Rpv10.2: A Haplotype Variant of Locus Rpv10 Enables New Combinations for Pyramiding Downy Mildew Resistance Traits in Grapevine
Abstract
:1. Introduction
2. Results
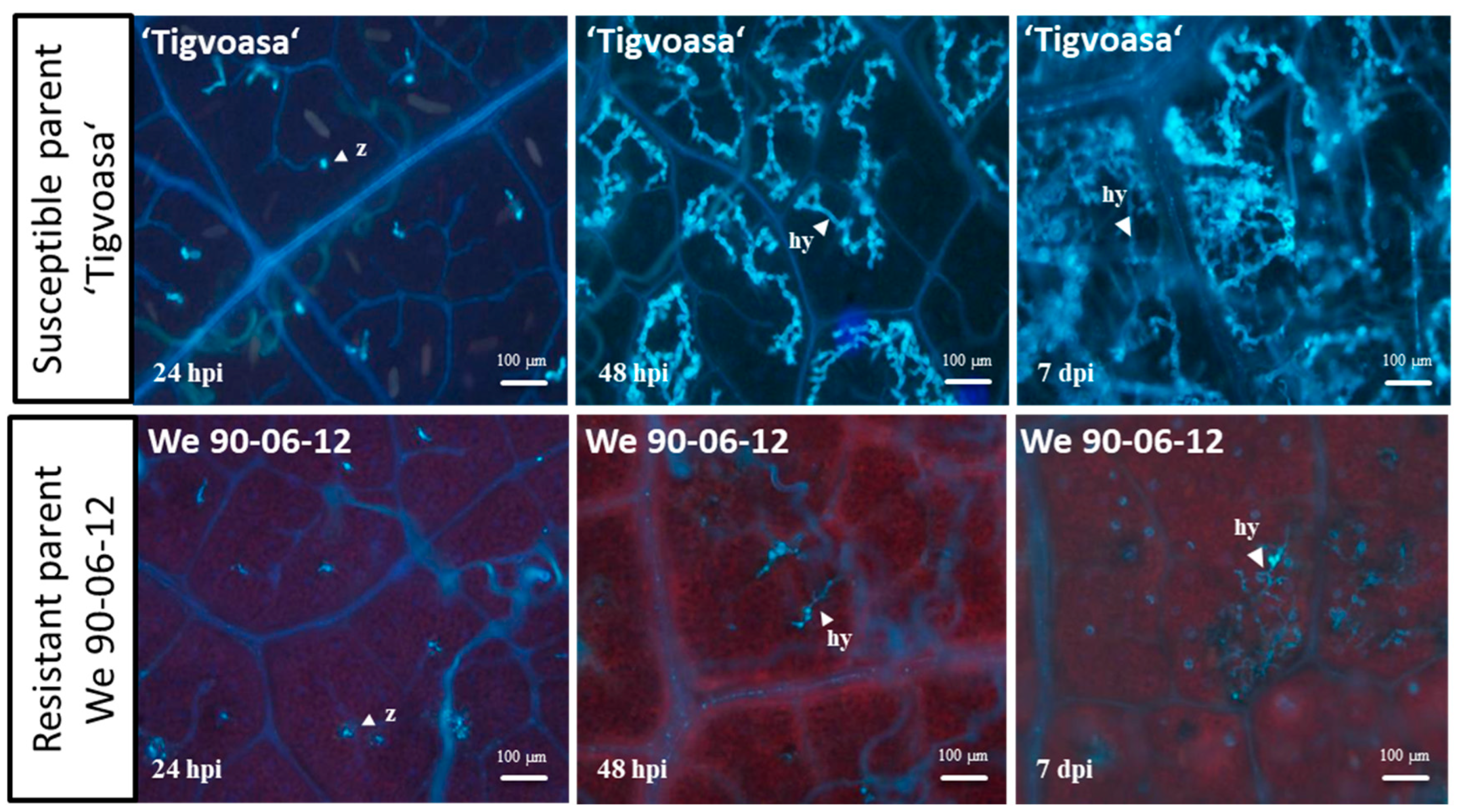
2.1. The Phenotype of Resistance of We 90-06-12
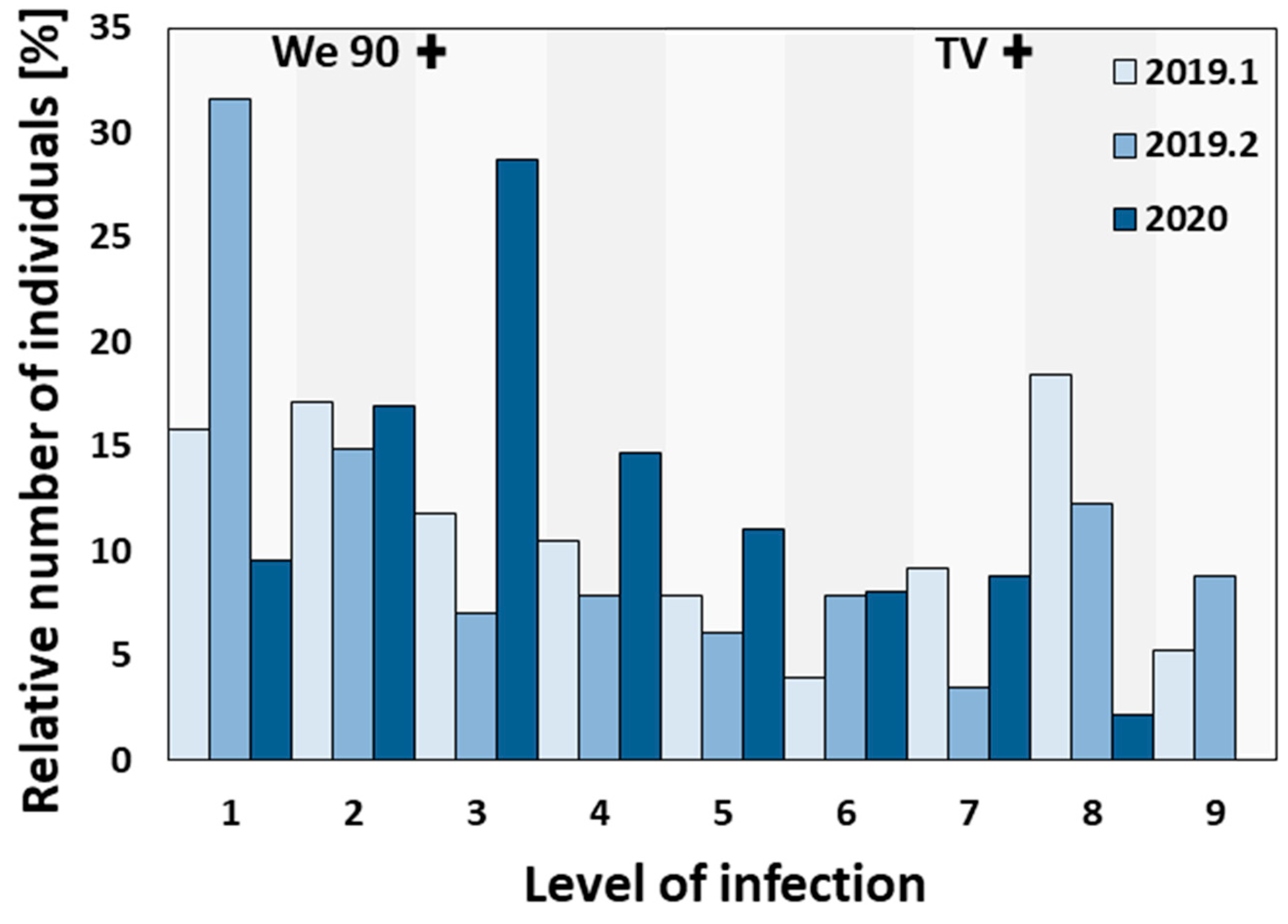
2.2. Segregation of the Phenotypes
2.3. Genetic Map
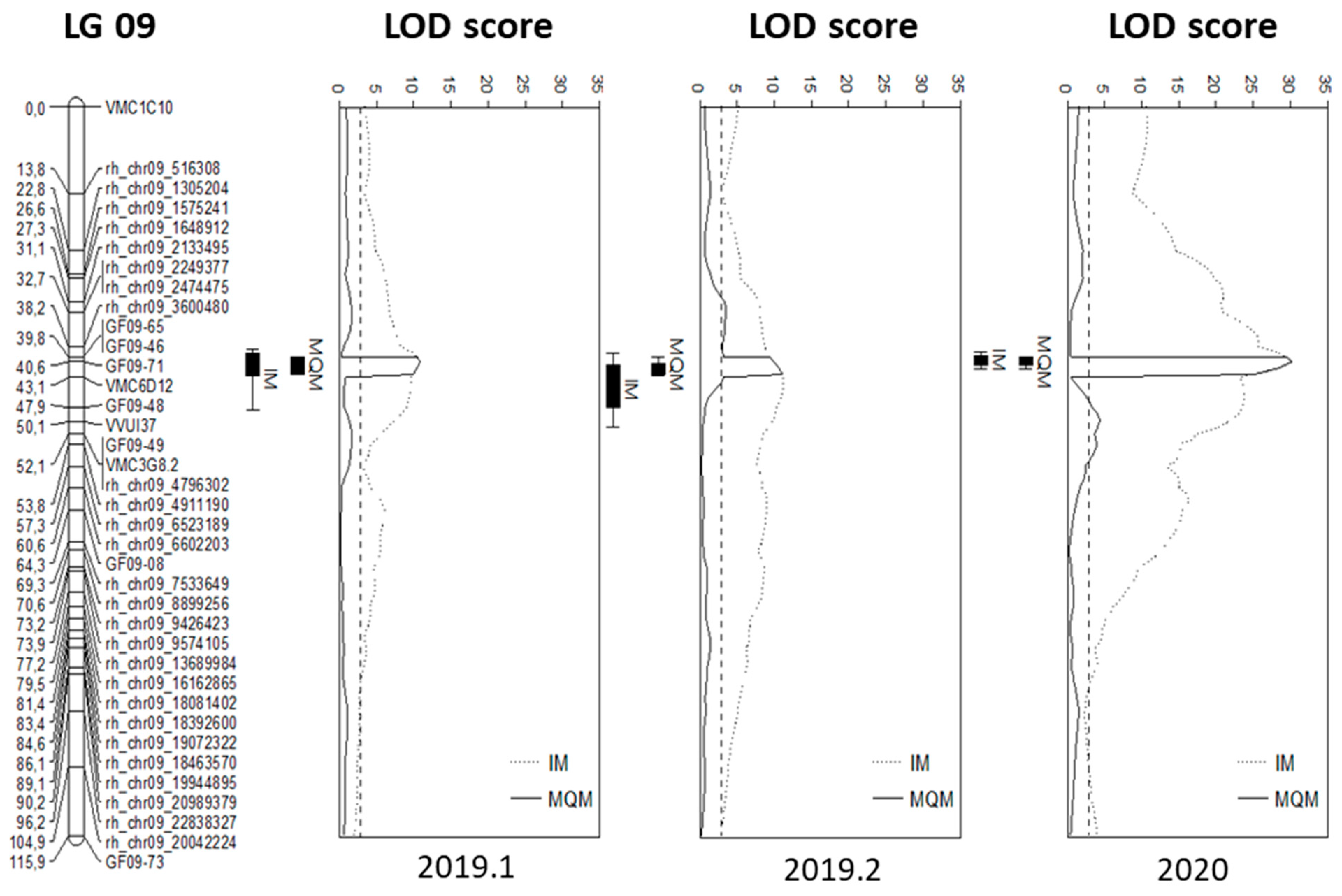
2.4. QTL Identification
2.5. Differences between Rpv10 and Rpv10.2
2.6. Sequence Comparison of Rpv10 Candidate Genes
3. Materials and Methods
3.1. Plant Material
3.2. Phenotyping
3.3. DNA Extraction
3.4. Microsatellite Analysis
3.5. Linkage Mapping
3.6. QTL Analysis
3.7. Sequence Analysis of the Rpv10 Candidate Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Azis, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine ‘Merlot’ (Vitis vinifera). Vitis 2010, 49, 77–85. [Google Scholar]
- Lamichhane, J.R. Pesticide use and risk reduction in European farming systems with IPM: An introduction to the special issue. Crop. Prot. 2017, 97, 1–6. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed, 2nd revised ed.; FRAC Monograph No. 1; Fungicide Resistance Action Committee 2007: Bristol, UK, 1995. [Google Scholar]
- Hall, R.J.; Gubbins, S.; Gilligan, C.A. Evaluating the Performance of Chemical Control in the Presence of Resistant Pathogens. Bull. Math. Biol. 2006, 69, 525–537. [Google Scholar] [CrossRef]
- Fischer, B.M.; Salakhutdinov, I.; Akkurt, M.; Eibach, R.; Edwards, K.J.; Töpfer, R.; Zyprian, E.M. Quantitative trait locus analysis of fungal disease resistance factors on a molecular map of grapevine. Theor. Appl. Genet. 2003, 108, 501–515. [Google Scholar] [CrossRef]
- Welter, L.J.; Göktürk-Baydar, N.; Akkurt, M.; Maul, E.; Eibach, R.; Töpfer, R.; Zyprian, E.M. Genetic mapping and localization of quantitative trait loci affecting fungal disease resistance and leaf morphology in grapevine (Vitis vinifera L.). Mol. Breed. 2007, 20, 359–374. [Google Scholar] [CrossRef]
- Bellin, D.; Peressotti, E.; Merdinoglu, D.; Wiedemann-Merdinoglu, S.; Adam-Blondon, A.-F.; Cipriani, G.; Morgante, M.; Testolin, R.; Di Gaspero, G. Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localized necrosis at the infection site. Theor. Appl. Genet. 2009, 120, 163–176. [Google Scholar] [CrossRef]
- Röckel, F.; Trapp, O.; Zyprian, E.; Hausmann, L.; Migliaro, D.; Vezzulli, S.; Töpfer, R.; Maul, E. A ‘Regent’ pedigree update: Ancestors, offspring and their confirmed resistance loci. Vitis 2021, 60, 189–193. [Google Scholar]
- Rouxel, M.; Mestre, P.; Comont, G.; Lehman, B.L.; Schilder, A.; Delmotte, F. Phylogenetic and experimental evidence for host-specialized cryptic species in a biotrophic oomycete. New Phytol. 2012, 197, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Toffolatti, S.L.; De Lorenzis, G.; Costa, A.; Maddelana, G.; Passera, A.; Bonza, M.C.; Pindo, M.; Stefani, E.; Cestaro, A.; Casati, P.; et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vits vinierfa). Sci. Rep. 2018, 8, 12523. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Huang, H.; Folta, K.M.; Lu, J. Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biol. 2010, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Schwander, F.; Eibach, R.; Fechter, I.; Hausmann, L.; Zyprian, E.; Töpfer, R. Rpv10: A new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor. Appl. Genet. 2011, 124, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Venuti, S.; Copetti, D.; Foria, S.; Falginella, L.; Hoffmann, S.; Bellin, D.; Cindrić, P.; Kozma, P.; Scalabrin, S.; Morgante, M.; et al. Historical Introgression of the Downy Mildew Resistance Gene Rpv12 from the Asian Species Vitis amurensis into Grapevine Varieties. PLoS ONE 2013, 8, e61228. [Google Scholar] [CrossRef]
- Wang, F.S.; Zhu, C.S.; Yang, D.B.; Zhang, H.D. Systematics study on the genus Chinese Vitis L. of China. J. Trop. Subtrop. Bot. 2000, 8, 1–10. [Google Scholar]
- Wan, Y.; Schwaninger, H.; Li, D.; Simon, C.J.; Wang, Y.; He, P. The eco-geographic distribution of wild grape germplasm in China. Vitis 2008, 47, 77–80. [Google Scholar]
- Wan, Y.; Schwaninger, H.; He, P.; Wang, Y. Comparison of resistance to powdery mildew and downy mildew in Chinese wild grapes. Vitis 2007, 46, 132–136. [Google Scholar]
- Dick, M.W. Towards an understanding of the evolution of the downy mildews. In Advances in Downy Mildew Research; Spencer-Phillips, P.T.N., Gisi, U., Lebeda, A., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; Volume 1, pp. 1–59. [Google Scholar]
- Jürges, G.; Kassemeyer, H.-H.; Dürrenberger, M.; Düggelin, M.; Nick, P. The mode of interaction between Vitis and Plasmopara viticola Berk. & Curt. Ex de Bary depends on host species. Plant Biol. 2008, 11, 886–898. [Google Scholar]
- Fröbel, S.; Dudenhöffer, J.; Töpfer, R.; Zyprian, E. Transcriptome analysis of early downy mildew (Plasmopara viticola) defense in grapevines carrying the Asian resistance locus Rpv10. Euphytica 2019, 215, 28. [Google Scholar] [CrossRef]
- Possamai, T.; Migliaro, D.; Gardiman, M.; Velasco, R.; De Nardi, B. Rpv mediated responses in grapevine offspring resistant to Plasmopara viticola. Plants 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Chitarrini, G.; Riccadonna, S.; Zulini, L.; Vecchione, A.; Stefanini, M.; Larger, S.; Pindo, M.; Cestaro, A.; Franceschi, P.; Magris, G.; et al. Two-omics data revealed commonalities and differences between Rpv12- and Rpv3-mediated resistance in grapevine. Sci. Rep. 2020, 10, 12193. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Kufer, T.A.; Schulze-Lefert, P. NLR functions in plant and animal immune systems: So far and yet so close. Nat. Immunol. 2011, 12, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.-Y.; Willingham, S.B.; Bergstralh, D.T. NLRs ar the intersection of cell death and immunity. Nat. Rev. Immunol. 2008, 8, 372–379. [Google Scholar] [CrossRef]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, L.; Chang, P.; Li, Z. Endophytic fungal community in grape is correlated to foliar age and domestication. Ann. Microbiol. 2020, 70, 30. [Google Scholar] [CrossRef]
- Musetti, R.; Vecchione, A.; Stringher, L.; Borselli, S.; Zulini, L.; Marzani, C.; D’Ambrosio, M.; di Toppi, L.S.; Pertot, I. Inhibition of sporulation and ultrastructural alterations of grapevine downy mildew by the endophytic fungus Alternaria alternata. Phytopathology 2006, 96, 689–698. [Google Scholar] [CrossRef]
- Wingerter, C.; Eisenmann, B.; Weber, P.; Dry, I.; Bogs, J. Grapevine Rpv3-, Rpv10- and Rpv12-mediated defense responses against Plasmopara viticola and the impact of their deployment on fungicide use in viticulture. BMC Plant Biol. 2021, 21, 470. [Google Scholar] [CrossRef]
- Peressotti, E.; Wiedemann-Merdinoglu, S.; Delmotte, F.; Bellin, D.; Di Gaspero, G.; Testolin, R.; Merdinoglu, D.; Mestre, P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Di Gaspero, G.; Copetti, D.; Coleman, C.; Castellarin, S.D.; Eibach, R.; Kozma, P.; Lacombe, T.; Gambetta, G.; Zvyagin, A.; Cindrić, P.; et al. Selective sweep at the *Rpv3* locus during grapevine breeding for downy mildew resistance. Theor. Appl. Genet. 2011, 124, 277–286. [Google Scholar] [CrossRef]
- R Core Team. A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Dudenhöffer, J.; Schwander, F.; Töpfer, R.; Zyprian, E. Sequence analysis of loci Rpv10 and Rpv3 for resistance against grapevine downy mildew (Plasmopara viticola). In Proceedings of the ISHS Proceedings of XIth International Conference on Grapevine Breeding and Genetics, ISHS Acta Horticulturae 1082, Beijing, China, 28 July–2 August 2014; Shao-Hua, L., Ed.; pp. 69–72. [Google Scholar]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Practical Flow Cytometry; John Wiley & Sons: New York, NY, USA, 1988; p. 129. [Google Scholar]
- Hood, M.E.; Shew, H.D. Applications of KOH-aniline blue fluorescence in the study of plant-fungal interactions. Phytopathology 1996, 86, 704–708. [Google Scholar] [CrossRef]
- Unger, S.; Büche, C.; Boso, S.; Kassemeyer, H.-H. The Course of Colonization of Two Different Vitis Genotypes by Plasmopara viticola Indicates Compatible and Incompatible Host-Pathogen Interactions. Phytopathology 2007, 97, 780–786. [Google Scholar] [CrossRef]
- Di Gaspero, G.; Cipriani, G.; Marrazzo, M.T.; Andreetta, D.; Castro, M.J.P.; Peterlunger, E.; Testolin, R. Isolation of (AC)n-microsatellites in Vitis vinifera L. and analysis of genetic background in grapevines under marker assisted selection. Mol. Breed. 2005, 15, 11–20. [Google Scholar] [CrossRef]
- Cipriani, G.; Marrazzo, M.T.; Di Gaspero, G.; Pfeiffer, A.; Morgante, M.; Testolin, R. A set of microsatellite markers with long core repeat optimized for grape (Vitis spp.) genotyping. BMC Plant Biol. 2008, 8, 127. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Merdinoglu, D.; Butterlin, G.; Bevilacqua, L.; Chiquet, V.; Adam-Blondon, A.-F.; Decroocq, S. Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol. Breed. 2005, 15, 349–366. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and Characterization of Additional Microsatellite DNA Markers for Grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar] [CrossRef]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analyzed as sequence-tagged sites. Theroretical Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef]
- Temnykh, S.; Lukashova, A.; Cartinhour, S.; DeClerck, G.; Lipovich, L.; McCouch, S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001, 11, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fresnedo-Ramírez, J.; Wang, M.; Cote, L.; Schweitzer, P.; Barba, P.; Takacs, E.M.; Clark, M.; Luby, J.; Manns, D.C.; et al. A next-generation marker genotyping platform (AmpSeq) in heterozygous crops: A case study for marker-assisted selection in grapevine. Hortic. Res. 2016, 3, 16002. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Karn, A.; Reisch, B.; Nguyen, A.; Sun, Y.; Bao, Y.; Campbell, M.S.; Church, D.; Williams, S.; Xu, X.; et al. Haplotyping the Vitis collinear core genome with rhAmpSeq improves marker transferability in a diverse genus. Nat. Commun. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, J.W. Accuracy of mapping quantitative trait loci in autogamous species. Theor. Appl. Genet. 1992, 84, 803–811. [Google Scholar] [CrossRef]
- Fu, P.; Wu, W.; Lai, G.; Li, R.; Peng, Y.; Yang, B.; Wang, B.; Yin, L.; Qu, J.; Song, S.; et al. Identifying Plasmopara viticola resistance Loci in grapevine (Vitis amurensis) via genotyping-by-sequencing-based QTL mapping. Plant Physiol. Biochem. 2020, 154, 75–84. [Google Scholar] [CrossRef]
- Lin, H.; Leng, H.; Guo, Y.; Kondo, S.; Zhao, Y.; Shi, G.; Guo, X. QTL and candidate genes for downy mildew resistance conferred by interspecific grape (V. vinifera L. × V. amurensis Rupr.) crossing. Sci. Hortic. 2019, 244, 200–207. [Google Scholar] [CrossRef]
- Zyprian, E.; Ochßner, I.; Schwander, F.; Šimon, S.; Hausmann, L.; Bonow-Rex, M.; Moreno-Sanz, P.; Grando, M.S.; Wiedemann-Merdinoglu, S.; Merdinoglu, D.; et al. Quantitative trait loci affecting pathogen resistance and ripening of grapevines. Mol. Genet. Genom. 2016, 291, 1573–1594. [Google Scholar] [CrossRef]
- Vezzulli, S.; Malacarne, G.; Masuero, D.; Vecchione, A.; Dolzani, C.; Goremykin, V.; Mehari, Z.H.; Banchi, E.; Velasci, R.; Stefanini, M.; et al. The Rpv3-3 hyplotype and stilbenoid induction mediate downy mildew resistance in a grapevine interspecific population. Front. Plant Sci. 2019, 10, 234. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Wiedeman-Merdinoglu, S.; Coste, P.; Dumas, V.; Haetty, S.; Butterlin, G.; Greif, C. Genetic analysis of downy mildew resistance derived from Muscadinia rotundifolia. Acta Hortic. 2003, 603, 451–456. [Google Scholar] [CrossRef]
- Lei, B.Z. The Investigation and Study of the Main Fungus Diseases on Grape in Xinjiang. Master’s Thesis., Shihezi University, Shihezi, China, 2004. [Google Scholar]
- Yin, L.; Zhang, Y.; Hao, Y.; Lu, J. Genetic diversity and population structure of Plasmopara viticola in China. Eur. J. Plant Pathol. 2014, 140, 365–376. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Burdett, H.; Bentham, A.R.; Williams, S.J.; Dodds, P.N.; Anderson, P.A.; Banfield, M.J.; Kobe, B. The plant “resistosome”: Structural insights into immune signaling. Cell Host Microbe 2019, 26, 193–201. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, 44. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.-L. Glycolisation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Delmotte, F.; Mestre, P.; Schneider, C.; Kassemeyer, H.-H.; Kozma, P.; Richart-Cervera, S.; Rouxel, M.; Delière, L. Rapid and multiregional adaptation to host partial resistance in a plant pathogenic oomycete: Evidence from European populations of Plasmopara viticola, the causal agent of grapevine downy mildew. Infect. Genet. Evol. 2014, 27, 500–508. [Google Scholar] [CrossRef]
- Heyman, L.; Höfle, R.; Kicherer, A.; Trapp, O.; Barka, E.A.; Töpfer, R.; Höfte, M. The Durability of Quantitative Host Resistance and Variability in Pathogen Virulence in the Interaction Between European Grapevine Cultivars and Plasmopara viticola. Front. Agron. 2021, 3, 684023. [Google Scholar] [CrossRef]
- Paineau, M.; Mazet, I.D.; Wiedemann-Merdinoglu, S.; Fabre, F.; Delmotte, F. The Characterization of Pathotypes in Grapevine Downy Mildew Provides Insights into the Breakdown of Rpv3, Rpv10, and Rpv12 Factors in Grapevines. Phytopathology 2022, 112, 2329–2340. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Entling, M.H.; Hoffmann, C. Differentiating the effects of organic management, pesticide reduction, and landscape diversification for arthropod conservation in viticulture. Biodivers. Conserv. 2023, 32, 2637–2653. [Google Scholar] [CrossRef]

| LG | No. of Markers | Average Distance [cM] | Total Length [cM] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I | TV | We90 | I | TV | We90 | I | TV | We90 | |
| 1 | 33 | 19 | 28 | 3.1 | 4.4 | 4.2 | 106 | 83.3 | 118.1 |
| 2 | 18 | 13 | 17 | 4.8 | 4.3 | 5 | 91.1 | 55.8 | 84.8 |
| 3 | 22 | 14 | 17 | 2.5 | 3.2 | 3.4 | 55.4 | 44.7 | 57 |
| 4 | 40 | 33 | 23 | 3 | 3.8 | 5 | 124.4 | 123.9 | 113.8 |
| 5 | 40 | 21 | 40 | 2.7 | 4.5 | 2.7 | 117.6 | 93.5 | 107.5 |
| 6 | 34 | 19 | 29 | 3.4 | 5 | 3.8 | 115.3 | 94.4 | 111.1 |
| 7 | 42 | 25 | 35 | 2.9 | 4.8 | 3.4 | 127.4 | 121.1 | 119.9 |
| 8 | 32 | 25 | 28 | 3.2 | 3.4 | 4.2 | 102.4 | 86 | 116.7 |
| 9 | 36 | 26 | 31 | 3.1 | 4.7 | 4.3 | 115.9 | 122.4 | 132.4 |
| 10 | 31 | 27 | 24 | 4.2 | 5.4 | 5.5 | 135.6 | 144.5 | 132.6 |
| 11 | 28 | 16 | 23 | 3.7 | 7.2 | 4.4 | 108.1 | 114.5 | 101.2 |
| 12 | 43 | 36 | 32 | 2.4 | 4.1 | 2.8 | 110 | 148.9 | 88.2 |
| 13 | 25 | 6 | 29 | 4.2 | 8.6 | 5.1 | 118.6 | 51.3 | 147.7 |
| 14 | 41 | 33 | 38 | 3.2 | 4.5 | 4.9 | 144.1 | 147.6 | 185.2 |
| 15 | 28 | 13 | 31 | 2 | 2.9 | 3.3 | 66.2 | 37.7 | 101.4 |
| 16 | 25 | 19 | 24 | 3.9 | 4.7 | 4.5 | 105.6 | 88.9 | 106.8 |
| 17 | 23 | 16 | 22 | 4.5 | 5 | 6.3 | 107.1 | 80.3 | 139 |
| 18 | 47 | 32 | 42 | 2.6 | 4 | 2.8 | 132.1 | 129.2 | 119 |
| 19 | 39 | 28 | 26 | 3 | 3.6 | 4.6 | 124.8 | 101.8 | 120.7 |
| Σ/. | 627 | 421 | 539 | 3.3 | 4.6 | 4.2 | 2107.7 | 1869.8 | 2203.1 |
| σ | 0.76 | 1.34 | 0.98 | ||||||
| Map | Test Inoculation | LG | Cofactor | Position [cM] | LOD Score | α = 0.05 | VE [%] |
|---|---|---|---|---|---|---|---|
| I | 2019.1 | 9 | GF09-71 | 40.64 | 10.86 | 3 | 48.7 |
| 2019.2 | 9 | GF09-71 | 40.64 | 9.9 | 2.9 | 35 | |
| 2020 | 9 | GF09-71 | 40.64 | 30.24 | 2.9 | 64 | |
| TV | 2019.1 | - | - | - | - | - | - |
| 2019.2 | - | - | - | - | - | - | |
| 2020 | - | - | - | - | - | - | |
| We 90 | 2019.1 | 9 | - | 51.8 | 10.95 | 1.7 | 49 |
| 2019.2 | 9 | - | 55.09 | 8.05 | 1.6 | 29.5 | |
| 2020 | 9 | GF09-71 | 50.08 | 30.3 | 1.7 | 64.2 |
| SSR Marker | VMC1C10 | GF09-11 | GF09-43 | GF09-62 | GF09-64 | GF09-65 | GF09-68 | GF09-46 | GF09-70 | GF09-71 | GF09-47 | VMC6D12 | GF09-48 | GF09-16 | DM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical position Chr.09 | 0.56 Mb | 2.95 Mb | 3.15 Mb | 3.5 Mb | 3.6 Mb | 3.62 Mb | 3.67 Mb | 3.67 Mb | 3.68 Mb | 3.69 Mb | 3.7 Mb | 3.8 Mb | 3.85 Mb | 5.92 Mb | |
| VA (Rpv10.2) allele size | 150 | 287 | 427 | 383 | 281 | 312 | 153 | 413 | 334 | 366 | 299 | 146 | 357 | 243 | |
| We90 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| TV | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2017-204-12 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 |
| 2017-204-19 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 |
| 2017-204-28 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | 3 |
| 2017-204-67 | + | + | + | + | + | + | + | + | + | + | + | − | − | − | 3 |
| 2017-204-72 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 |
| 2017-204-78 | − | − | − | + | + | + | + | + | + | + | + | + | + | + | 3 |
| 2017-204-94 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | 2 |
| 2017-204-168 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 |
| 2017-204-195 | + | + | − | + | + | + | + | + | + | + | + | + | + | − | 3 |
| 2017-204-240 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 |
| 2017-204-413 | + | − | n/a | + | + | + | + | + | + | n/a | + | + | + | − | 1 |
| 2017-204-423 | − | + | + | + | + | + | + | + | + | + | + | n/a | − | + | 1 |
| 2017-204-630 | − | + | + | + | + | + | + | + | + | + | + | + | − | + | 2 |
| 2017-204-24 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | 6 |
| 2017-204-178 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | 7 |
| 2017-204-209 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | 7 |
| 2017-204-243 | + | + | + | − | − | − | − | − | − | − | − | − | − | − | 6 |
| SSR Marker | GF09-62 | GF09-64 | GF09-65 | GF09-68 | GF09-46 | GF09-70 | GF09-71 | GF09-47 | GF09-48 | Rpv Allele |
|---|---|---|---|---|---|---|---|---|---|---|
| Physical Position Chr.09 | 3.5 Mb | 3.6 Mb | 3.62 Mb | 3.67 Mb | 3.67 Mb | 3.68 Mb | 3.69 Mb | 3.7 Mb | 3.85 Mb | |
| V. amurensis Rpv10.2 allele size | 383 | 281 | 312 | 153 | 413 | 334 | 366 | 299 | 357 | Rpv10.2 |
| We 73-45-84 | + | + | + | + | + | + | + | + | + | |
| We 90-06-12 | + | + | + | + | + | + | + | + | + | |
| We 75-103-07 | + | + | + | + | + | + | n/a | + | + | |
| We 73-40-27 | + | + | + | + | + | + | + | + | n/a | |
| We 75-115-07 | + | + | + | + | + | + | + | + | + | |
| We 75-90-03 | + | + | + | + | + | + | + | + | + | |
| We 75-14-23 | + | + | + | + | + | + | + | + | n/a | |
| We 75-36-26 | + | + | + | + | + | + | + | + | + | |
| We 75-34-13 | + | + | + | + | + | + | + | + | + | |
| We 75-108-10 | + | + | + | + | + | + | + | + | + | |
| ‘Sauvitage’ | + | + | + | + | + | + | + | + | + | |
| ‘Solaris’ | + | + | + | 155 | 415 | + | + | + | 359 | Rpv10 |
| ‘Baron’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Monarch’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Muscaris’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Rondo’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Cabernet Cantor’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Cabernet Carbon’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Cabernet Carol’ | + | + | + | 155 | 415 | + | + | + | n/a | |
| ‘Cabernet Cortis’ | + | + | + | 155 | 415 | + | + | + | 359 | |
| ‘Cabernet franc’ | − | − | − | − | − | − | − | − | − | Control |
| ‘Muscat a Petits Grains’ | − | − | − | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höschele, T.; Malagol, N.; Bori, S.O.; Müllner, S.; Töpfer, R.; Sturm, J.; Zyprian, E.; Trapp, O. Rpv10.2: A Haplotype Variant of Locus Rpv10 Enables New Combinations for Pyramiding Downy Mildew Resistance Traits in Grapevine. Plants 2024, 13, 2624. https://doi.org/10.3390/plants13182624
Höschele T, Malagol N, Bori SO, Müllner S, Töpfer R, Sturm J, Zyprian E, Trapp O. Rpv10.2: A Haplotype Variant of Locus Rpv10 Enables New Combinations for Pyramiding Downy Mildew Resistance Traits in Grapevine. Plants. 2024; 13(18):2624. https://doi.org/10.3390/plants13182624
Chicago/Turabian StyleHöschele, Tim, Nagarjun Malagol, Salvador Olivella Bori, Sophia Müllner, Reinhard Töpfer, Jürgen Sturm, Eva Zyprian, and Oliver Trapp. 2024. "Rpv10.2: A Haplotype Variant of Locus Rpv10 Enables New Combinations for Pyramiding Downy Mildew Resistance Traits in Grapevine" Plants 13, no. 18: 2624. https://doi.org/10.3390/plants13182624







