Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential
Abstract
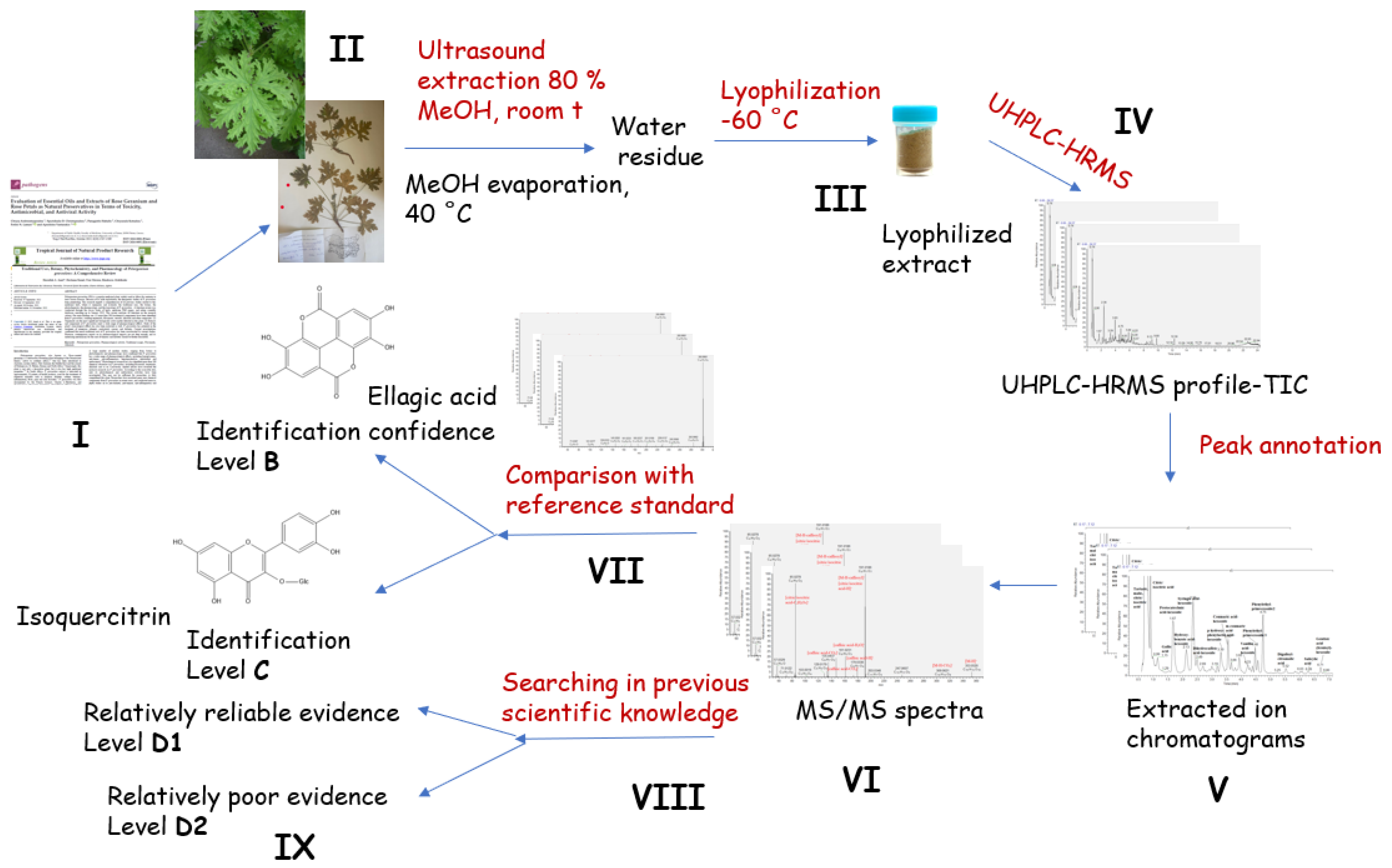
:1. Introduction
2. Results and Discussion
2.1. Dereplication and Annotation of Specialized Metabolites in Pelargonium roseum Extracts
2.1.1. Phenolic Acids and Their Glycosides, Coumarins and Aliphatic Acids
2.1.2. Acyltartaric Acids
2.1.3. Acylcitric/Acylisocitric Acids
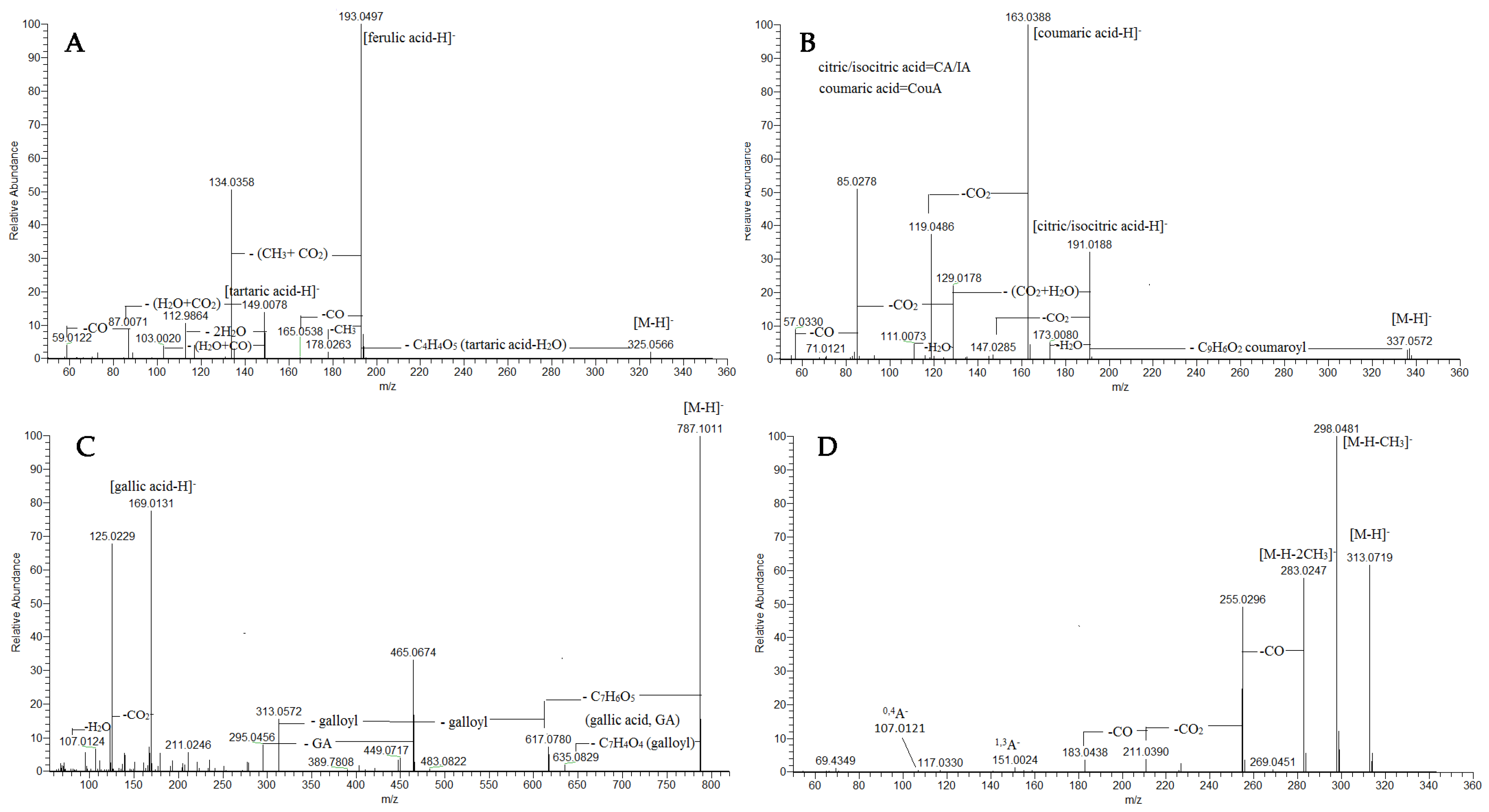
2.1.4. Gallotannins
2.1.5. Flavonoids
2.2. Total Phenolic and Flavonoid Content
2.3. Antioxidant Properties
2.4. Enzyme-Inhibitory Properties
3. Materials and Methods
3.1. Plant Material
3.2. Sample Extraction
3.3. Chemicals
3.4. UHPLC-HRMS
3.5. Assay for Total Phenolic and Flavonoid Contents
3.6. Assays for In Vitro Antioxidant Capacity
3.7. Inhibitory Effects against Some Key Enzymes
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amel, H.A.; Kamel, H.; Meriem, F.; Abdelkader, K. Traditional Uses, Botany, Phytochemistry, and Pharmacology of Pelargonium graveolens: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2022, 6, 1547–1569. [Google Scholar]
- Available online: www.ipni.org (accessed on 1 January 2024).
- Narnoliya, L.K.; Jadaun, J.S.; Singh, S.P. The phytochemical composition, biological effects and biotechnological approaches to the production of high-value essential oil from geranium. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 327–352. [Google Scholar]
- Available online: http://www.worldfloraonline.org (accessed on 1 January 2024).
- Roman, S.; Voaides, C.; Babeanu, N. Exploring the Sustainable Exploitation of Bioactive Compounds in Pelargonium sp.: Beyond a Fragrant Plant. Plants 2023, 12, 4123. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Lothe, N.B.; Bawitlung, L.; Singh, S.; Singh, M.K.; Kumar, P.; Verma, R.K.; Tandon, S.; Pal, A.; Verma, R.S. Secondary metabolic profile of rose-scented geranium: A tool for characterization, distinction and quality control of Indian genotypes. Ind. Crops Prod. 2022, 187, 115487. [Google Scholar] [CrossRef]
- Androutsopoulou, C.; Christopoulou, S.D.; Hahalis, P.; Kotsalou, C.; Lamari, F.N.; Vantarakis, A. Evaluation of essential oils and extracts of rose geranium and rose petals as natural preservatives in terms of toxicity, antimicrobial, and antiviral activity. Pathogens 2021, 10, 494. [Google Scholar] [CrossRef]
- El-Sonbaty, A.E.; Farouk, S.; Al-Yasi, H.M.; Ali, E.F.; Abdel-Kader, A.A.; El-Gamal, S.M. Enhancement of rose scented geranium plant growth, secondary metabolites, and essential oil components through foliar applications of iron (nano, sulfur and chelate) in alkaline soils. Agronomy 2022, 12, 2164. [Google Scholar] [CrossRef]
- Nazarideljou, M.J. Biomass and volatile compounds of rose-scented geranium under various elicitors and cultivation systems. J. Essent. Oil Bear. Plants 2023, 26, 987–1007. [Google Scholar] [CrossRef]
- Lothe, N.B.; Verma, R.K. A study on geranium (Pelargonium graveolens L′ Herit ex Aiton) cultivars’ productivity and economics as intervening by diverse climatic conditions of the western peninsular region of India. Ind. Crops Prod. 2023, 200, 116882. [Google Scholar] [CrossRef]
- Fraisse, D.; Scharf, C.; Vermin, G.; Metzer, J. SPECMA Bank application to the study of geranium essential oils of various origins. In Proceedings of the IXth International Essential Oil Congress, Singapore, 13–17 March 1983; pp. 100–120. [Google Scholar]
- Pandith, S.A.; Dhar, N.; Wani, T.A.; Razdan, S.; Bhat, W.W.; Rana, S.; Khan, S.; Verma, M.K.; Lattoo, S.K. Production dynamics in relation to ontogenetic development and induction of genetic instability through in vitro approaches in Pelargonium graveolens: A potential essential oil crop of commercial significance. Flavour Fragr. J. 2017, 32, 376–387. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Martiskainen, O.; Seif el-Din, S.H.; Sabra, A.-N.A.; Hammam, O.A.; El-Lakkany, N.M. Protective effect of Pelargonium graveolens against carbon tetrachloride-induced hepatotoxicity in mice and characterization of its bioactive constituents by HPLC–PDA–ESI–MS/MS analysis. Med. Chem. Res. 2015, 24, 1438–1448. [Google Scholar] [CrossRef]
- Boukhris, M.; Bouaziz, M.; Feki, I.; Jemai, H.; El Feki, A.; Sayadi, S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis. 2012, 11, 81. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Geranium and Pelargonium: The Genera Geranium and Pelargonium; Taylor & Francis: London, UK, 2002. [Google Scholar]
- Marmouzi, I.; Karym, E.M.; Alami, R.; El Jemli, M.; Kharbach, M.; Mamouch, F.; Attar, A.; Faridi, B.; Cherrah, Y.; Faouzi, M.E.A. Modulatory effect of Syzygium aromaticum and Pelargonium graveolens on oxidative and sodium nitroprusside stress and inflammation. Orient. Pharm. Exp. Med. 2019, 19, 201–210. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Y.; Jiao, M.; Ye, C.; Li, Y.; Liu, C.; Wang, J. Integration of high-throughput omics technologies in medicinal plant research: The new era of natural drug discovery. Front. Plant Sci. 2023, 14, 1073848. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.S.; Mangoni, A.; Hanschen, F.S.; Agerbirk, N.; Zidorn, C. Essentials in the acquisition, interpretation, and reporting of plant metabolite profiles. Phytochemistry 2024, 220, 114004. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C. Tanacetum vulgare L.(Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef] [PubMed]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Zheleva-Dimitrova, D.; Picot-Allain, C.; Mahomoodally, M.F.; Imran, M.; Dall’Acqua, S. UHPLC-MS Characterization and biological insights of different solvent extracts of two Achillea species (A. aleppica and A. santolinoides) from Turkey. Antioxidants 2021, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Haramiishi, R.; Okuyama, S.; Yoshimura, M.; Nakajima, M.; Furukawa, Y.; Ito, H.; Amakura, Y. Identification of the characteristic components in walnut and anti-inflammatory effect of glansreginin A as an indicator for quality evaluation. Biosci. Biotechnol. Biochem. 2020, 84, 187–197. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Petrova, A.; Zengin, G.; Sinan, K.I.; Balabanova, V.; Joubert, O.; Zidorn, C.; Voynikov, Y.; Simeonova, R.; Gevrenova, R. Metabolite profiling and bioactivity of Cicerbita alpina (L.) Wallr. (Asteraceae, Cichorieae). Plants 2023, 12, 1009. [Google Scholar] [CrossRef]
- Cech, N.B.; Eleazer, M.S.; Shoffner, L.T.; Crosswhite, M.R.; Davis, A.C.; Mortenson, A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromatogr. A 2006, 1103, 219–228. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl flavonol glycosides and more in marketed green teas: An intrinsic value beyond much-lauded catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Picot-Allain, M.C.N.; Dall’Acqua, S.; Behl, T.; Goh, B.H.; Ying, P.T.S.; Mahomoodally, M.F. Exploring the chemical profiles and biological values of two Spondias species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products. Antioxidants 2021, 10, 1771. [Google Scholar] [CrossRef]
- Gevrenova, R.; Kostadinova, I.; Stefanova, A.; Balabanova, V.; Zengin, G.; Zheleva-Dimitrova, D.; Momekov, G. Phytochemical Profiling, Antioxidant and Cognitive-Enhancing Effect of Helichrysum italicum ssp. italicum (Roth) G. Don (Asteraceae). Plants 2023, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of ultrasound-assisted extraction of phloretin and other phenolic compounds from apple tree leaves (Malus domestica Borkh.) and comparison of different cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- de Rijke, E.; Out, P.; Niessen, W.M.; Ariese, F.; Gooijer, C.; Udo, A. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Bendaif, H.; Mrabti, H.; Elmsellem, H.; Kadmi, Y.; Shariati, M.; Abdel-Rahman, I.; Hammouti, B.; Bouyanzer, A. Antioxidant activity of phenols and flavonoids contents of aqueous extract of Pelargonium graveolens orgin in the North-East Morocco. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1218. [Google Scholar] [CrossRef]
- Boukhris, M.; Simmonds, M.S.J.; Sayadi, S.; Bouaziz, M. Chemical Composition and Biological Activities of Polar Extracts and Essential Oil of Rose-scented Geranium, Pelargonium graveolens. Phytother. Res. 2013, 27, 1206–1213. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Hamdi, N. Phytochemical composition and antimicrobial activities of the essential oils and organic extracts from pelargonium graveolens growing in Tunisia. Lipids Health Dis. 2012, 11, 167. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Mammen, D. Chemical perspective and drawbacks in flavonoid estimation assays. Front. Nat. Prod. Chem 2022, 10, 189–228. [Google Scholar]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; Bouyahya, A.; Yildiztugay, E.; Carretero, A.S.; Mahomoodally, M.F.; Ponniya, S.K.M.; Nilofar; Koyuncu, I.; et al. UHPLC-ESI-QTOF-MS metabolite profiles of different extracts from Pelargonium endlicherianum parts and their biological properties based on network pharmacological approaches. Arch. Pharm. 2024, 357, 2300728. [Google Scholar] [CrossRef] [PubMed]
- Ennaifer, M.; Bouzaiene, T.; Messaoud, C.; Hamdi, M. Phytochemicals, antioxidant, anti-acetyl-cholinesterase, and antimicrobial activities of decoction and infusion of Pelargonium graveolens. Nat. Prod. Res. 2020, 34, 2634–2638. [Google Scholar] [CrossRef] [PubMed]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of polyphenol content and antioxidant capacity of aqueous extracts from eight medicinal plants from reunion island: Protection against oxidative stress in red blood cells and preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Golmei, P.; Kasna, S.; Roy, K.P.; Kumar, S. A Review on Pharmacological Advancement of Ellagic Acid. J. Pharmacol. Pharmacother. 2024, 15, 0976500X241240634. [Google Scholar] [CrossRef]
- Tošović, J.; Bren, U. Antioxidative Action of Ellagic Acid—A Kinetic DFT Study. Antioxidants 2020, 9, 587. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Kamisah, Y.; Jalil, J.; Yunos, N.M.; Zainalabidin, S. Cardioprotective properties of kaempferol: A review. Plants 2023, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Świderski, G.; Gołębiewska, E.; Kalinowska, M.; Świsłocka, R.; Kowalczyk, N.; Jabłońska-Trypuć, A.; Lewandowski, W. Comparison of Physicochemical, Antioxidant, and Cytotoxic Properties of Caffeic Acid Conjugates. Materials 2024, 17, 2575. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Najm, S.; Barkat, K.; Nafidi, H.-A.; Jardan, Y.A.B.; Bourhia, M. Caftaric Acid Ameliorates Oxidative Stress, Inflammation, and Bladder Overactivity in Rats Having Interstitial Cystitis: An In Silico Study. ACS Omega 2023, 8, 28196. [Google Scholar]
- Koriem, K. Caftaric acid: An overview on its structure, daily consumption, bioavailability and pharmacological effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. Enzym. Inhib. Act. 2017, 165, 177. [Google Scholar]
- Taqui, R.; Debnath, M.; Ahmed, S.; Ghosh, A. Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomed. Plus 2022, 2, 100184. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Louckova, A.; Polak, J.; Hajslova, J. Identifying edible plants with high anti-obesity potential: In vitro inhibitory effect against pancreatic lipase followed by bioactive compound metabolomic screening. Food Biosci. 2023, 56, 103453. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Santa-arthampreecha, S.; Samakthanasan, S.; Kitphati, W.; Pratuangdejkul, J.; Nukoolkam, V. Gallic acid and derivatives as acetylcholinesterase inhibitors. Thai J. Pharm. Sci. 2012, 36, 35–37. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Medica 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Yin, P.; Yang, L.; Xue, Q.; Yu, M.; Yao, F.; Sun, L.; Liu, Y. Identification and inhibitory activities of ellagic acid-and kaempferol-derivatives from Mongolian oak cups against α-glucosidase, α-amylase and protein glycation linked to type II diabetes and its complications and their influence on HepG2 cells’ viability. Arab. J. Chem. 2018, 11, 1247–1259. [Google Scholar]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Si, Y.-X.; Yin, S.-J.; Oh, S.; Wang, Z.-J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef]
- Akhlaghipour, I.; Shad, A.N.; Askari, V.R.; Maharati, A.; Rahimi, V.B. How caffeic acid and its derivatives combat diabetes and its complications: A systematic review. J. Funct. Foods 2023, 110, 105862. [Google Scholar] [CrossRef]
- Casanova, L.M.; Gu, W.; Costa, S.S.; Jeppesen, P.B. Phenolic substances from Ocimum species enhance glucose-stimulated insulin secretion and modulate the expression of key insulin regulatory genes in mice pancreatic islets. J. Nat. Prod. 2017, 80, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A multifunctional flavonol in biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, W.; Yang, F.; Ai, F.; Du, D.; Li, Y. Discovery of caffeoylisocitric acid as a Keap1-dependent Nrf2 activator and its effects in mesangial cells under high glucose. J. Enzym. Inhib. Med. Chem. 2022, 37, 178–188. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Guan, X.-Q. Research progress on pharmacological effects of p-coumaric acid. Chin. Tradit. Herb. Drugs 2018, 49, 4162–4170. [Google Scholar]
- Romanik, G.; Gilgenast, E.; Przyjazny, A.; Kamiński, M. Techniques of preparing plant material for chromatographic separation and analysis. J. Biochem. Biophys. Methods 2007, 70, 253–261. [Google Scholar] [CrossRef]
- Djordjevic, S.M. From medicinal plant raw material to herbal remedies. Aromat. Med. Plants Back Nat. 2017, 25, 269–288. [Google Scholar]
- Nikolov, S. Specialized Encyclopedia of medicinal plants in Bulgaria. In “Bulgarian encyclopedia”; Ed. Trud: Sofia, Bulgaria, 2006. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs—I. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Bel.g 1994, 49, 462–468. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Šafašík, I. Rapid Detection of Alpha-Amylase Inhibitors. J. Enzym. Inhib. 1990, 3, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.; Zhang, X.-D.; Song, Y.-W.; Liu, J.-W. A microplate-based screening method for alpha-glucosidase inhibitors. Chin. J. Clin. Pharmacol. Ther. 2005, 10, 1128. [Google Scholar]
| № | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− | tR (min) | Identification Confidence Level (Çiçek et al. [18]) |
|---|---|---|---|---|---|
| Hydroxybenzoic, Hydroxycinnamic Acids and Their Glycosides, Coumarins and Aliphatic Acids | |||||
| 1. | tartaric acid | C4H6O6 | 149.0092 | 0.70 | D1 |
| 2. | mallic acid | C4H6O5 | 133.0143 | 0.74 | D1 |
| 3. | citric/isocitric acid | C6H8O7 | 191.0197 | 0.75 | D1 |
| 4. | citric/isocitric acid | C6H8O7 | 191.0197 | 0.90 | D1 |
| 5. | gallic acid * | C7H6O5 | 169.0143 | 1.15 | B |
| 6. | protocatechuic acid-O-dihexoside | C19H26O14 | 477.1250 | 1.52 | D1 |
| 7. | protocatechuic acid-O-hexoside 1 | C13H16O9 | 315.0727 | 1.67 | D1 |
| 8. | vanillic acid-O-hexoside 1 | C14H18O9 | 329.0875 | 1.77 | D1 |
| 9. | protocatechuic acid * | C7H6O4 | 153.0181 | 2.03 | B |
| 10. | protocatechuic acid-O-hexoside 2 | C13H16O9 | 315.0727 | 2.10 | D1 |
| 11. | hydroxybenzoic acid-O-hexoside 1 | C13H16O8 | 299.0778 | 2.15 | D1 |
| 12. | syringic acid-O-hexoside | C15H20O10 | 359.0985 | 2.26 | D1 |
| 13. | vanilloyl-O-hexose | C14H18O9 | 329.0875 | 2.47 | D1 |
| 14. | dihydrocaffeic acid-O-hexoside | C15H20O9 | 343.1035 | 2.49 | D2 |
| 15. | coumaric acid-O-hexoside 1 | C15H18O8 | 325.0930 | 2.51 | D1 |
| 16. | caffeic acid–O-hexoside 1 | C15H18O9 | 341.0871 | 2.63 | D1 |
| 17. | p-hydroxyphenylacetic acid O-hexoside 1 | C14H18O8 | 313.0929 | 2.66 | D1 |
| 18. | aesculetin-O-hexoside | C15H15O9 | 339.0724 | 2.69 | D1 |
| 19. | syringyl-O-hexose | C15H20O10 | 359.0984 | 2.76 | D1 |
| 20. | 4-hydroxybenzoic acid * | C7H6O3 | 137.0230 | 2.84 | D1 |
| 21. | 3-hydroxybenzoic acid * | C7H6O3 | 137.0230 | 2.99 | D1 |
| 22. | hydroxybenzoic acid-O-hexoside 2 | C13H16O8 | 299.0778 | 2.99 | D1 |
| 23. | caffeic acid O-hexoside 2 | C15H18O9 | 341.0871 | 3.08 | D1 |
| 24. | p-coumaric acid * | C9H8O3 | 163.0389 | 3.10 | B |
| 25. | scopoletin O-hexoside | C16H18O9 | 353.0878 | 3.16 | D1 |
| 26. | dihydrocaffeic acid-O-hexoside | C15H20O9 | 343.1035 | 3.19 | D1 |
| 27. | p-hydroxyphenylacetic acid O-hexoside 2 | C14H18O8 | 313.0929 | 3.30 | D1 |
| 28. | coumaric acid-O-hexoside 2 | C15H18O8 | 325.0930 | 3.34 | D1 |
| 29. | m-coumaric acid * | C9H8O3 | 163.0389 | 3.35 | B |
| 30. | vanillic acid-O-hexoside 2 | C14H18O9 | 329.0875 | 3.39 | D1 |
| 31. | vanillyl alcohol-(acetyl)-hexoside | C16H22O9 | 357.1191 | 3.41 | D1 |
| 32. | aesculetin | C9H6O4 | 177.0193 | 3.45 | D1 |
| 33. | scopoletin O-hexoside isomer | C16H18O9 | 353.0878 | 3.48 | D1 |
| 34. | ferulic acid * | C10H10O4 | 193.0494 | 3.55 | B |
| 35. | caffeic acid * | C9H8O4 | 179.0339 | 3.56 | B |
| 36. | gentisic acid * | C7H6O4 | 153.0180 | 3.67 | B |
| 37. | vanillic acid-O-hexoside 3 | C14H18O9 | 329.0875 | 4.22 | D1 |
| 38. | vanillin * | C8H8O3 | 151.0401 | 4.34 | B |
| 39. | phenylethyl-O-pentosylhexoside (primeveroside) | C20H30O12 | 461.1665 | 4.48 | D1 |
| 40. | o-coumaric acid * | C9H8O3 | 163.0389 | 4.55 | B |
| 41. | coumaric acid-O-hexoside 3 | C15H18O8 | 325.0929 | 4.70 | D1 |
| 42. | phenylethyl-O-pentosylhexoside (primeveroside) | C20H30O12 | 461.1665 | 4.75 | D1 |
| 43. | vanillic acid * | C8H8O4 | 167.0338 | 4.79 | B |
| 44. | scopoletin–(caffeoyl)-hexoside | C25H26O13 | 533.1301 | 5.12 | D1 |
| 45. | glansreginic acid O-hexoside | C18H28O10 | 403.1609 | 5.43 | D2 |
| 46. | digalloylcitramalic acid | C19H18O14 | 469.0624 | 5.52 | D2 |
| 47. | salicylic acid * | C7H6O3 | 137.0230 | 6.28 | B |
| 48. | ferulic acid-(vanillyl)-hexoside | C24H26O12 | 505.1352 | 6.45 | D1 |
| 49. | gentisic acid-(feruloyl)-hexoside | C23H24O12 | 491.1195 | 6.71 | D1 |
| Acyltartaric Acids | |||||
| 50. | caftaric acid * | C13H12O9 | 311.0409 | 2.16 | C |
| 51. | caffeoyltartaric acid-hexoside- | C19H22O14 | 473.0937 | 2.28 | D2 |
| 52. | caftaric acid isomer | C13H12O9 | 311.0409 | 2.36 | D1 |
| 53. | cafeoyltartaric acid dimer | C26H23O18 | 623.0892 [2M-H]− | 2.36 | D2 |
| 54. | dicaffeoyltartaric acid 1 | C22H20O13 | 491.0831 | 2.58 | D2 |
| 55. | dicaffeoyltartaric acid 2 | C22H20O13 | 491.0831 | 2.79 | D2 |
| 56. | coumaroyltartaric acid | C13H12O8 | 295.0459 | 3.10 | D2 |
| 57. | feruloyltartaric acid | C14H14O9 | 325.0565 | 3.55 | D2 |
| Citric/Isocitric Acid Esters (Acylcitric Acids) | |||||
| 58. | caffeoylcitric/isocitric acid 1 | C15H14O10 | 353.0514 | 2.41 | D1 |
| 59. | caffeoylcitric/isocitric acid 2 | C15H14O10 | 353.0514 | 2.76 | D1 |
| 60. | caffeoylcitric/isocitric acid 3 | C15H14O10 | 353.0514 | 2.90 | D1 |
| 61. | caffeoylcitric/isocitric acid 4 | C15H14O10 | 353.0514 | 3.28 | D1 |
| 62. | caffeoylcitric/isocitric acid 5 | C15H14O10 | 353.0514 | 3.95 | D1 |
| 63. | coumaroylcitric/isocitric acid 1 | C15H14O9 | 337.0565 | 4.16 | D1 |
| 64. | caffeoylcitric/isocitric acid 6 | C15H14O10 | 353.0514 | 4.29 | D1 |
| 65. | coumaroylcitric/isocitric acid 2 | C15H14O9 | 337.0565 | 4.85 | D2 |
| 66. | coumaroylcitric/isocitric acid 3 | C15H14O9 | 337.0565 | 5.26 | D2 |
| 67. | feruloylcitric/isocitric acid 1 | C16H16O10 | 367.0671 | 5.30 | D2 |
| 68. | feruloylcitric/isocitric acid 2 | C16H16O10 | 367.0671 | 5.63 | D2 |
| Gallotannins | |||||
| 69. | galloyl-O-hexoside 1 | C13H16O10 | 331.0671 | 1.22 | D1 |
| 70. | galloyl-O-hexoside 2 | C13H16O10 | 331.0671 | 1.58 | D1 |
| 71. | gallocatechin | C15H14O7 | 305.0667 | 1.80 | D1 |
| 72. | digalloyl-O-hexose | C20H20O14 | 483.0780 | 2.99 | D1 |
| 73. | digallic acid | C14H10O9 | 321.0252 | 3.10 | D1 |
| 74. | methylgallate | C8H8O5 | 183.0299 | 3.16 | D1 |
| 75. | tetragalloyl-hexoside | C34H28O22 | 787.1000 | 4.94 | D1 |
| 76. | ellagic acid * | C14H6O8 | 300.9990 | 5.01 | B |
| Flavonoids | |||||
| 77. | eriodyctiol O-hexoside | C21H22O11 | 449.1089 | 4.03 | D1 |
| 78. | myricitin 3-O-pentosylhexoside | C26H28O17 | 611.1254 | 4.18 | D1 |
| 79. | myricetin 3-O-rutinoside | C27H30O17 | 625.1410 | 4.48 | D1 |
| 80. | quercetin 3-O-pentosylhexoside | C26H28O16 | 595.1305 | 4.71 | D1 |
| 81. | myricetin O-pentoside | C20H18O12 | 449.0726 | 4.96 | D1 |
| 82. | rutin * | C27H30O16 | 609.1464 | 5.08 | C |
| 83. | myricetin O-rhamnoside (myricitrin) | C21H20O12 | 463.0885 | 5.11 | C |
| 84. | hyperoside * | C21H20O12 | 463.0885 | 5.19 | C |
| 85. | quercetin O-hexuronide | C22H22O12 | 477.1039 | 5.23 | D1 |
| 86. | kaempferol O-pentosylhexoside | C26H28O15 | 579.1355 | 5.27 | D1 |
| 87. | Isoquercitrin * | C21H20O12 | 463.0885 | 5.30 | C |
| 88. | myricetin methylether O-hexoside | C22H22O13 | 493.0988 | 5.35 | D1 |
| 89. | phloretin 3′, 5–diC-hexoside | C27H34O15 | 597.18249 | 5.37 | D1 |
| 90. | kaempferol O-deoxyhexosyl-O-hexoside | C27H30O15 | 593.1512 | 5.41 | D1 |
| 91. | quercetin 3-O-pentoside | C20H18O11 | 433.0776 | 5.64 | D1 |
| 92. | kaempferol 3-O-rutinoside * | C27H30O15 | 593.1512 | 5.64 | C |
| 93. | phloretin C-hexoside | C21H24O10 | 435.1297 | 5.74 | D1 |
| 94. | isorhamnetin 3-O-rutinoside * | C28H32O17 | 623.1618 | 5.78 | C |
| 95. | kaempferol 3-O-glucoside * | C21H19O11 | 447.0934 | 5.88 | C |
| 96. | isorhamnetin 3-O-glucoside * | C22H22O12 | 477.1044 | 6.03 | C |
| 97. | naringenin 7-O-hexoside | C21H20O10 | 431.0984 | 6.06 | D1 |
| 98. | kaempferol 3-O-pentoside | C20H18O10 | 417.0827 | 6.07 | D1 |
| 99. | eriodyctiol * | C15H12O6 | 287.0561 | 6.29 | B |
| 100. | myricetin * | C15H10O8 | 317.0303 | 6.29 | B |
| 101. | luteolin 7-O-caffeoylhexoside | C30H26O14 | 609.1250 | 7.01 | D1 |
| 102. | quercetin * | C15H10O7 | 301.0354 | 7.61 | B |
| 103. | isorhamnetin * | C16H12O7 | 315.0512 | 8.09 | B |
| 104. | myricetin dimethyl ether | C17H14O8 | 345.0616 | 8.11 | D1 |
| 105. | naringenin * | C15H12O5 | 271.0612 | 8.58 | B |
| 106. | kaempferol dimethyl ether O-hexoside | C31H22O8 | 521.1242 | 8.79 | D1 |
| 107. | kaempferol * | C15H10O6 | 285.0405 | 8.84 | B |
| 108. | kaempferol methyl ether (kaempferide) | C16H12O6 | 299.0561 | 9.33 | D1 |
| 109. | quercetin dimethyl ether | C17H14O7 | 329.0677 | 9.61 | D1 |
| 110. | myricetin trimethyl ether | C18H16O8 | 359.0772 | 10.68 | D1 |
| 111. | kaempferol dimethyl ether | C17H14O6 | 313.0718 | 12.30 | D1 |
| Other Compounds | |||||
| 112. | bergenin O-coumaric acid | C23H22O11 | 473.1089 | 8.03 | D1 |
| Parameters | Results |
|---|---|
| Total bioactive compounds | |
| Total phenolic content (mg GAE/g) | 83.86 ± 1.67 |
| Total flavonoid content (mg RE/g) | 9.49 ± 0.26 |
| Antioxidant properties | |
| DPPH scavenging ability (mg TE/g) | 273.45 ± 4.31 |
| ABTS•+ scavenging ability (mg TE/g) | 531.97 ± 10.97 |
| CUPRAC (mg TE/g) | 413.22 ± 7.22 |
| FRAP (mg TE/g) | 292.21 ± 4.77 |
| Metal chelating (mg EDTAE/g) | 13.44 ± 0.44 |
| Phosphomolybdenum (mmol TE/g) | 2.71 ± 0.15 |
| Enzyme-inhibitory properties | |
| AChE inhibition (mg GALAE/g) | 2.80 ± 0.02 |
| BChE inhibition (mg GALAE/g) | 2.20 ± 0.11 |
| Tyrosinase inhibition (mg KAE/g) | 75.49 ± 0.49 |
| Amylase inhibition (mmol ACAE/g) | 0.79 ± 0.01 |
| Glucosidase inhibition (mmol ACAE/g) | 3.75 ± 0.01 |
| Lipase inhibition (mg OE/g) | 28.91 ± 4.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevrenova, R.; Zengin, G.; Balabanova, V.; Szakiel, A.; Zheleva-Dimitrova, D. Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential. Plants 2024, 13, 2612. https://doi.org/10.3390/plants13182612
Gevrenova R, Zengin G, Balabanova V, Szakiel A, Zheleva-Dimitrova D. Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential. Plants. 2024; 13(18):2612. https://doi.org/10.3390/plants13182612
Chicago/Turabian StyleGevrenova, Reneta, Gokhan Zengin, Vessela Balabanova, Anna Szakiel, and Dimitrina Zheleva-Dimitrova. 2024. "Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential" Plants 13, no. 18: 2612. https://doi.org/10.3390/plants13182612









