with their original diploid counterparts. Furthermore, both tetraploid lines accumulated a higher total soluble protein [42]. Under drought stress, higher soluble sugar, protein, and proline content were monitored in autotetraploid Ziziphus jujuba Mill. var. spinosawere compared to its diploid counterpart, suggesting the former had better drought resistance [43].
After chromosomes doubled, changes in pigment content in the plant leaves were observed, which may change the color of plant leaves and affect the photosynthesis of plants. Multiple researchers found that increased chlorophyll content was measured in polyploid plants, which resulted in darker green leaves [44,45], suggesting darker leaves are a common characteristic of tetraploids. The chlorophyll content is directly related to chloroplast numbers; the latter could be used as the identification index of tetraploid [46] and plays a vital role in the photosynthesis system [47]. Our results showed that the chlorophyll a and chlorophyll b, total chlorophyll content, and carotenoid content in tetraploid were significantly higher in triploid leaves compared to diploid leaves with varying degrees of increase (Table 2), indicating that the tetraploid P. edulis possessed a stronger light absorption capacity and photosynthetic capacity than the diploid P. edulis. Our findings were consistent with a report on tetraploid Lilium FO hybrids [48]. Moreover, the Fv/Fm and Fv/Fo values in tetraploid were significantly higher than diploid, suggesting that tetraploid had a higher light conversion efficiency. In terms of physiological characteristics, both our result and Wang’s study referred to polyploid plants as having better physiology parameters. However, Wang’s analysis focused on the monthly changes in triploid and diploid “Mantianxing” [25].
3.3. Analysis of Different Ploidy Antioxidant Enzymes, MDA, and Pro Contents
The activity of antioxidant enzymes, malondialdehyde, and proline can reflect the sensitivity of plants to environmental factors and are often used as indicators to judge the strength of plant self-regulation. Plants usually remove excess ROS by enhancing their antioxidant enzyme activity to maintain cellular homeostasis and reduce the damage caused by stress [49,50]. In this study, the SOD, POD, and CAT activities in tetraploid P. edulis lines significantly increased compared with those in diploid, indicating that tetraploid P. edulis had a more robust capacity for removing free radicals and restoring equilibrium. This finding was consistent with the responses of the enzyme activity in tetraploid Brassica. Juncea under salinity stress [51]. Proline can protect and stabilize reactive oxygen species scavenging enzymes, and stabilize protein and cell structure, thereby maintaining cell osmotic potential. MDA is widely used to indicate the degree of injury caused by abiotic stress [52]. In this study, the content of MDA in tetraploid was lower than in diploid, while the former had higher Pro content (Figure 5). Therefore, we proposed that tetraploid P. edulis had lower damage and stronger adjustment ability to adverse environments than diploids.
3.4. Photosynthetic Characteristics and Chlorophyll Fluorescence between Diploid and Tetraploid P. edulis
The stoma and leaf play critical roles in photosynthesis. The stoma controls the gaseous exchange between the leaf and the external atmosphere [53]. The leaf is the principal place of photosynthesis in plants. In our study, tetraploids had a significantly longer and broader stoma and larger leaf area than the diploids. In addition, their chlorophyll and carotenoid content were significantly higher, contributing to plants’ photosynthesis. These findings indicated that the photosynthetic characteristics of tetraploid P. edulis were significantly better than diploid. Similar findings have been reported in tetraploid Anoectochilus roxburghii [54] and tetraploid Liriodendron sino-americanum [55].
In the diurnal variation of photosynthesis of most plants, the variation of photosynthetic rate is a “single peak” or “double peak” curve with a midday depression feature. This can be seen, for example, in the reports of Camellia sinensis (L.) O. Kuntze [56] and marula Sclerocarya birrea (A. Rich.) [57]. In this study, the diurnal variation of net photosynthetic rate, transpiration rate, and stomatal conduction degree day of diploid and tetraploid plants of P. edulis presented a “double peak” curve, and the diurnal variation of intercellular CO2 concentration presented a “U-shaped” curve. The lowest value was reached at 14:00 (Supplementary Tables S4–S7). This is consistent with the daily variation trend in plant photosynthesis studied by predecessors.
In the current study, the PS II primary photochemical efficiency and initial fluorescence parameters in the tetraploid lines were significantly higher than in the diploid line. The energy distribution ratio in the PS II reaction center and “OJIP” curve of the tetraploid lines were better than those of the diploid line (Figure 7), indicating that the tetraploid line had higher photochemical efficiency, fluorescence yield, and photosynthetic efficiency. The fluorescence parameters of triploid P. edulis also showed polyploid superiority [25]. Compared with diploid, the increase in chromosomes in Plumbago auriculata Lam. changed the physiology characteristics, and tetraploid plants showed better PSII activity and cold resistance [58].
3.5. Effects of Low-Temperature Stress on P. edulis
Temperature plays a critical role in the photosynthesis of plants [59]. Most plants can adjust their photosynthetic characteristics to acclimate their growth temperatures (temperature acclimation) [60]. Distinct plant species with varying ploidies exhibit differing regulatory capacities under abiotic stress conditions. Thus, the cold resistance evaluation of tetraploid P. edulis enriches the understanding of its physiological and biochemical adaptation to low temperatures, and provides insight for breeders to expand the range of P. edulis cultivation. Our study revealed that, under low-temperature stress, tetraploid P. edulis showed a superior Pn (Figure 9), indicating stronger photosynthetic capacity, higher intrinsic photochemical activity, enhanced electron transfer efficiency, and photochemical efficiency, all of which contribute to the net photosynthetic rate. Our findings were supported by similar results obtained in low-temperature experiments. For example, triploid citrus varieties appeared more tolerant to naturally low temperatures than diploid ones, as evidenced by better photosynthetic properties (Pnet, Gs, Fv/Fm, ETR/Pnet ratio) [61]. Furthermore, Dianthus broteri had particular photochemical adaptations in higher ploidy levels under climate change [62].
Antioxidant enzyme variations in plants are considered scientific indicators for their response to cold stress. In the evaluations of the abiotic stress resistance of polyploid plants, it can be found that most plants with high ploidy often have higher antioxidant enzyme activity to maintain better resistance than ones with low ploidy. For example, autotetraploid Poncirus trifoliata enhanced its drought resistance by maintaining ROS detoxification and osmotic adjustment via elevating antioxidant activity and sugar accumulation compared its diploid counterpart [63]. Our findings demonstrated that tetraploid P. edulis was more adaptable to low-temperature stress.
Plant polyploidization is an essential force in plant evolution. Studies about plant polyploidization help biologists understand how plants have evolved and diversified and assist breeders in designing new strategies for crop improvement [64]. Thus, polyploid P. edulis with excellent properties can be used as experimental material to produce new polyploid P. edulis varieties.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
The sterile seedlings (2n = 2x = 18) were obtained from the mature seeds of P. edulis “Tai-Nung No. 1”, provided by the Institute of Tropical Crop Science of Yunnan Province. Tai-Nung No. 1 P. edulis is a new hybrid variety (purple fruit species (female parent) × yellow fruit species (male parent)) bred by the Fengshan Tropical Horticultural Research Institute in Taiwan in 1981 [65].
Polyploid plants were obtained by treating the indefinite buds of P. edulis sterile seedlings with colchicine in the previous study of our research group [66]. Flow cytometric analysis further confirmed the ploidy levels of the sterile seedlings and plants treated with colchicine. After determination, the diploid sterile seedlings served as contrast, and three tetraploid plants (2n = 4x = 36) were named T1, T2, and T3 plant lines, respectively. Then, all the seedlings were subcultured in MS medium (MS + 1.0 mg/L 6 − BA + 0.2 mg/L IAA, Sucrose 30 g/L, Agar 3.75 g/L, pH = 5.8~6.0) by using the indefinite bud proliferation method [66]. All experimental seedlings were cultivated in the tissue culture room of Southwest Forestry University with a temperature of (25 ± 2) °C and an average of 12 h/d of light/darkness, and the light intensity was 1000~1500 Lux.
4.2. Ploidy Verification of Tetraploid P. edulis
4.2.1. Flow Cytometric Analysis
Two young leaves harvested from the top of each plant, including P. edulis sterile seedlings and variant seedlings treated with colchicine, were sent to Kunming Medical University for flow cytometry detection using the CyFlow Ploidy Analyzer (Sysmex Parec, Görlitz, Germany). The DNA content of P. edulis sterile seedlings was used as a contrast to detect the changes in the DNA content of the variant plants.
4.2.2. Chromosome Counting
Chromosome counting was conducted to identify the chromosome numbers of the potential plants. From 10:00 a.m. to 11:00 a.m., 1–2 cm root tips of the diploid plants and plants treated with colchicine were collected and soaked in a mixed solution, which was made from the equivalent volume of 0.1% colchicine solution and 0.002 mol·L−1 8-hydroxyquinoline to fix for three h. The pre-treated root tips were washed with distilled water 2~3 times, then fixed in Carnoy Fluid for 10 min and then rinsed with 90% and 70% alcohol successively for later use. The fixed root tips were cleaned with distilled water 2~3 times, followed by 1 mol·L−1 hydrochloric acid and a constant temperature water bath at 60 °C for 30 min to dissociate the root tips. The root crown from the root tip was removed, and 1–2 mm of the root tip was taken and placed on a microscope slide, mashed with tweezers, and stained in 1 drop of modified phenol fuchsin-staining solution, then we covered the slid with coverslip and gently tapped the coverslip with a pencil eraser to make the cells evenly dispersed and to observe the chromosomes.
4.3. Morphological Characterizations Measurement of Polyploidy P. edulis
The same batch of 45-day-old subculture seedlings with different ploidy was randomly selected for the comparison of morphological characteristics regarding plant height, ground diameter, leaf thickness, internodes, and root numbers. The Plant Image Analyzer (WanShen LA-S series; Hangzhou Wseen testing Technology Co., LTD, Hangzhou, China, http://www.wseen.com/, accessed on 27 August 2024) was used to scan the leaf samples and record leaf length, leaf width, petiole length, leaf area, and leaf serration number. Each plant was considered an independent biological replicate. We set three biological replicates.
4.4. Measurement of Physiological and Biochemical Attributes
Three full-expanded leaves with different leaf positions were collected from the same plant and thoroughly mixed. Then, they were divided into 3 independent biological replicates (weighted 0.1 g of fresh material per replicate total). All samples were weighed to determine the physiological and biochemical attributes of P. edulis.
The contents of chlorophyll a, chlorophyll b, and carotenoids have been determined by vis spectrophotometer (7230G, Shanghai Jinghua Technology Instrument Co., LTD, Shanghai, China, http://www.jinghuatec.cn/, accessed on 27 August 2024) according to Arnon [61]. The absorbance was determined at 663 nm, 645 nm, and 470 nm, respectively.
Following the manufacturer’s guidelines, we used corresponding commercial kits bought from Suzhou Michy Biomedical Technology Co., Ltd., (Suzhou, China, www.michybio.com, accessed on 27 August 2024) to measure the contents of soluble sugar (anthrone colorimetry), soluble protein (Coomassie Brilliant Blue G-250 method), malondialdehyde (MDA, thiobarbituric acid reactive substance assay), proline (Pro, sulfosalicylic acid (SA) method) content, catalase (CAT, Ammonium Molybdate Colorimetry method), superoxide dismutase (SOD, WST-8 method), and peroxidase (POD, Spectrophotometer, SpectraMax Plus 384, Moleculardevices (Shanghai) Co., LTD, China, https://www.moleculardevices.cn/, accessed on 27 August 2024). The specific operation steps were strictly implemented free of charge, following the instructions provided by the company. All the parameters were measured using the enzyme-linked immunosorbent assay.
4.5. Analysis of Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters
The diurnal changes in photosynthetic characteristics of P. edulis were measured with the LI-6800 Portable Photosynthesis System (LI-COR, Lincoln, NE, USA), and the net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) of the leaves were mainly determined. Three seedlings in each plant line were collected, and three leaves with stable growth at the top of each seedling were used to determine the photosynthetic characteristics at 8:00, 10:00, 12:00, 14:00, 16:00, and 18:00, respectively. A plant efficiency analyzer (Handy PEA, Hansatech Instruments Ltd., Narborough Road, Pentney, King’s Lynn, Norfolk PE32 1JL, UK) was used to determine the chlorophyll fluorescence of diploid and tetraploid leaves. After 30 min dark adaptation, the leaves were measured using the instrument’s probe.
4.6. Comparison of Diploid and Tetraploid P. edulis under Low-Temperature Stress
The same batch of rooting seedlings with stable growth for 45 days was randomly selected and moved into a 4 °C refrigerator to implement cold stress, and the treatment time was set at 0 h, 6 h, 12 h, 24 h, 36 h, and 48 h, respectively. Photosynthetic characteristics and chlorophyll fluorescence parameters were determined immediately after cold stress treatment. Three leaves from the same plant were mixed to be divided into three independent biological replicates, and 0.1 g of material was weighed per replicate. All samples of different plant lines were loaded into labeled freezer tubes. These leaves samples were quickly cooled with liquid nitrogen and stored in a −80 °C refrigerator for further analysis. We determined the activity of SOD, POD, and CAT enzymes and the content of MDA and Pro by using the kit of Suzhou Michy Biomedical Technology Co., Ltd (Suzhou, China, www.michybio.com, accessed on 27 August 2024).
4.7. Data Processing and Analysis
Excel (2019) was used for data sorting and mapping, IBM SPSS statistics 22.0 was used for single-factor analysis of variance and two-factor variance analysis, the Duncan method was used for multiple comparisons, and Pearson correlation analysis was used to analyze the correlation. Origin (2021) and PowerPoint (2019) were used to draw diagrams. An independent samples t-test was used to analyze the mean value difference and test the significance of diploidy and tetraploidy. Each index’s calculation and analysis were conducted via average value, and each average value was the mean of three independent biological replicates.
5. Conclusions
To summarize, this study conducted a series of comparisons to investigate the differences in morphology, stoma, photosynthesis characteristics, chlorophyll content, chlorophyll fluorescence, antioxidant enzyme activity, and resistance in cold stress of tetraploid and diploid P. edulis. The results reveal that polyploidization not only leads to the dwarfing phenotype, bigger stoma with lower density, larger leaf with a darker color, and longer root, but also makes tetraploid P. edulis perform better in physiological and biochemical traits than its diploid counterpart. The improved soluble sugar and protein, chlorophyll content, and the active antioxidant enzyme are useful for tetraploids when it comes to developing an effective protective system under temperature stress. Our findings provide understanding for researching the differences between diploid and tetraploid P. edulis and a reference for breeding programs involving P. edulis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13182603/s1; Figure S1: PCA analysis on traits of diploid and tetraploid P. edulis; Table S1: Stomatal parameters of diploid and tetraploid P. edulis; Table S2: Growth index of diploid and tetraploid plants of P. edulis; Table S3: Leaf parameters of diploid and tetraploid P. edulis; Table S4: Analysis of diurnal variation difference of net photosynthetic rate (μmol m−2·s−1) between diploid and tetraploid P. edulis; Table S5: Analysis of diurnal variation difference of transpiration rate (mmol m−2·s−1) between diploid and tetraploid P. edulis; Table S6: Analysis of diurnal variation difference of stomatal conductance (mol m−2s−1) between diploid and tetraploid P. edulis; Table S7: Analysis of diurnal variation of intercellular CO2 concentration (μmol m−2·s−1) between diploid and tetraploid P. edulis.
Author Contributions
Conceptualization, X.S., N.C. and Y.X.; formal analysis, X.S., X.W., R.L. and C.Z.; investigation, X.S., C.Z., L.C., S.C. and Y.X.; methodology, X.W., R.L. and L.C.; resources, Y.X.; writing—original draft, X.S. and X.W.; writing—review and editing, X.S. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Yunnan Young & Elite Talents Projects (YNWR-QNBJ-2019-075), and the Yunnan Graduate Tutor Team Building Project (2022-97).
Data Availability Statement
All data generated or analyzed during this study are included in this article. All data are available from the first author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Araya, S.; Martins, A.M.; Junqueira, N.T.V.; Costa, A.M.; Faleiroa, F.G.; Ferreira, M.E. Microsatellite marker development by partial sequencing of the sour passion fruit genome (Passiflora edulis Sims). BMC Genom. 2017, 18, 549. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, J.; Myiake Kato, E.T. Leaf and stem morphoanatomy of Passiflora edulis Sims, Passifloraceae. Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2010, 20, 233–239. [Google Scholar] [CrossRef]
- Kawakami, S.; Morinaga, M.; Tsukamoto-Sen, S.; Mori, S.; Matsui, Y.; Kawama, T. Constituent Characteristics and Functional Properties of Passion Fruit Seed Extract. Life 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Rothfels, C.J. Polyploid phylogenetics. New Phytol. 2021, 230, 66–72. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2015, 243, 281–296. [Google Scholar] [CrossRef]
- Catalano, C.; Abbate, L.; Motisi, A.; Crucitti, D.; Cangelosi, V.; Pisciotta, A.; Di Lorenzo, R.; Carimi, F.; Carra, A. Autotetraploid Emergence via Somatic Embryogenesis in Vitis vinifera Induces Marked Morphological Changes in Shoots, Mature Leaves, and Stomata. Cells 2021, 10, 1336. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Liu, H.; Zhang, X.; Ye, Q.; Zhang, H. Induction, identification and genetics analysis of tetraploid Actinidia chinensis. R. Soc. Open Sci. 2019, 6, 191052. [Google Scholar] [CrossRef]
- Wu, F.H.; Yu, X.D.; Zhuang, N.S.; Liu, G.D.; Liu, J.P. Induction and identification of Stylosanthes guianensis tetraploids. Genet. Mol. Res. 2015, 14, 12692–12698. [Google Scholar] [CrossRef] [PubMed]
- Zahumenická, P.; Fernández, E.; Šedivá, J.; Žiarovská, J.; Ros-Santaella, J.L.; Martínez-Fernández, D.; Russo, D.; Milella, L. Morphological, physiological and genomic comparisons between diploids and induced tetraploids in Anemone sylvestris L. Plant Cell Tissue Organ Cult. 2018, 132, 317–327. [Google Scholar] [CrossRef]
- Yahata, S.; Sato, S.; Ohara, H.; Matsui, H. Differences of morphological and fruit bearing characteristics among the different ploidy plants and triploid production by crossings between diploid and triploid plants in loquat. Hortic. Res. 2005, 4, 379–384. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Gao, J. In vitro tetraploid induction from multigenotype protocorms and tetraploid regeneration in Dendrobium officinale. Plant Cell Tissue Organ Cult. 2020, 141, 289–298. [Google Scholar] [CrossRef]
- Li, J.; Suzuki, Y.; Hoshino, Y. Phenotypic analysis of polyploid and aneuploid haskap (Lonicera caerulea L. subsp. edulis (Turcz. ex Herder) Hulten) plants and their progeny production. Euphytica 2023, 219, 52. [Google Scholar]
- Liu, Z.; Wang, J.; Qiu, B.; Ma, Z.; Lu, T.; Kang, X.; Yang, J. Induction and Characterization of Tetraploid Through Zygotic Chromosome Doubling in Eucalyptus urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef] [PubMed]
- Thong-on, W.; Arimatsu, P.; Pitiporn, S.; Soonthornchareonnon, N.; Prathanturarug, S. Field evaluation of in vitro-induced tetraploid and diploid Centella asiatica (L.) Urban. J. Nat. Med. 2014, 68, 267–273. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, K.; Liu, W.; Feng, G.; Peng, Y.; Li, Z. Adaptive Responses of Common and Hybrid Bermudagrasses to Shade Stress Associated with Changes in Morphology, Photosynthesis, and Secondary Metabolites. Front. Plant Sci. 2022, 13, 817105. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Jin, J.; Ma, X.; Li, K. Physiological and Transcriptome Analysis on Diploid and Polyploid Populus ussuriensis Kom. under Salt Stress. Int. J. Mol. Sci. 2022, 23, 7529. [Google Scholar] [CrossRef]
- Zhang, X. Triploid Hybrid Watermelon Plants. U.S. Patent 7,238,866, 3 July 2007. [Google Scholar]
- Baack, E.J. Ecological factors influencing tetraploid, establishment in snow buttercups (Ranunculus adoneus, Ranunculaceae): Minority cytotype exclusion and barriers to triploid formation. Am. J. Bot. 2005, 92, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Vilcherrez-Atoche, J.A.; Iiyama, C.M.; Cardoso, J.C. Polyploidization in Orchids: From Cellular Changes to Breeding Applications. Plants 2022, 11, 469. [Google Scholar] [CrossRef]
- Marangelli, F.; Pavese, V.; Vaia, G.; Lupo, M.; Bashir, M.A.; Cristofori, V.; Silvestri, C. In Vitro Polyploid Induction of Highbush Blueberry through de novo Shoot Organogenesis. Plants 2022, 11, 2349. [Google Scholar] [CrossRef]
- Wu, J.-H.; Ferguson, A.R.; Murray, B.G.; Jia, Y.; Datson, P.M.; Zhang, J. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Ann. Bot. 2012, 109, 169–179. [Google Scholar] [CrossRef]
- Wang, P.; Mu, X.; Gao, Y.G.; Zhang, J.; Du, J. Successful induction and the systematic characterization of tetraploids in cerasus humilis for subsequent breeding. Sci. Hortic. 2020, 265, 109216. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Wang, L.; Deng, W.; Wei, H.; Liu, P.; Liu, M. Morphological, cytological and nutritional changes of autotetraploid compared to its diploid counterpart in Chinese jujube (Ziziphus jujuba Mill.). Sci. Hortic. 2019, 249, 263–270. [Google Scholar] [CrossRef]
- Zhang, Q. Study on Technology of Breeding Polyploid of Passiflora edulis by Chromosome Engineering. Master’s Thesis, Southwest University, Chongqing, China, 2002. [Google Scholar]
- Wang, X.; Zhu, Y.; Chen, S.; Yang, Z. Comparison of Morphology, Chlorophyll and Chlorophyll Fluorescence Parameters of Passiflora edulis with Different Ploidy. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2022, 53, 755–762. [Google Scholar]
- Davik, J.; Wilson, R.C.; Njah, R.G.; Grini, P.E.; Randall, S.K.; Alsheik, M.K.; Sargent, D.J. Genetic mapping and identification of a QTL determining tolerance to freezing stress in Fragaria vesca L. PLoS ONE 2021, 16, e0248089. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, T.; Zhang, C.; Zhang, T.; Pu, L.; Zhao, W. Effects of exogenous zinc on the physiological characteristics and enzyme activities of Passiflora edulis Sims f. edulis seedlings. PeerJ 2023, 11, e16280. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; He, T.; Gao, R.; Guo, G.; Lu, R.; Chen, Z.; Liu, C. Comparative Analysis of Morphology, Photosynthetic Physiology, and Transcriptome between Diploid and Tetraploid Barley Derived from Microspore Culture. Front. Plant Sci. 2021, 12, 626916. [Google Scholar] [CrossRef]
- Moetamedipoor, S.A.; Jowkar, A.; Saharkhiz, M.J.; Hassani, H.S. Hexaploidy induction improves morphological, physiological and phytochemical characteristics of mojito mint (Mentha × villosa). Sci. Hortic. 2022, 295, 110810. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.S.; Schwarzacher, T.; Liu, Q. Polyploidy: Its consequences and enabling role in plant diversification and evolution. Ann. Bot. 2023, 131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.; Wang, W.; Liu, G.; Yang, C.; Jiang, J. Differences in Leaf Morphology and Related Gene Expression between Diploid and Tetraploid Birch (Betula pendula). Int. J. Mol. Sci. 2022, 23, 12966. [Google Scholar] [CrossRef]
- Certner, M.; Sudová, R.; Weiser, M.; Suda, J.; Kolár, F. Ploidy-altered phenotype interacts with local environment and may enhance polyploid establishment in Knautia serpentinicola (Caprifoliaceae). New Phytol. 2019, 221, 1117–1127. [Google Scholar] [CrossRef]
- Udall, J.A.; Wendel, J.F. Polyploidy and crop improvement. Crop Sci. 2006, 46, S3–S14. [Google Scholar] [CrossRef]
- Gu, X.F.; Yang, A.F.; Meng, H.; Zhang, J.R. In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. cv. Zhanhua. Plant Cell Rep. 2005, 24, 671–676. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.C.; Pires, J.C.; Birchler, J.A.; Auger, D.L.; Chen, Z.J.; Lee, H.-S.; Comai, L.; Madlung, A.; Doerge, R.W.; Colot, V.; et al. Understanding mechanisms of novel gene expression in polyploids. Trends Genet. 2003, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, G.; Dong, Y.; Zhai, X.; Deng, M.; Zhao, Z.; Liu, W.; Cao, Y. Implications of polyploidy events on the phenotype, microstructure, and proteome of Paulownia australis. PLoS ONE 2017, 12, e0172633. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Hayat, S. Interaction of glucose and phytohormone signaling in plants. Plant Physiol. Biochem. 2019, 135, 119–126. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, R.; Yuan, Z.; Cao, H.; Martin, J.J.J. Physiology Response and Resistance Evaluation of Twenty Coconut Germplasm Resources under Low Temperature Stress. Horticulturae 2021, 7, 234. [Google Scholar] [CrossRef]
- Abdolinejad, R.; Shekafandeh, A.; Jowkar, A. In vitro tetraploidy induction creates enhancements in morphological, physiological and phytochemical characteristics in the fig tree (Ficus carica L.). Plant Physiol. Biochem. 2021, 166, 191–202. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Hou, L.; Yang, W.; Liu, S.; Pang, X.; Li, Y. Multiple responses contribute to the enhanced drought tolerance of the autotetraploid Ziziphus jujuba Mill. var. spinosa. Cell Biosci. 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhao, Y.; Huang, M.; Chen, C.; Cao, C.; Wang, J.; Shi, Z.; Gao, J. Polyploid Induction and Identification of Begonia × benariensis. Horticulturae 2024, 10, 47. [Google Scholar] [CrossRef]
- Beck, S.L.; Fossey, A.; Mathura, S. Ploidy determination of black wattle (Acacia mearnsii) using stomatal chloroplast counts. S. Afr. For. J. 2003, 198, 79–82. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles to Augment Photosynthesis of Chloroplasts. Angew. Chem. (Int. Ed. Engl.) 2017, 56, 5308–5311. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, X.; Gao, X.; Wang, L.; Jia, G. Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol. Biochem. 2018, 133, 50–56. [Google Scholar] [CrossRef]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Bread wheat progenitors: Aegilops tauschii (DD genome) and Triticum dicoccoides (AABB genome) reveal differential antioxidative response under water stress. Physiol. Mol. Biol. Plants 2017, 23, 99–114. [Google Scholar] [CrossRef]
- Hinge, P.; Kale, A.; Pawar, B.; Jadhav, A.; Chimote, V.; Gadakh, S. Effect of PEG induced water stress on chlorophyll content, membrane injury index, osmoprotectants and antioxidant enzymes activities in sorghum (Sorghum bicolor (L) Moench). Maydica 2015, 60, M8. [Google Scholar]
- Shahbazi, E.; Jamei, S.; Meratan, A.A.; Pour Mohammadi, P. Effect of salt stress on some physio-biochemical traits and antioxidative enzymes of two Brassica species under callus culture. Plant Cell Tissue Organ Cult. 2021, 147, 453–465. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, A.; Kaur, K.; Gupta, A.K.; Singh, I. DPPH radical scavenging activity and contents of H2O2, malondialdehyde and proline in determining salinity tolerance in chickpea seedlings. Indian J. Biochem. Biophys. 2014, 51, 407–415. [Google Scholar]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ouyang, K.; Luo, Y.; Xie, G.; Yang, Y.; Zhang, J. A comparative study of characteristics in diploid and tetraploid Anoectochilus roxburghii. Front. Nutr. 2022, 9, 1034751. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Sheng, Y.; Hao, Z.; Long, X.; Fu, F.; Liu, Y.; Tang, Z.; Ali, A.; Peng, Y.; Lu, L.; et al. Transcriptome and proteome analysis suggest enhanced photosynthesis in tetraploid Liriodendron sino-americanum. Tree Physiol. 2021, 41, 1953–1971. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Feng, W.; Wang, Y.; Chen, X.; Fang, W.; Li, X.; Xiao, R. Diurnal variations of photosynthetic characteristic parameters in strains of tea plants under fruit-tea intercropping models. In Advances in Biomedical Engineering; Hu, J., Ed.; Information Engineering Research Inst: Newark, DE, USA, 2011. [Google Scholar]
- Li, T.; Ding, Y.; Hu, Y.; Sun, L.; Jiang, C.; Liu, Y. Diurnal changes in photosynthesis in Sclerocarya birrea from South Africa and Israel after introduction and acclimatization in Wenshan, Yunnan Province, China. S. Afr. J. Bot. 2015, 100, 101–107. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, S.; Hu, J.; He, G.; Liu, Y.; Chen, X.; Lei, T.; Li, Q.; Yang, L.; Li, W.; et al. Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tissue Organ Cult. 2020, 140, 315–325. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2018, 221, 32–49. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid Citrus Genotypes Have a Better Tolerance to Natural Chilling Conditions of Photosynthetic Capacities and Specific Leaf Volatile Organic Compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef]
- Lopez-Jurado, J.; Balao, F.; Mateos-Naranjo, E. Polyploidy-mediated divergent light-harvesting and photoprotection strategies under temperature stress in a Mediterranean carnation complex. Environ. Exp. Bot. 2020, 171, 103956. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Xie, Z.; Guo, D.; Chen, C.; Fan, Q.; Deng, X.; Liu, J.-H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef]
- Yang, X.H.; Ye, C.Y.; Cheng, Z.M.; Tschaplinski, T.J.; Wullschleger, S.D.; Yin, W.L.; Xia, X.L.; Tuskan, G.A. Genomic aspects of research involving polyploid plants. Plant Cell Tissue Organ Cult. 2011, 104, 387–397. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Gesangpingcuo; Hong, Y.; Zhao, F.; Li, Y. Introduction Performance and Cultivation Key Points of ‘Tai-Nung No. 1’and ‘flavicarpa Deg’ Passion Fruit in Greenhouse of Lhasa, Tibet. Tibet J. Agric. Sci. 2020, 42, 44–46. [Google Scholar]
- Su, X.; Wang, X.; He, B.; Yang, Z.; Zhou, C.; Chen, S.; Wang, D.; Cai, N. Mutagenesis of Passiflora edulis Sims Based on Tissue Culture Technology. J. West China For. Sci. 2023, 52, 138–145. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).