Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation
Abstract
1. Introduction
2. Results
2.1. Morphological Observations
2.2. Lipid Peroxidation Level
2.3. Antioxidant Plant Enzymes
2.4. Non-Enzymatic Plant Antioxidants
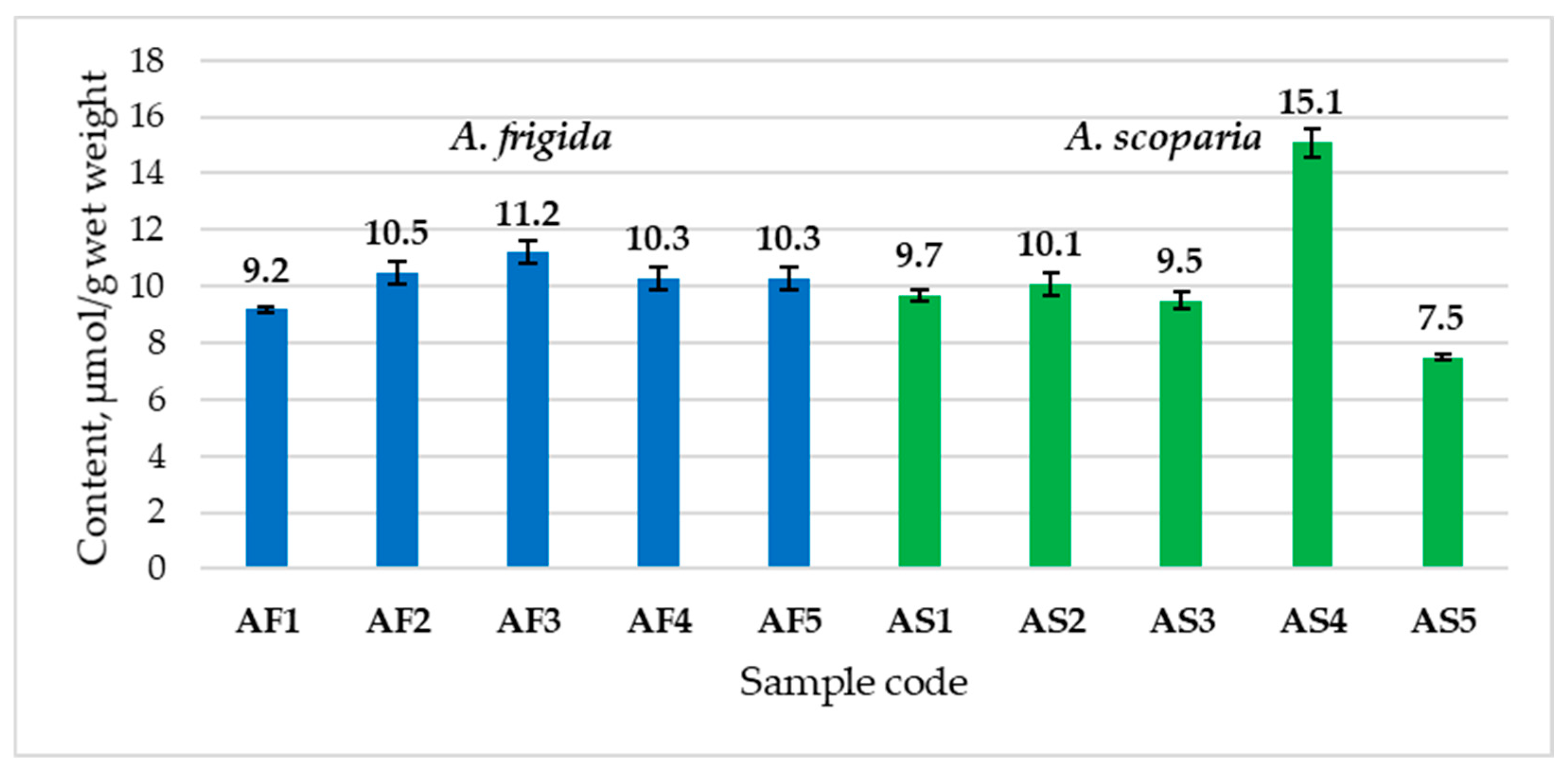
2.4.1. Ascorbic Acid and Glutathione Content
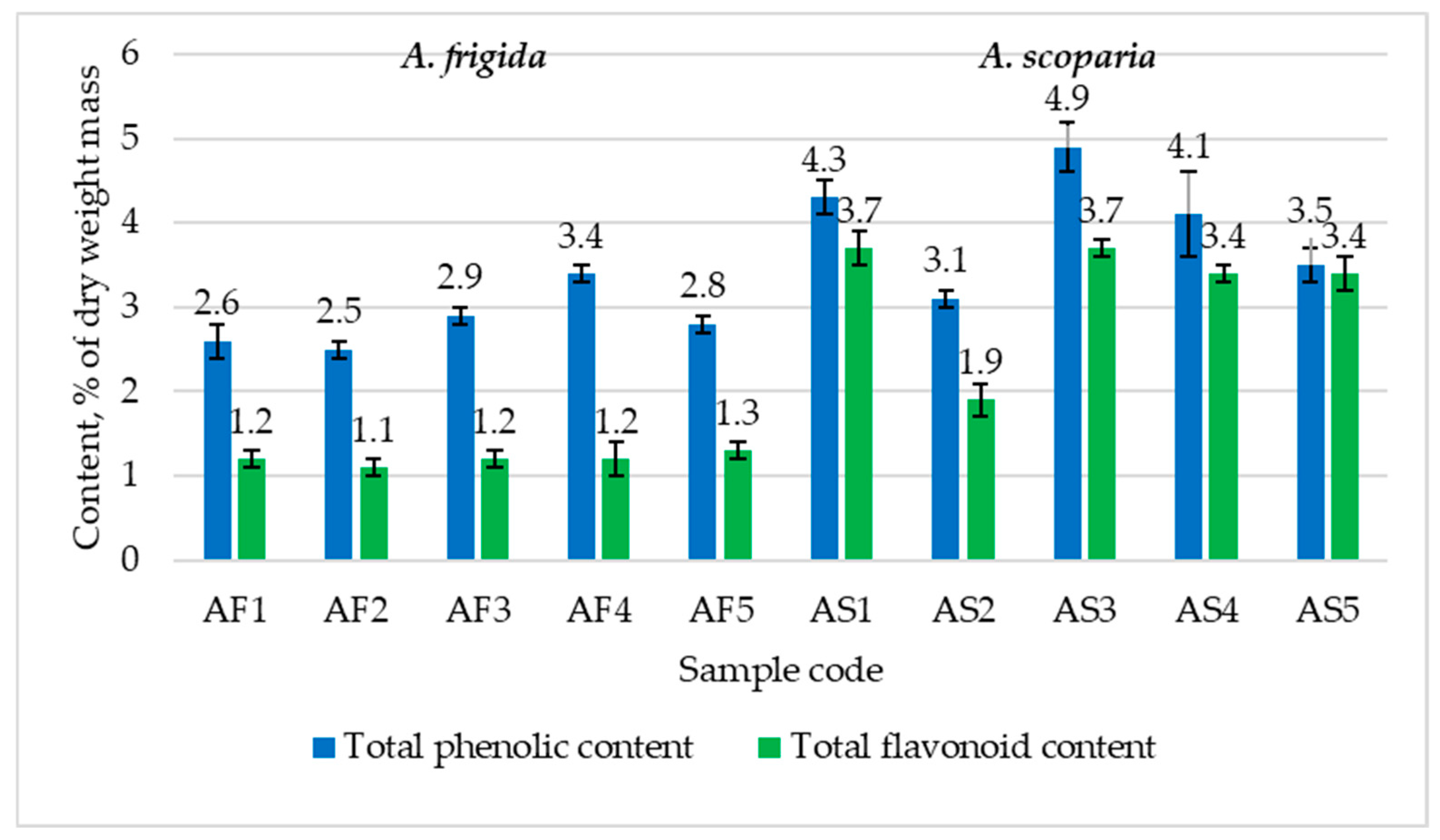
2.4.2. Total Phenolic Content and Total Flavonoid Content
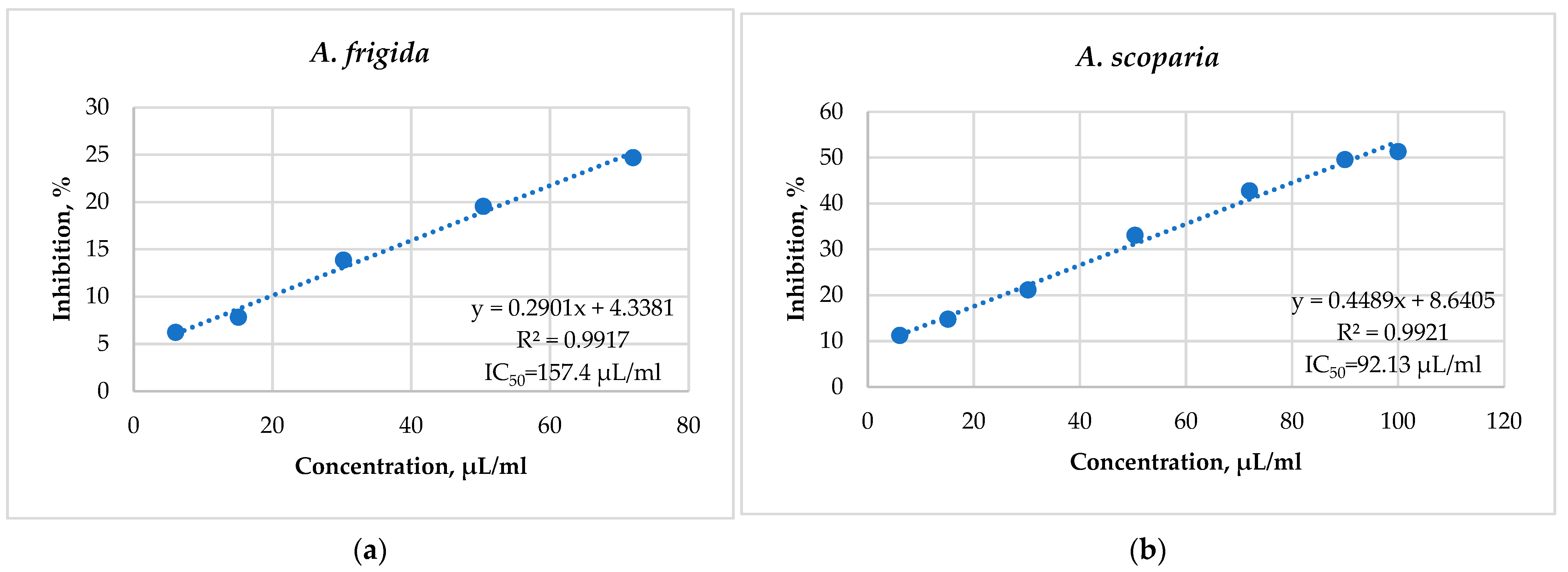
2.5. Antiradical Activity of Alcohol Extracts
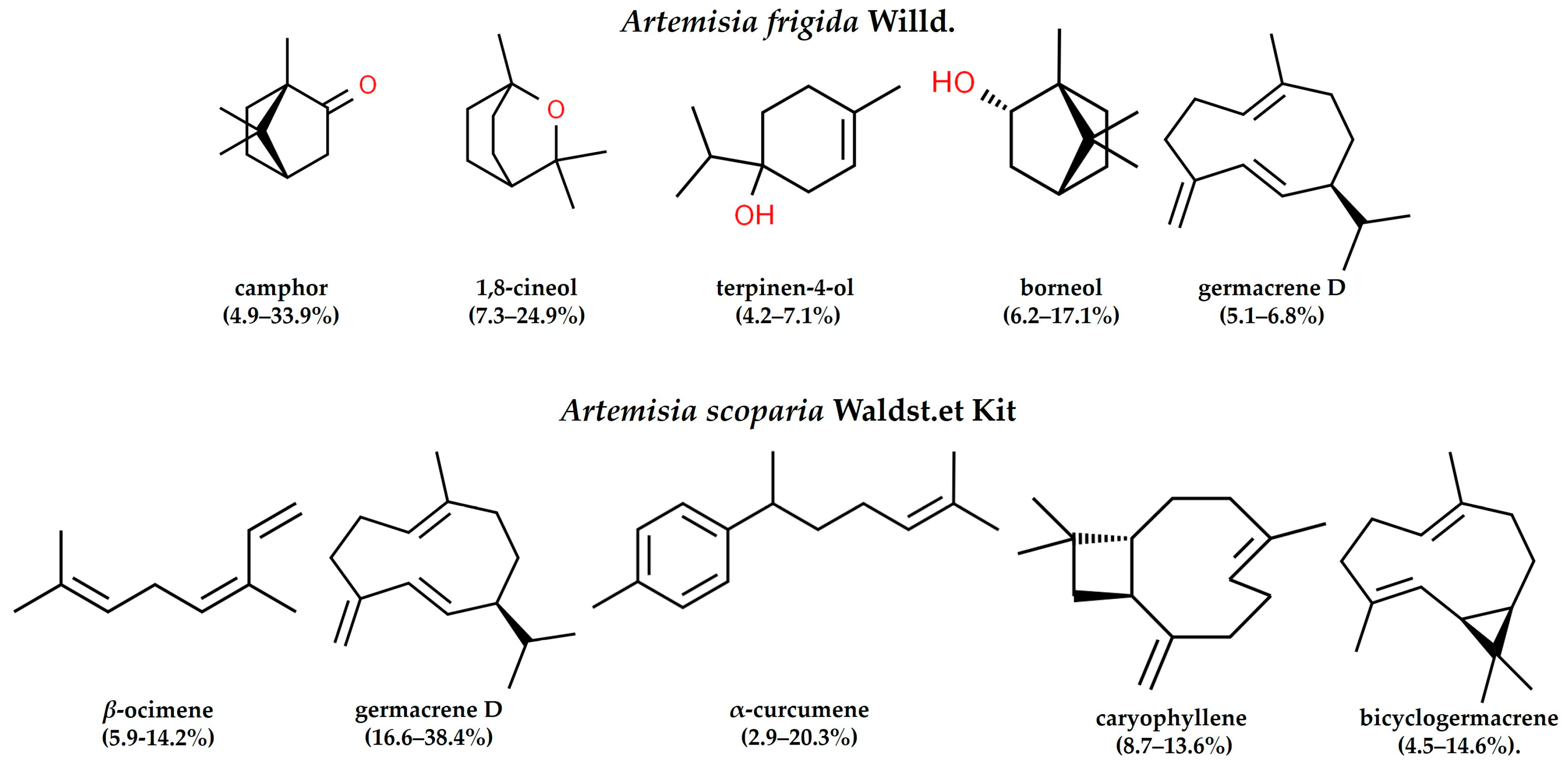
2.6. Composition of Essential Oils by GC-MS and PCA-Analysis
3. Discussions
4. Materials and Methods
4.1. Description of Studied Area
4.2. Plant Materials
4.3. Measurement of Lipid Peroxidation
4.4. Enzymic Antioxidants
Measurement of Superoxide Dismutase (SOD, EC 1.15.1.1.) and Catalase (CAT. EC 1.11.1.6) Activities
4.5. Non-Enzymic Antioxidants
4.5.1. Determination of Ascorbic Acid and Glutathione
4.5.2. Determination of Total Phenolic Content, Total Flavonoid Content
4.5.3. Composition of Essential Oils
4.6. Antiradical Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADS | antioxidant defense system |
| AOS | active oxygen species |
| CAT | catalase |
| EO | essential oils |
| HTC | Selyaninov hydrothermal coefficient |
| Kext | temperature–moisture extremity coefficient |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| MEAN | arithmetic mean |
| PDSI | Palmer Drought Severity Index |
| RIHMI-WDC | All-Russian Research Institute of Hydrometeorological Information—World Data Center |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SOD | superoxide dismutase |
| SPEI | Standardized Precipitation–Evapotranspiration Index |
| TBA-RS | thiobarbituric acid reactive substances |
| TFC | total flavonoid content |
| TPC | total phenolic content |
References
- Zagoskina, N.V.; Nazarenko, L.V. Active Oxygen Species and Antioxidant System of Plants. MCU J. Nat. Sci. 2016, 2, 9–23. (In Russian) [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Karpets, Y.V.; Kabashnikova, L.F. Antioxidative system of plants: Cellular compartmentalization, protective and signaling functions, mechanisms of regulation (review). Appl. Biochem. Microbiol. 2019, 55, 441–459. [Google Scholar] [CrossRef]
- Berwal, M.K.; Kumar, R.; Prakash, K.; Rai, G.K.; Hebbar, K.B. Antioxidant defense system in plants against abiotic stress. In Abiotic Stress Tolerance Mechanisms in Plants, 1st ed.; Rai, G.K., Kumar, R.R., Bagati, S., Eds.; CRC Press: London, UK, 2021; p. 28. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Satish, L.; Pandian, S.K.; Chen, J.-T.; Ahmar, S.; Wang, X.; Mora-Poblete, F.; Ramesh, M. An Overview of Abiotic Stress in Cereal Crops: Negative Impacts, Regulation, Biotechnology and Integrated Omics. Plants 2021, 10, 1472. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef]
- Habuš Jerčić, I.; Bošnjak Mihovilović, A.; Matković Stanković, A.; Lazarević, B.; Goreta Ban, S.; Ban, D.; Major, N.; Tomaz, I.; Banjavčić, Z.; Kereša, S. Garlic Ecotypes Utilise Different Morphological, Physiological and Biochemical Mechanisms to Cope with Drought Stress. Plants 2023, 12, 1824. [Google Scholar] [CrossRef]
- Zlatić, N.; Jakovljević, D.; Stanković, M. Temporal, Plant Part, and Interpopulation Variability of Secondary Metabolites and Antioxidant Activity of Inula helenium L. Plants 2019, 8, 179. [Google Scholar] [CrossRef]
- Ryu, H.W.; Song, H.-H.; Kim, K.O.; Park, Y.J.; Lim, D.-Y.; Kim, J.-H.; Oh, S.R. Secondary metabolite profiling and modulation of antioxidants in wild cultivated Euphorbia supina. Ind. Crops Prod. 2016, 89, 215–224. [Google Scholar] [CrossRef]
- Beresneva, I.A. Klimaty Aridnoj Zony Azii (Climates of the Arid Zone of Asia); Gunin, P.D., Ed.; Nauka: Moscow, Russia, 2006; p. 285. (In Russian) [Google Scholar]
- Namzalov, B.B.; Zhigzhitzhapova, S.V.; Dubrovsky, N.G.; Sakhyaeva, A.B.; Radnaeva, L.D. Wormwoods of Buryatia: Diversity analysis, ecological-geographical features, and chemotaxonomy of section Abrotanum. Acta Biol. Sib. 2019, 5, 178–187. [Google Scholar] [CrossRef][Green Version]
- Bisht, D.; Kumar, D.; Kumar, D.H.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Borisova, G.G.; Maleva, M.G.; Nekrasova, G.F.; Chukina, N.V. Metody Ocenki Antioksidantnogo Statusa Rastenij (Methods for Assessing the Antioxidant Status of Plants); Federal State Autonomous Educational Institution of Higher Education—Ural Federal University named after the first President of Russia Yeltsin, B.N.: Ekaterinburg, Russia, 2012; p. 72. (In Russian) [Google Scholar]
- Nikonova, A.A.; Vorobyeva, S.S. Nonspecific response of Lake Baikal phytoplankton to anthropogenic impact. Vavilov J. Genet. Breed. 2022, 26, 467–476. [Google Scholar] [CrossRef]
- Nikerova, K.M. Aktivnost’ fermentov antioksidantnoj sistemy pri izmenenii scenariev ksilogeneza u Betula pendula Roth i Pinus sylvestris L. (Activity of enzymes of the antioxidant system when changing xylogenesis scenarios in Betula pendula Roth and Pinus sylvestris L.). In Candidate of Biological Sciences; Komarov Botanocal Institute: Saint-Petersburg, Russia, 2020. (In Russian) [Google Scholar]
- Zhigzhitzhapova, S.V.; Dylenova, E.P.; Zhigzhitzhapov, B.V.; Goncharova, D.B.; Tykheev, Z.A.; Taraskin, V.V.; Anenkhonov, O.A. Essential Oils of Artemisia frigida Plants (Asteraceae): Conservatism and Lability of the Composition. Plants 2023, 12, 3422. [Google Scholar] [CrossRef]
- Miroshnichenko, Y.M. O rasprostranenii Artemisia frigida Willd. v MNR (On the distribution of Artemisia frigida Willd. in the Mongolian People’s Republic). Bot. Zhurnal 1965, 50, 420–424. (In Russian) [Google Scholar]
- Korolyuk, E.A.; Tkachev, A.V. Chemical composition of the essential oil from two wormwood species Artemisia frigida and Artemisia argyrophylla. Russ. J. Bioorg. Chem. 2010, 36, 884–893. [Google Scholar] [CrossRef]
- Anenkhonov, O.A.; Sandanov, D.V.; Naidanov, B.B.; Chimitov, D.G.; Liu, H.; Xu, C.; Guo, W.; Korolyuk, A.Y.; Zvereva, A.A. Using data on the thermal conditions of soils for the differentiation of vegetation in the exposure-related forest-steppe of Transbaikalia. Contemp. Probl. Ecol. 2020, 13, 522–532. [Google Scholar] [CrossRef]
- Liu, H.; He, S.; Anenkhonov, O.A.; Hu, G.; Sandanov, D.V.; Badmaeva, N.K. Topography-Controlled Soil Water Content and the Coexistence of Forest and Steppe in Northern China. Phys. Geogr. 2012, 33, 561–573. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Randalova, T.E.; Radnaeva, L.D. Composition of essential oil of Artemisia scoparia Waldst. et Kit. from Buryatia and Mongolia. Russ. J. Bioorg. Chem. 2016, 42, 730–734. [Google Scholar] [CrossRef]
- Yunatov, A.A. Osnovnye Cherty Rastitel’Nogo Pokrova Mongol’Skoj Narodnoj Respubliki (Main Features of the Vegetation Cover of the Mongolian People’s Republic); Izd-vo AN SSSR (Publishing House of the USSR Academy of Sciences): Moscow, Russia, 1950; p. 224. (In Russian) [Google Scholar]
- Gorshkova, A.A. Biologija Stepnyh Pastbishhnyh Rastenij Zabajkal’Ja (Biology of Steppe Pasture Plants of Transbaikalia); Izdatel’stvo “Nauka” (Publishing house “Science”): Moscow, Russia, 1966; p. 274. (In Russian) [Google Scholar]
- Namzalov, B.B. Stepi Yuzhnoj Sibiri (Steppes of Southern Siberia); BSC SB RAS: Novosibirsk, Russia; Ulan-Ude, Russia, 1994; p. 309. (In Russian) [Google Scholar]
- Garifzyanov, A.R.; Zhukov, N.N.; Ivanishchev, V.V. Formation and physiological reactions of oxygen active forms in plants cells. Sovrem. Probl. Nauk. I Obraz. (Mod. Probl. Sci. Educ.) 2011, 2, 2. (In Russian) [Google Scholar]
- Samusik, E.A.; Marchik, T.P.; Golovatyi, S.E. The intensity of oxidative processes and the activity of the antioxidant system in the leaves of woody plants growing in conditions of technogenic pollution. Environ. Hum. Ecol. Stud. 2022, 12, 418–438. (In Russian) [Google Scholar] [CrossRef]
- Nesterov, V.N.; Bogdanova, E.S.; Tabalenkova, G.N.; Rozentsvet, O.A. Lipid peroxidation of wild-growing halophytes in the conditions of the Prieltonye. Izv. Samar. Nauchnogo Cent. Ross. Akad. Nauk. (News Samara Sci. Cent. Russ. Acad. Sci.) 2014, 16, 299–303. (In Russian) [Google Scholar]
- Rakhmatullina, N.S.; Akinshina, N.G.; Azizov, A.A.; Radzhabova, G.G. Indicators of antioxidant system of Artemisia annau L., Matricaria chamomillia L., and Tanacetum vulgare L. under natural growing conditions and in the urban environment. Nauchnoe Obozr. Biol. Nauk. (Sci. Rev. Biol. Sci.) 2020, 4, 28–32. (In Russian) [Google Scholar]
- Michailova, I.D.; Lukatkin, A.S. Lipid Peroxidation in Cucumber and Radish Seedlings Affected by Heavy Metals. Izv. Saratov Univ. New Ser. Chemistry. Biology. Ecol. 2016, 16, 206–210. (In Russian) [Google Scholar] [CrossRef]
- Batova, Y.V.; Kaznina, N.M.; Titov, A.F. Effect of low temperature in the intensity of oxidative processes and activity of antioxidant enzymes in wheat plants at optimal and excessive zinc concentrations in the root medium. Biol. Bull. 2021, 48, 156–164. [Google Scholar] [CrossRef]
- Baranenko, V.V. Superoxide dismutase in plant cells. Tsitologiya 2006, 48, 465–475. (In Russian) [Google Scholar]
- Kirgizova, I.V.; Gajimuradova, A.M.; Omarov, R.T. Accumulation of antioxidant enzymes in potato plants under the conditions of biotic and abiotic stress. Proc. Univ. Appl. Chem. Biotechnol. 2018, 8, 42–54. (In Russian) [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.X.; Chen, J.; Yuan, R.; Deng, F.N.; Shen, F.F. Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochem. Mosc. 2016, 81, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef]
- Nene, T.; Yadav, M.; Yadav, H.S. Plant catalase in silico characterization and phylogenetic analysis with structural modeling. J. Genet. Eng. Biotechnol. 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Lin, C.-C.; Lett, C.; Karpinska, B.; Wright, M.H. Catalase: A critical node in the regulation of cell fate. Free Radic. Biol. Med. 2023, 199, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Breusegem, F.V.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 16, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Gryshko, V.H.; Syshchikov, D.V.; Ivanova, A. The products of peroxide lipids oxidations and bioantioxidants—Glutathione and ascorbic acid of galophytes Salsola soda L., Gaucium flavum Crambs. and Euphorbia peplus L. Plant Introd. 2012, 56, 72–77. (In Russian) [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Denisenko, T.A.; Vishnikin, A.B.; Tsiganok, L.P. Spectrophotometric determinatiom of sum of phenolic compounds in plants using aluminum chloride, 18-molybdodiphosphate and Folin-Cocalteu reagents. Anal. I Kontrol’ (Anal. Control.) 2015, 19, 373–380. (In Russian) [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic Acids and Flavonoids of Fringed Sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS Profile, HPLC-DAD Quantification, in Vitro Digestion Stability, and Antioxidant Capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Korokulkin, D.Y.; Abilov, Z.A.; Muzychkina, R.A.; Tolstikov, G.A. Prirodnye Flavonoidy (Natural Flavonoids); Akademicheskoe izdatel’stvo “Geo” (Academic publishing house “Geo”): Novosibirsk, Russia, 2007; p. 232. ISBN 978-5-9747-0119-1. (In Russian) [Google Scholar]
- Tian, M.; Row, K.H. Separation of four bioactive compounds from Herba artemisiae scopariae by HPLC with ionic liquid-based silica colimn. J. Anal. Chem. 2011, 66, 580–585. [Google Scholar] [CrossRef]
- Boakye, Y.D.; Shaheen, S.; Nawaz, H.; Nisar, S.; Azeem, M.W. Artemisia scoparia: A review on traditional uses, phytochemistry and pharmacological properties. Int. J. Chem. Biochem. Sci. 2017, 12, 92–97. [Google Scholar]
- Airapetyan, E.E.; Leonova, V.N.; Konovalov, D.A. Development of method for quantitative determination of the amount of flavonoids in Artemisia scoparia herb. Hum. Their Health 2022, 25, 105–112. (In Russian) [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Topuzovic, M.; Solujic, S.; Mihailovic, V. Antioxidant activity and concentration of phenols and flavonoids in the whole plant and plant parts of Teucrium chamaerdys L. var. glanduliferum Haussk. J. Med. Plants Res. 2010, 4, 2092–2098. [Google Scholar]
- Terao, J. Dietary Flavonoids as Antioxidants. In Forum of Nutrition. Food Factors for Health Promotion; Yoshikawa, S., Ed.; S Karger AG: Basel, Switzerland, 2009; Volume 61, pp. 87–94. [Google Scholar] [CrossRef]
- Markovskaya, E.F.; Galibina, N.A.; Ilyinova, M.K.; Nikerova, K.M.; Shmakova, N.Y. Lipid composition and functional state of membrane systems in Stellaria humifusa. Tr. Karel’’skogo Nauchnogo Cent. Ross. Akad. Nauk. (Proc. Karelian Sci. Cent. Russ. Acad. Aciences) 2017, 5, 99–110. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Shaldaeva, T.M.; Vysochina, G.I. Flavonoid content in representatives of the genus Artemisia L. from natural populations of Siberia. Khimija Rastit. Syr’ja 2012, 2, 79–84. (In Russian) [Google Scholar]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Zhao-Jiang, Z.; Ru-Min, Z.; Pei-Jun, G.; Guo-Sheng, W.; Ping, H.; Yan, G. Allelopathic effects of Artemisia frigida Willd. on growth of pasture grasses in Inner Mongolia, China. Biochem. Syst. Ecol. 2011, 39, 377–383. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Zuo, Z.; Li, R.; Wu, J.; Gao, Y. Inhibition effects of volatile organic compounds from Artemisia frigida illd. on pasture grass intake by lambs. Small Rumin. Res. 2014, 121, 248–254. [Google Scholar] [CrossRef]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Veit, D.; Gershenzon, J.; Schuman, M.C. The Sesquiterpenes (E)-ß-Farnesene and (E)-α-Bergamotene Quench Ozone but Fail to Protect the Wild Tobacco Nicotiana attenuata from Ozone, UVB, and Drought Stresses. PLoS ONE 2015, 10, e0127296. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Florensov, N.A. Mezozojskie I Kajnozojskie Vpadiny Pribajkal’Ja (Mesozoic and Cenozoic Basins of the Baikal Region); Izdatel’stvo Akademii nauk SSSR (Publishing House of the USSR Academy of Sciences): Leningrad, Russia, 1960; p. 258. (In Russian) [Google Scholar]
- Obyazov, V.A. Izmenenija Sovremennogo Klimata I Ocenka Ih Posledstvij Dlja Prirodnyh I Prirodno-Klimaticheskih Sistem Zabajkal’Ja (Changes in Modern Climate and Assessment of Their Consequences for Natural and Climatic Systems of Transbaikalia). Ph.D. Thesis, Kazan Federal University, Kazan, Russia, 29 May 2014. (In Russian). [Google Scholar]
- Belodubrovskaya, G.A.; Goncharov, M.Y.; Zhokhova, E.V.; Krupkina, L.I.; Mistrova, A.A.; Nadel’, N.N.; Povydysh, M.N.; Pryakhina, N.I.; Sklyarevskaya, N.V.; Sokolova, I.V.; et al. Bol’shoj Jenciklopedicheskij Slovar’ Lekarstvennyh Rastenij (Big Encyclopedic Dictionary of Medicinal Plants), 3rd ed.; Yakovlev, G.P., Ed.; SpecLit: Saint-Petersburg, Russia, 2015; pp. 16–27. ISBN 978-5-299-00528-8. (In Russian) [Google Scholar]
- Anenkohonov, O.A.; Pykhalova, T.D.; Osipov, K.I.; Sekulich, I.R.; Badmaeva, N.K.; Namzalov, B.B.; Krivobokov, L.V.; Munkueva, M.S.; Sutkin, A.V.; Tubshinova, D.B.; et al. Opredelitel’ Rastenij Burjatii (Key to Plants of Buryatia); Anenkhonov, O.A., Ed.; Respublikanskaja tipografija: Ulan-Ude, Russia, 2001; p. 671. (In Russian) [Google Scholar]
- Singleton, V.L.; Orthifer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Tykheev, Z.A.; Zhigzhitzhapova, S.V.; Zhang, F.; Taraskin, V.V.; Anenkhonov, O.A.; Radnaeva, L.D.; Chen, S. Constituents of Essential Oil and Lipid Fraction from the Aerial Part of Bupleurum scorzonerifolium Willd. (Apiaceae) from Different Habitats. Molecules 2018, 23, 1496. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Karstang, T.V. A General-Purpose Program for Multivariate Data Analysis; Elsevier: New York, NY, USA, 1987. [Google Scholar]
- Esbenson, K.H. Multivariate Data Analysis—In Practice. Selected Chapters; Publishing House IPKHF RAN: Chernogolovka, Russia, 2005. (In Russian) [Google Scholar]

| Sample Code | Morphological Parameters | ||
|---|---|---|---|
| Plant Height, mm * | A. frigida—Diameter of the Bush Base, cm; A. scoparia—Inflorescence Length, mm. | A. frigida—Number of Generative Shoots; A. scoparia—Inflorescence Width, mm. | |
| A. frigida | |||
| AF1 | 246.8 ± 27.1 | 128.5 ± 19.1 | 12.2 ± 3.0 |
| AF2 | 211.1 ± 35.4 | 117.4 ± 28.3 | 2.6 ± 1.4 |
| AF3 | 212.9 ± 36.3 | 123.5 ± 30.3 | 1.9 ± 1.3 |
| AF4 | 247.7 ± 30.2 | 162.3 ± 20.9 | 13.3 ± 2.7 |
| AF5 | 268.7 ± 18.3 | 160.4 ± 19.4 | 13.8 ± 2.8 |
| A. scoparia | |||
| AS1 | 390.8 ± 81.4 | 240.2 ± 30.8 | 135.9 ± 26.1 |
| AS2 | 385.7 ± 77.5 | 230.0 ± 35.1 | 145.3 ± 22.1 |
| AS3 | 388.8 ± 80.6 | 236.2 ± 22.8 | 136.9 ± 22.8 |
| AS4 | 386.5 ± 79.1 | 236.2 ± 29.0 | 122.1 ± 26.5 |
| AS5 | 391.7 ± 81.5 | 239.0 ± 28.0 | 135.1 ± 25.8 |
| Sample Code | A. frigida | A. scoparia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AF1 | AF2 | AF3 | AF4 | AF5 | AS1 | AS2 | AS3 | AS4 | AS5 | |
| SOD | 7.40 ± 0.51 * | 0.2 ± 0.01 | 0.7 ± 0.03 | 0.8 ± 0.02 | 1.4 ± 0.04 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.02 |
| CAT | 0.7 ± 0.03 | 1.0 ± 0.04 | 3.8 ± 0.03 | 3.2 ± 0.03 | 1.8 ± 0.04 | 6.5 ± 0.04 | 7.9 ± 0.05 | 4.8 ± 0.03 | 4.3 ± 0.03 | 6.2 ± 0.04 |
| Plant Species | Ascorbic Acid, μg/g of Fresh Mass | Glutathione, mMol/g of Fresh Mass |
|---|---|---|
| A. frigida | 64.3 ± 0.6 * | 64.5 ± 0.5 |
| A. scoparia | 42.9 ± 0.2 | 50.2 ± 0.9 |
| Meteorological Station (Index, Name) | Climatic Parameters | Year | |||
|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | ||
| 30823 Ulan-Ude | ∑t (°C) | 1794.9 | 1731 | 1655.3 | 1641.5 |
| ∑ precipitation (mm) | 195.3 | 164 | 219.7 | 123.8 | |
| HTC | 1.08 | 0.95 | 1.33 | 0.75 | |
| PSEI | 0 | −0.38 | 1.42 | −0.28 | |
| PDSI | 1.46 | 1.34 | 1.69 | −1.92 | |
| Tav. daily (°C) | 19.5 | 18.8 | 18.0 | 17.8 | |
| Kext (°C/mm) | 0.1 | 0.11 | 0.08 | 0.14 | |
| 30825 Ivolginsk | ∑t (°C) | 1742.6 | 1675 | 1603.6 | 1620.2 |
| ∑ precipitation (mm) | 134.7 | 144.9 | 234.1 | 100.8 | |
| HTC | 0.77 | 0.86 | 1.46 | 0.62 | |
| PSEI | −0.16 | 0.38 | 1.87 | −0.49 | |
| PDSI | −0.84 | −0.91 | 3.95 | −2.38 | |
| Tav. daily (°C) | 18.9 | 18.2 | 17.4 | 17.6 | |
| Kext (°C/mm) | 0.14 | 0.13 | 0.07 | 0.17 | |
| 30745 Sosnovo-Ozersk | ∑t (°C) | 1466 | 1405 | 1443.7 | 1405.3 |
| ∑ precipitation (mm) | 146.3 | 275.5 | 155.8 | 154.9 | |
| HTC | 0.10 | 1.96 | 1.08 | 1.10 | |
| PSEI | −0.34 | 0.23 | 0.92 | −0.32 | |
| PDSI | 0.57 | −0.29 | 1.71 | −2.00 | |
| Tav. daily (°C) | 15.9 | 15.3 | 15.7 | 15.3 | |
| Kext (°C/mm) | 0.11 | 0.06 | 0.10 | 0.10 | |
| Location | Sample | Sample Code | Collection Date | Latitude Longitude |
|---|---|---|---|---|
| Ivolginsky district, Cheremukhovaya narrow valley * | A. frigida | AF1 | 23 August 2023 | 51°93′ N 106°48′ E |
| A. scoparia | AS1 | |||
| Ivolginsky district, foothill of Ganzurinsky ridge | A. frigida | AF2 | 24 August 2023 | 51°42′ N 107°12′ E |
| A. scoparia | AS2 | |||
| Selenginsky district, surroundings of the Novoselenginsk village ** | A. frigida | AF3 | 24 August 2023 | 51°06′ N 106°39′ E |
| A. scoparia | AS3 | |||
| Khorinsky district, surroundings of the Oninoborsk village | A. frigida | AF4 | 30 August 2023 | 52°24′ N 110°00′ E |
| A. scoparia | AS4 | |||
| Eravninsky district, surroundings of Mozhayka village | A. frigida | AF5 | 30 August 2023 | 52°40′ N 110°78′ E |
| A. scoparia | AS5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhigzhitzhapova, S.V.; Dylenova, E.P.; Goncharova, D.B.; Zhigzhitzhapov, B.V.; Emelyanova, E.A.; Polonova, A.V.; Tykheev, Z.A.; Bazarsadueva, S.V.; Taraskina, A.S.; Pintaeva, E.T.; et al. Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation. Plants 2024, 13, 2630. https://doi.org/10.3390/plants13182630
Zhigzhitzhapova SV, Dylenova EP, Goncharova DB, Zhigzhitzhapov BV, Emelyanova EA, Polonova AV, Tykheev ZA, Bazarsadueva SV, Taraskina AS, Pintaeva ET, et al. Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation. Plants. 2024; 13(18):2630. https://doi.org/10.3390/plants13182630
Chicago/Turabian StyleZhigzhitzhapova, Svetlana V., Elena P. Dylenova, Danaya B. Goncharova, Bato V. Zhigzhitzhapov, Elena A. Emelyanova, Anastasiya V. Polonova, Zhargal A. Tykheev, Selmeg V. Bazarsadueva, Anna S. Taraskina, Evgeniya T. Pintaeva, and et al. 2024. "Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation" Plants 13, no. 18: 2630. https://doi.org/10.3390/plants13182630
APA StyleZhigzhitzhapova, S. V., Dylenova, E. P., Goncharova, D. B., Zhigzhitzhapov, B. V., Emelyanova, E. A., Polonova, A. V., Tykheev, Z. A., Bazarsadueva, S. V., Taraskina, A. S., Pintaeva, E. T., & Taraskin, V. V. (2024). Functional Activity of the Antioxidant System of Artemisia Genus Plants in the Republic of Buryatia (Russia) and Its Significance in Plant Adaptation. Plants, 13(18), 2630. https://doi.org/10.3390/plants13182630






