Koliella bifissiva sp. nov (Chlorellaceae, Chlorophyta) and Analysis of Its Organelle Genomes
Abstract
1. Introduction
2. Results
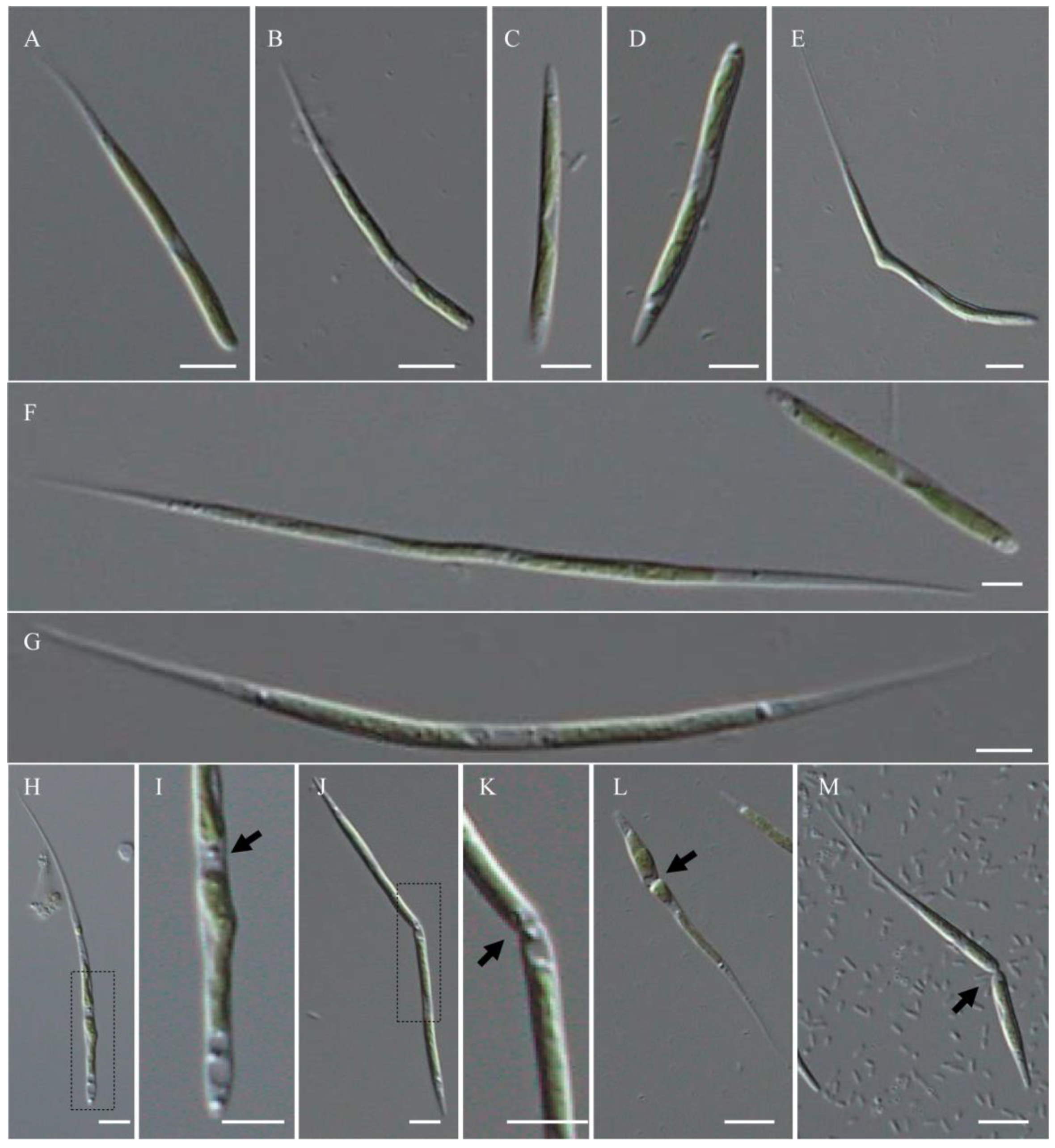
2.1. Morphological Observation and Taxonomic Implication
- Koliella bifissiva H. Song, Y. Hu, and G. Liu sp. nov. (Figure 1).
- Description
- Live microscopy
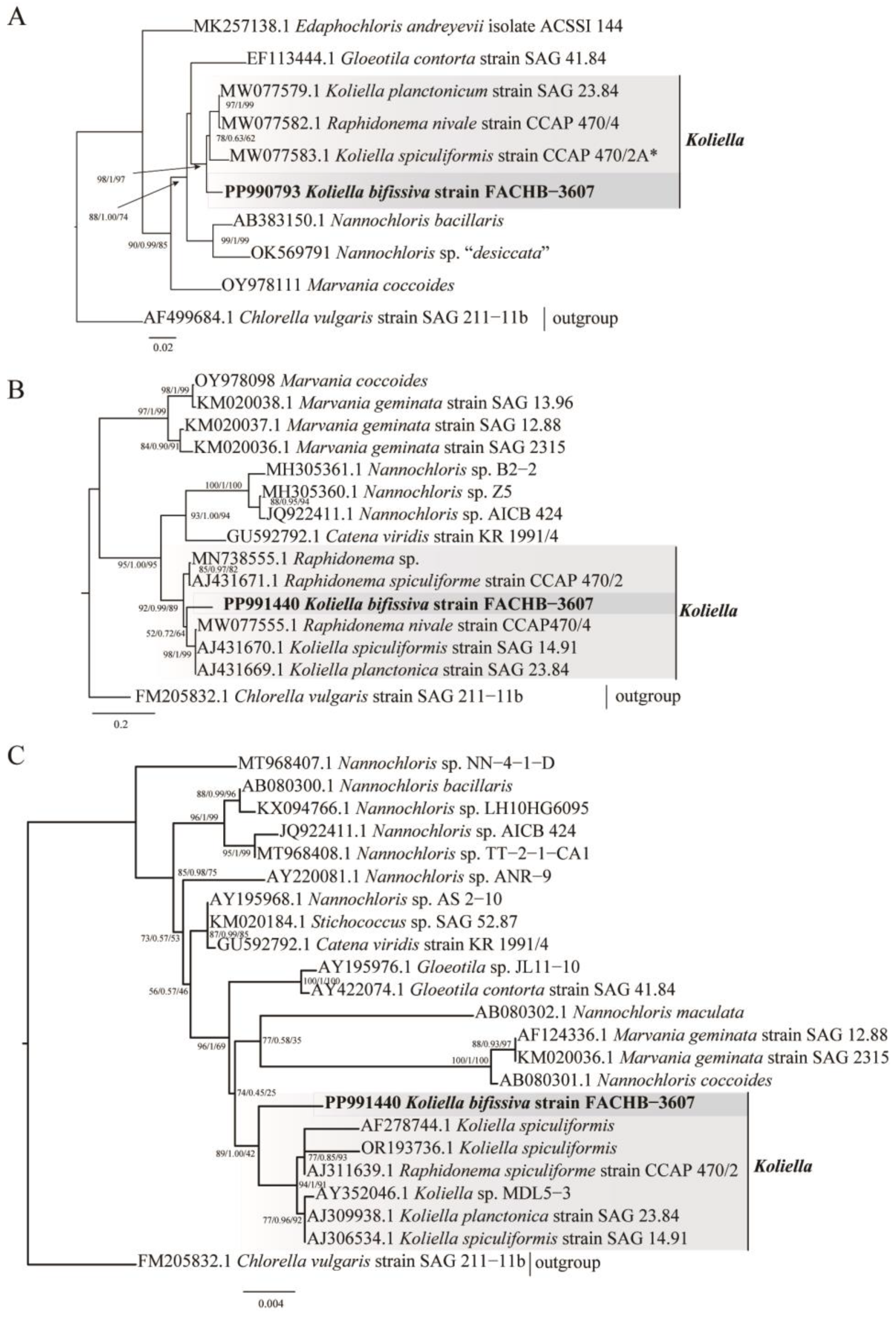
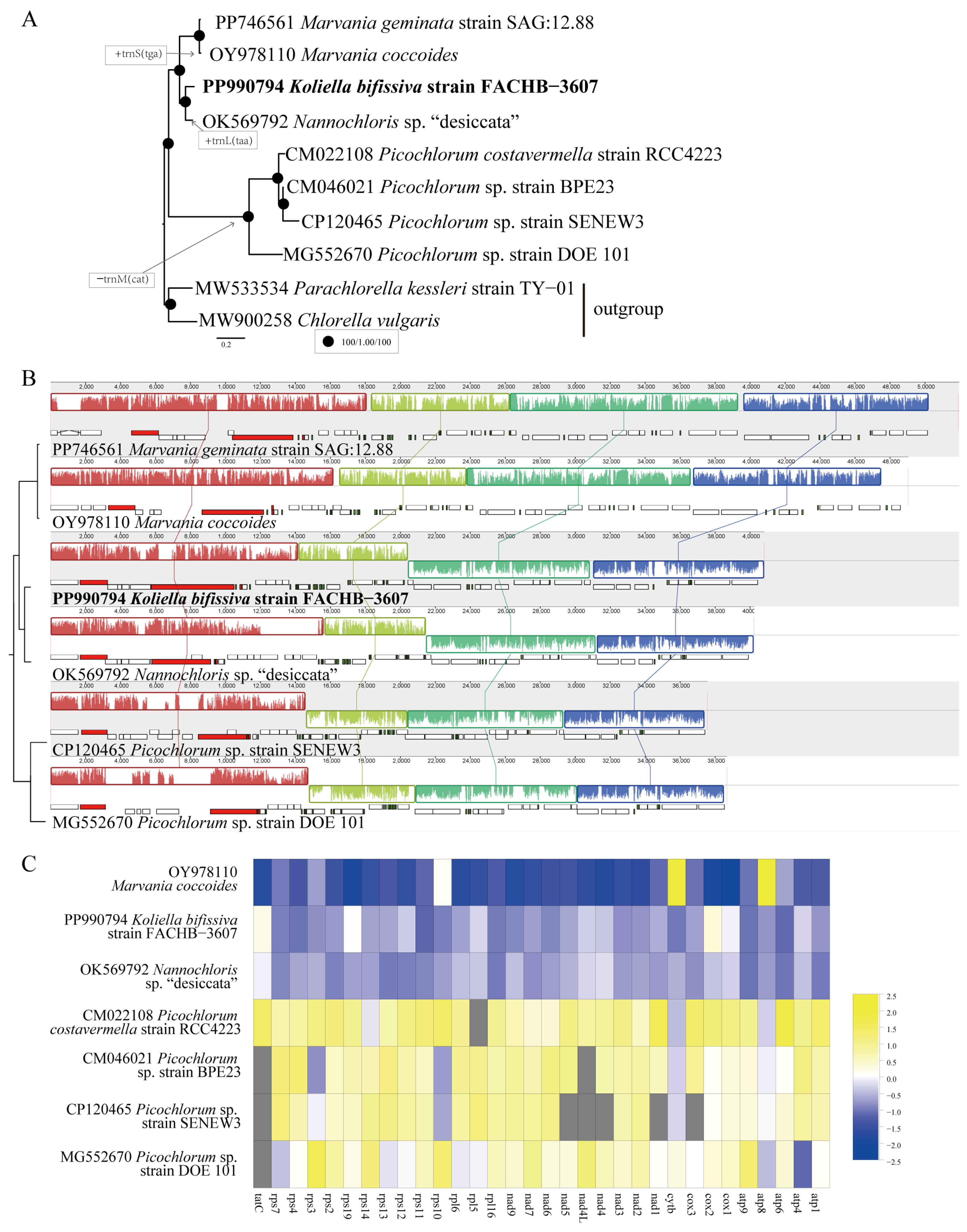
2.2. Phylogenetic Analyses
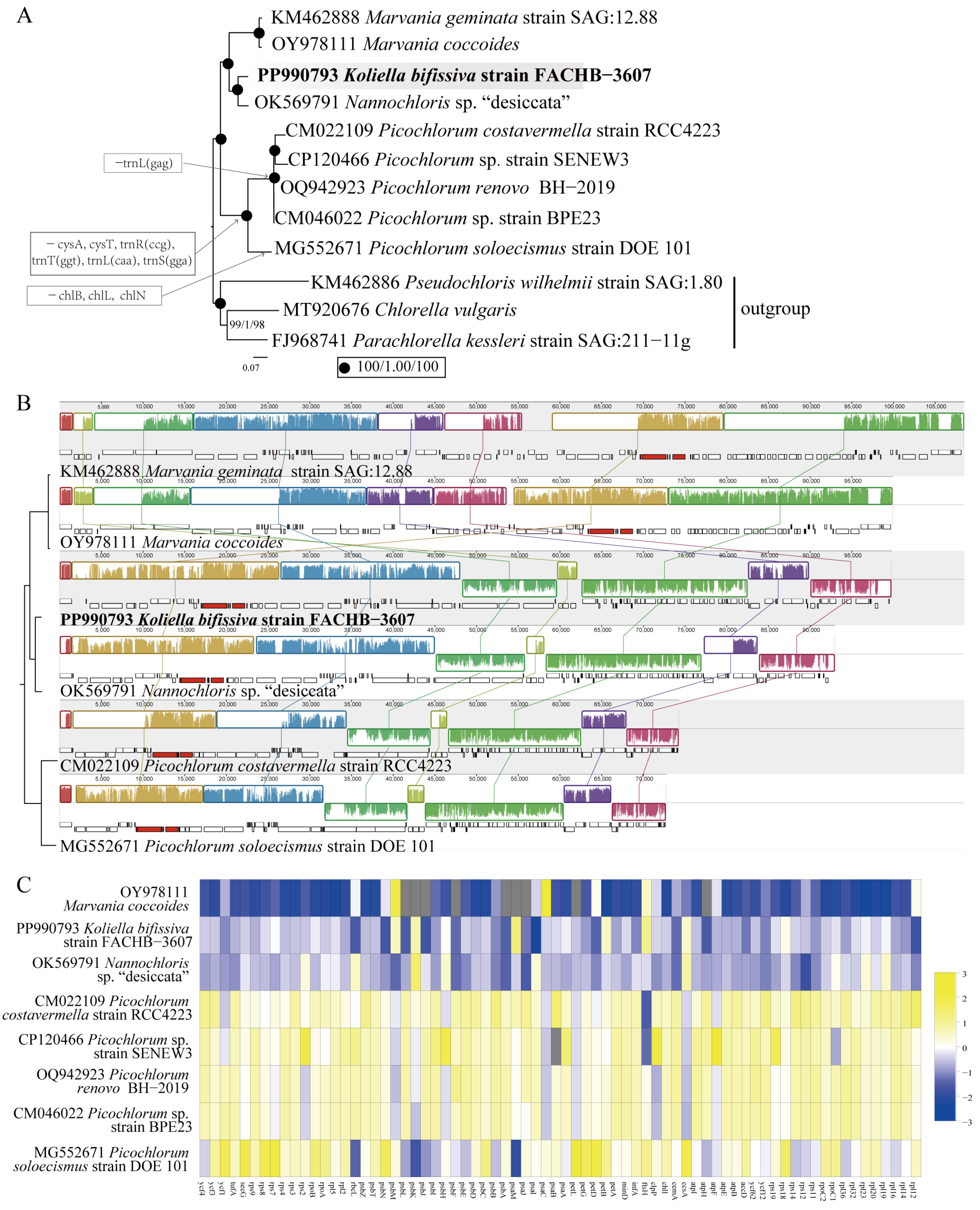
2.3. Characteristics of the Chloroplast Genome
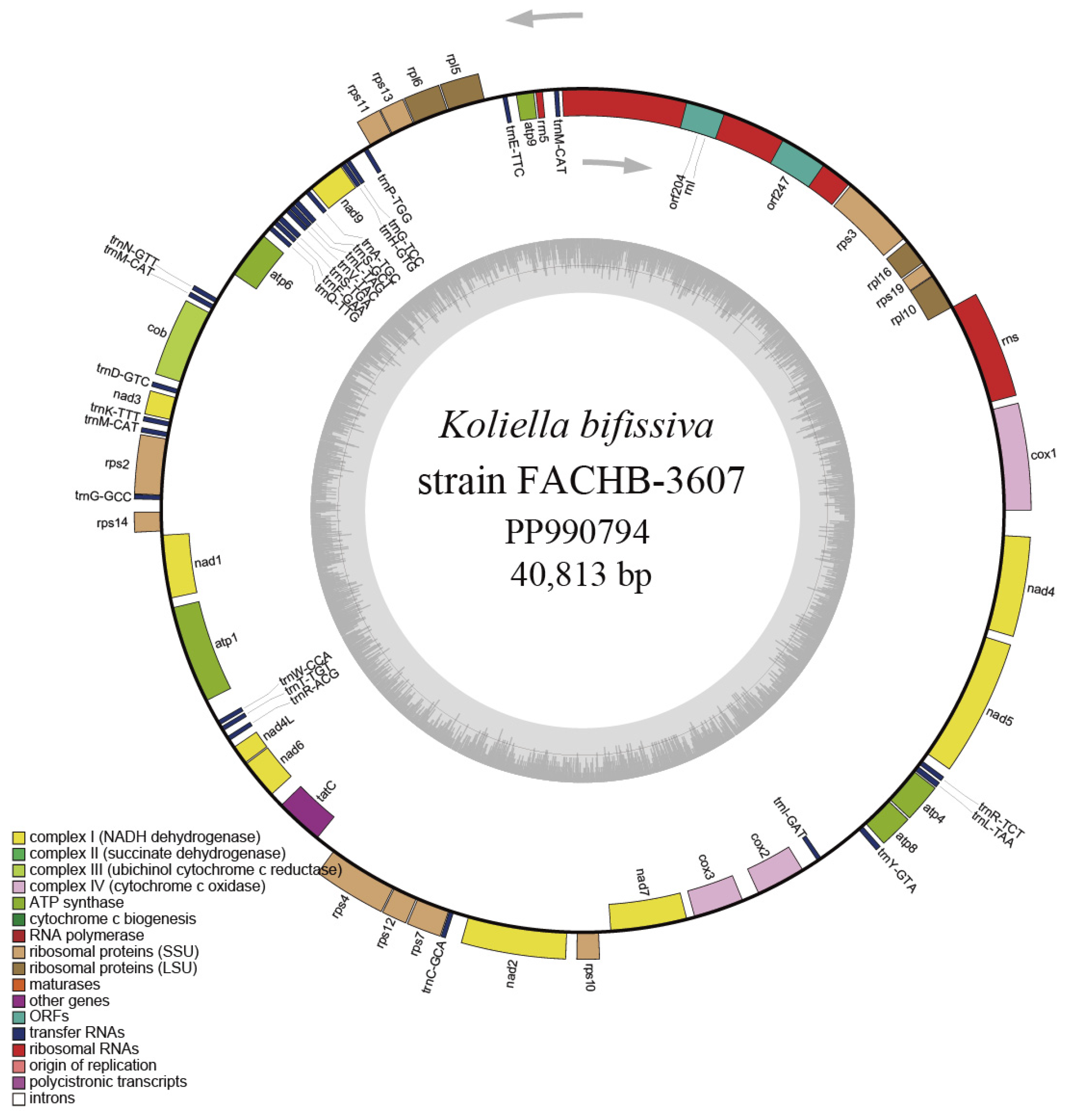
2.4. Characteristics of the Mitochondrial Genome
2.5. Sequence Variations between K. bifissiva Strain FACHB-3607 and Nannochloris sp. “desiccata”
3. Discussion
4. Materials and Methods
4.1. Strain Isolation and Culture
4.2. Light Microscopy
4.3. DNA Extraction and Whole Genome Sequencing
4.4. Quality Control, Assembly, and Annotation
4.5. Phylogenetic Inference
4.6. Phylogenomic Analysis
4.7. Synteny Analysis and Selective Pressure Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krienitz, L.; Huss, V.A.; Bock, C. Chlorella: 125 years of the green survivalist. Trends Plant Sci. 2015, 20, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, Z.; Liu, G. Assessing advances in taxonomic research on Chlorellaceae (Chlorophyta) (in Chinese with English abstract). Biodivers. Sci. 2023, 31, 22083. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Md Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of microalga in aquaculture. Rev. Aquacult 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohrs, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Chodat, R. Sur trois genres nouveaux de Protococcoidées et sur la florule planktonique d–un Étang du Danemark. Mémoires De L–herbier Boissier 1900, 8, 1–10. [Google Scholar]
- Henley, W.J.; Hironaka, J.L.; Guillou, L.; Buchheim, M.A.; Buchheim, J.A.; Fawley, M.W.; Fawley, K.P. Phylogenetic analysis of the ‘Nannochloris-like’ algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov.(Trebouxiophyceae, Chlorophyta). Phycologia 2004, 43, 641–652. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, T.; Kajitani, H.; Kawano, S. Patterns of asexual reproduction in Nannochloris bacillaris and Marvania geminata (Chlorophyta, Trebouxiophyceae). Planta 2007, 226, 917–927. [Google Scholar] [CrossRef]
- Darienko, T.; Pröschold, T. Genetic variability and taxonomic revision of the genus Auxenochlorella (Shihira et Krauss) Kalina et Puncocharova (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 394–400. [Google Scholar] [CrossRef]
- Somogyi, B.; Felföldi, T.; Solymosi, K.; Makk, J.; Homonnay, Z.G.; Horváth, G.; Turcsi, E.; Böddi, B.; Márialigeti, K.; Vörös, L. Chloroparva pannonica gen. et sp. nov. (Trebouxiophyceae, Chlorophyta) – a new picoplanktonic green alga from a turbid, shallow soda pan. Phycologia 2011, 50, 1–10. [Google Scholar] [CrossRef]
- Borovsky, D.; Sterner, A.; Powell, C.A. Cloning and expressing trypsin modulating oostatic factor in Chlorella desiccata to control mosquito larvae. Arch. Insect Biochem. 2016, 91, 17–36. [Google Scholar] [CrossRef]
- Dahlin, L.R.; Gerritsen, A.T.; Henard, C.A.; Van Wychen, S.; Linger, J.G.; Kunde, Y.; Hovde, B.T.; Starkenburg, S.R.; Posewitz, M.C.; Guarnieri, M.T. Development of a high-productivity, halophilic, thermotolerant microalga Picochlorum renovo. Commun. Biol. 2019, 2, 388. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.K.; Hanschen, E.R.; Biondi, T.C.; Hovde, B.T.; Kunde, Y.A.; Eng, W.L.; Kwon, T.; Dale, T. Phylogenetic analyses and reclassification of the oleaginous marine species Nannochloris sp. "desiccata" (Trebouxiophyceae, Chlorophyta), formerly Chlorella desiccata, supported by a high-quality genome assembly. J. Phycol. 2022, 58, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Yakimovich, K.M.; Gauthier, N.P.G.; Engstrom, C.B.; Leya, T.; Quarmby, L.M. A molecular analysis of microalgae from around the globe to revise Raphidonema (Trebouxiophyceae, Chlorophyta). J. Phycol. 2021, 57, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Hindák, F. Systematik der Gattungen Koliella gen. nov. und Raphidonema Lagerh. Nova Hedwig. 1963, 6, 95–125. [Google Scholar]
- Nygaard, G. New or interesting plankton algae. With a contribution on their ecology. Det. K. Dan. Vidensk. Selskab. Biol. Skr. 1977, 21, 1–107. [Google Scholar]
- Nygaard, G. Freshwater phytoplankton from the Narssaq area, South Greenland. Bot. Tidsskr. 1979, 73, 191–238. [Google Scholar]
- Hortobágyi, T. Uj Ulothrichales a Dunabol: Koliella budapestinensis Hortob. n. sp. [New Ulotrichales in the Danube: Koliella budapestinensis Hortob. n. sp. Bot. Közlemenyek 1983, 70, 103–104. [Google Scholar]
- Hindák, F. Four new planktic species of the genus Koliella (Ulotrichales, Chlorophyceae. Preslia 1984, 56, 1–11. [Google Scholar]
- Kuosa, H. Observations on the taxonomy and ecology of Monoraphidium (Chlorophyceae, Chlorococcales) and Koliella (Chlorophyceae, Ulotrichales) species in the Tvärminne Sea area, SW coast of Finland. Arch. Für Protistenkd. 1988, 135, 45–53. [Google Scholar] [CrossRef]
- Andreoli, C.; Lokhorst, G.M.; Mani, A.M.; Scarabel, L.; Moro, I.; Rocca, N.L. Koliella antarctica sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea (Antarctica). Arch. Für Hydrobiologie. Suppl. Algol. Stud. 1998, 125, 1–8. [Google Scholar]
- Hindák, F. New taxa and nomenclatorical changes in the Ulotrichineae (Ulotrichales, Chlorophyta). Biol. Bratisl. 1996, 51, 357–364. [Google Scholar]
- Katana, A.; Kwiatowski, J.; Spalik, K.; Zakrys, B.; Szalacha, E.; Szymanska, H. Phylogenetic position of Koliella (Chlorophyta) as inferred from nuclear and chloroplast small subunit rDNA. J. Phycol. 2001, 37, 443–451. [Google Scholar] [CrossRef]
- Lemieux, C.; Otis, C.; Turmel, M. Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evol. Biol. 2014, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Krasovec, M.; Vancaester, E.; Rombauts, S.; Bucchini, F.; Yau, S.; Hemon, C.; Lebredonchel, H.; Grimsley, N.; Moreau, H.; Sanchez-Brosseau, S.; et al. Genome analyses of the microalga Picochlorum provide insights into the evolution of thermotolerance in the green lineage. Genome Biol. Evol. 2018, 10, 2347–2365. [Google Scholar] [CrossRef]
- Kufferath, H. Algues et Protistes muscicoles, corticoles et terrestres récoltés sur la montagne de Barba (Costa-Rica). Ann. De Cryptogam. Exot. 1929, 2, 23–52. [Google Scholar]
- Škaloud, P.; Němcová, Y.; Pytela, J.; Bogdanov, N.I.; Bock, C.; Pickinpaugh, S.H. Planktochlorella nurekis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga carrying significant biotechnological potential. Fottea 2014, 14, 53–62. [Google Scholar] [CrossRef]
- Malavasi, V.; Skvorova, Z.; Nemcova, Y.; Skaloud, P. Laetitia sardoa gen. & sp. nov., a new member of the Chlorellales (Trebouxiophyceae, Chlorophyta) isolated from Sardinia Island. Phycologia 2022, 61, 375–383. [Google Scholar] [CrossRef]
- Lyubetsky, V.A.; Seliverstov, A.V.; Zverkov, O.A. Transcription regulation of plastid genes involved in sulfate transport in Viridiplantae. Biomed. Res. Int. 2013, 2013, 413450. [Google Scholar] [CrossRef]
- Turmel, M.; Otis, C.; Lemieux, C. The chloroplast genomes of the green algae Pedinomonas minor, Parachlorella kessleri, and Oocystis solitaria reveal a shared ancestry between the Pedinomonadales and Chlorellales. Mol. Biol. Evol. 2009, 26, 2317–2331. [Google Scholar] [CrossRef]
- Pombert, J.F.; Otis, C.; Lemieux, C.; Turmel, M. The chloroplast genome sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) reveals unusual structural features and new insights into the branching order of chlorophyte lineages. Mol. Biol. Evol. 2005, 22, 1903–1918. [Google Scholar] [CrossRef]
- Song, H.; Chen, Y.; Liu, F.; Chen, N. Large differences in the haptophyte Phaeocystis globosa mitochondrial genomes driven by repeat amplifications. Front. Microbiol. 2021, 12, 676447. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; dePamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdottir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Peng, H.; Fang, Z.; Zhang, B.; Zhu, Z.; Xiao, Z.; Liu, G.; Hu, Y. Koliella bifissiva sp. nov (Chlorellaceae, Chlorophyta) and Analysis of Its Organelle Genomes. Plants 2024, 13, 2604. https://doi.org/10.3390/plants13182604
Song H, Peng H, Fang Z, Zhang B, Zhu Z, Xiao Z, Liu G, Hu Y. Koliella bifissiva sp. nov (Chlorellaceae, Chlorophyta) and Analysis of Its Organelle Genomes. Plants. 2024; 13(18):2604. https://doi.org/10.3390/plants13182604
Chicago/Turabian StyleSong, Huiyin, Hai Peng, Zhiwei Fang, Baolong Zhang, Zhaolu Zhu, Zilan Xiao, Guoxiang Liu, and Yuxin Hu. 2024. "Koliella bifissiva sp. nov (Chlorellaceae, Chlorophyta) and Analysis of Its Organelle Genomes" Plants 13, no. 18: 2604. https://doi.org/10.3390/plants13182604
APA StyleSong, H., Peng, H., Fang, Z., Zhang, B., Zhu, Z., Xiao, Z., Liu, G., & Hu, Y. (2024). Koliella bifissiva sp. nov (Chlorellaceae, Chlorophyta) and Analysis of Its Organelle Genomes. Plants, 13(18), 2604. https://doi.org/10.3390/plants13182604





