Metabolome Shift in Centella asiatica Leaves Induced by the Novel Plant Growth-Promoting Rhizobacterium, Priestia megaterium HyangYak-01
Abstract
1. Introduction
2. Results
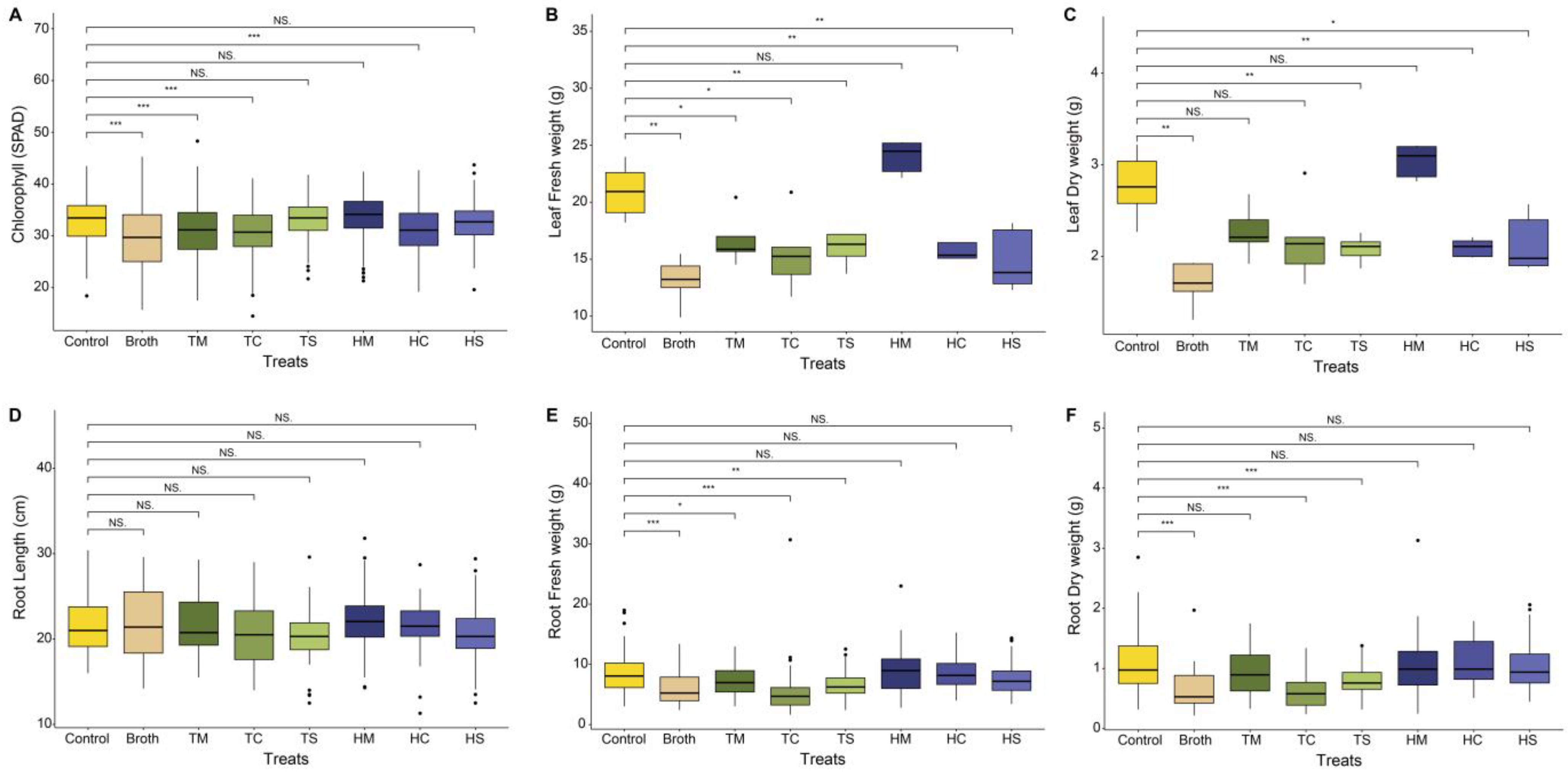
2.1. Field Experiment Results
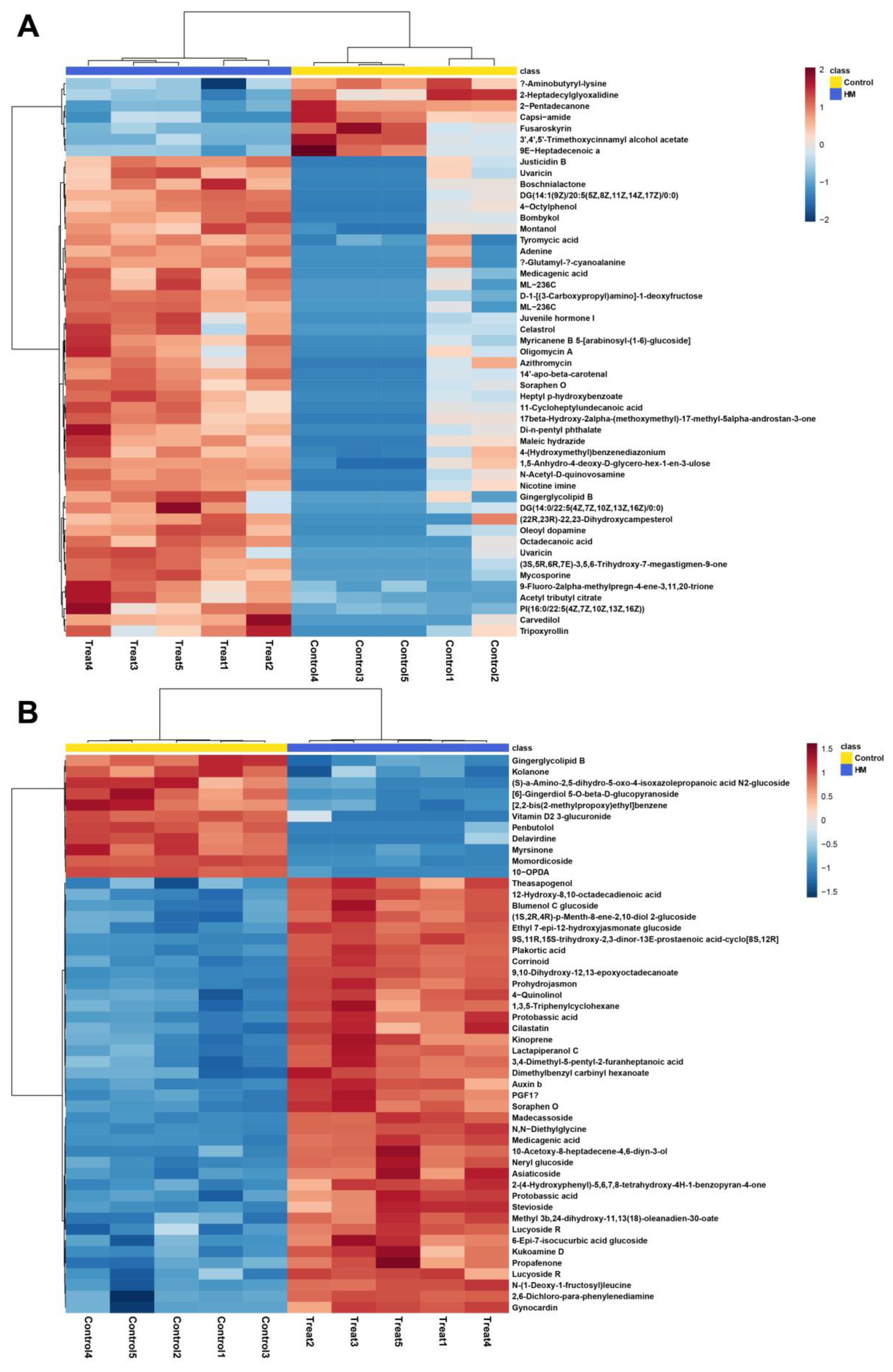
2.2. Untargeted Metabolomic Analysis of C. asiatica Leaf Metabolome
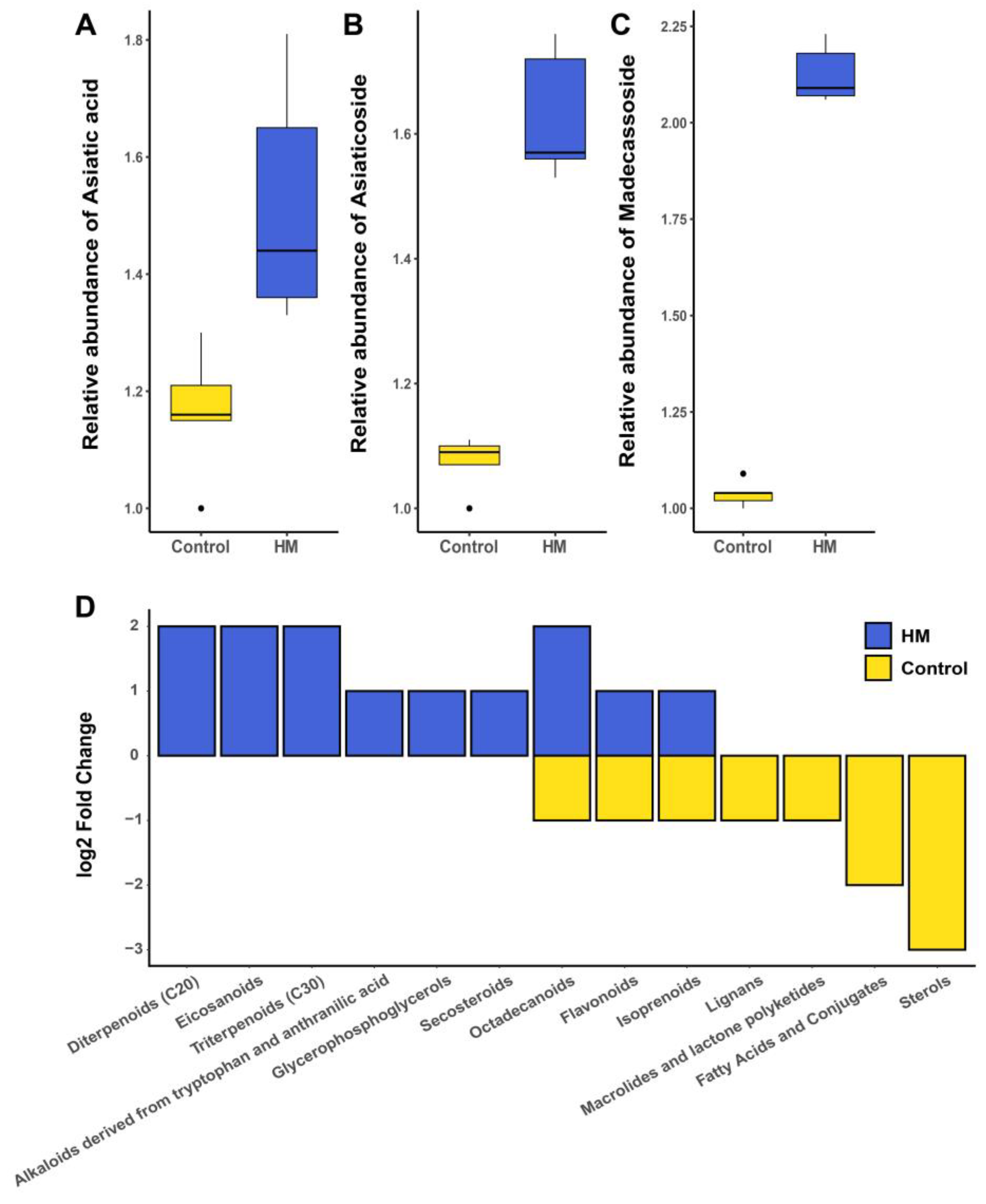
2.3. Relative Quantification of Targeted Compounds
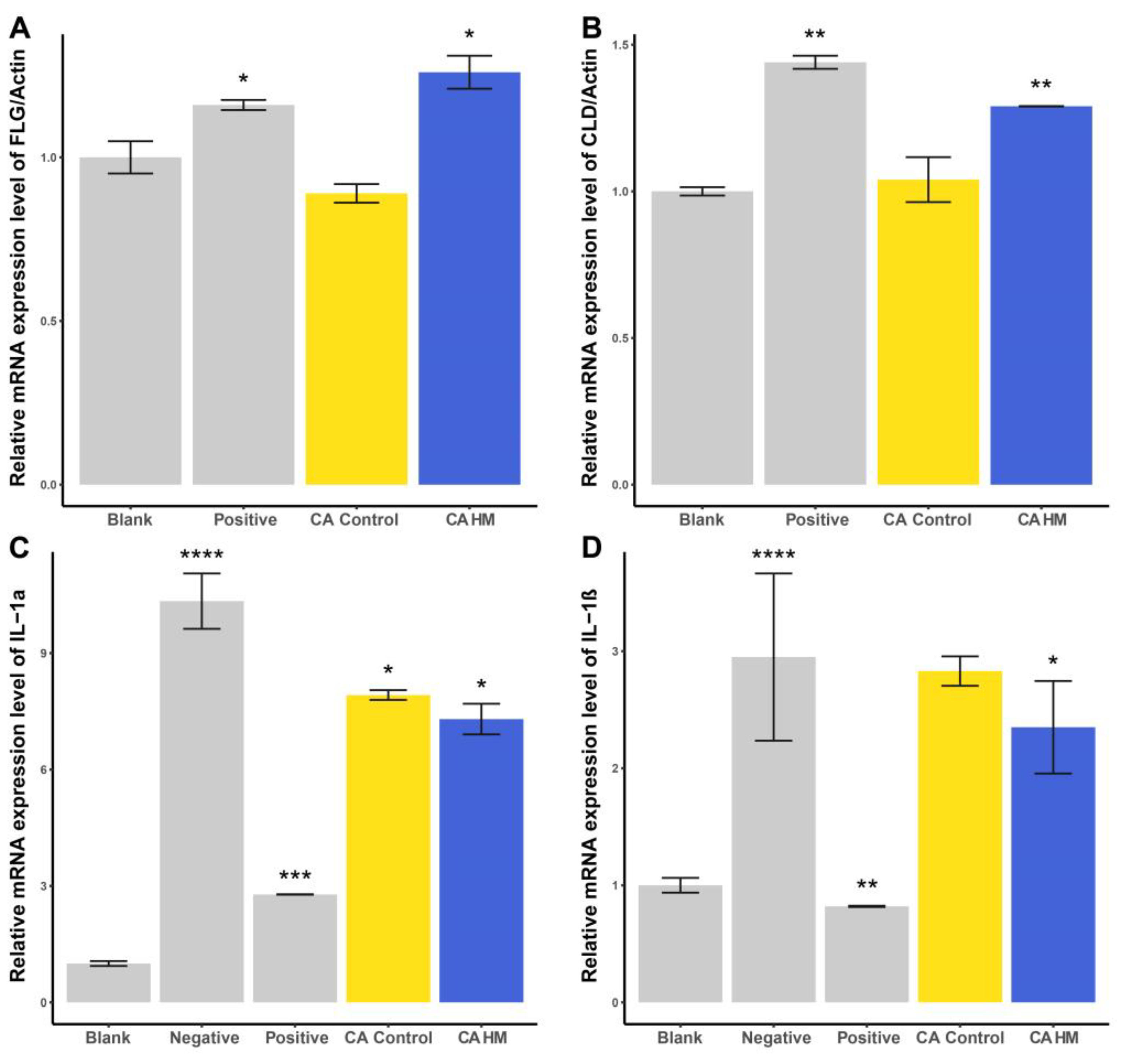
2.4. Cell Treatments
3. Discussion
4. Materials and Methods
4.1. Field Experiment and Sampling
4.1.1. Experimental Design
4.1.2. Plant Material Sampling
4.2. Leaf Extract Preparation
4.3. Metabolomic Analysis
4.3.1. Ultra-High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-Q TOF-MS) Analysis
4.3.2. Metabolite Identification and Statistical Analysis
4.4. Cell Culture and Sample Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Poovizhi, T. Perspective study on Pharmacological and Therapeutic potential of Centella asiatica. Medicon Med. Sci. 2022, 2, 02–11. [Google Scholar]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in Sustainable Agriculture: A Critical Sustainable Development Driver Governed by Green Chemistry Principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Okselni, T.; Septama, A.W.; Pamungkas, R.A.; Rahmi, E.P.; Efdi, M.; Koketsu, M. A Systematic Review and Meta-Analysis Extraction Techniques to Reach the Optimum Asiaticoside Content from the Edible Plant of Centella asiatica. S. Afr. J. Bot. 2023, 155, 261–273. [Google Scholar] [CrossRef]
- Devkota, A.; Jha, P.K. Effect of integrated manuring on growth and yield of Centella asiatica (L.) Urb. Trop. Ecol. 2013, 54, 89–95. [Google Scholar]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Rani, L.; Kumar, P.; Dutt, I.; Maddodi, B.S.; Chaudhary, V.K. Sustainable Options for Fertilizer Management in Agriculture to Prevent Water Contamination: A Review. Environ. Dev. Sustain. 2024, 26, 8303–8327. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef] [PubMed]
- Walder, F.; Schmid, M.W.; Riedo, J.; Valzano-Held, A.Y.; Banerjee, S.; Büchi, L.; Bucheli, T.D.; van Der Heijden, M.G. Soil microbiome signatures are associated with pesticide residues in arable landscapes. Soil Biol. Biochem. 2022, 174, 108830. [Google Scholar] [CrossRef]
- DiBartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A hazard assessment. Front. Environ. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Neher, D.A.; Barbercheck, M.E. Soil microarthropods and soil health: Intersection of decomposition and pest suppression in agroecosystems. Insects 2019, 10, 414. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the Crucial Role of Soil Microorganisms in Carbon Cycling: A Review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Verdú, M.; Alcántara, J.M.; Navarro-Cano, J.A.; Goberna, M. Transitivity and intransitivity in soil bacterial networks. ISME J. 2023, 17, 2135–2139. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, M.P. Plant-microbe interactions in alleviating abiotic stress—A mini review. Front. Agron. 2021, 3, 667903. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.; Wang, J.; Wei, Q.; Zhang, W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Angon, P.B.; Anjum, N.; Akter, M.M.; KC, S.; Suma, R.P.; Jannat, S. An overview of the impact of tillage and cropping systems on soil health in agricultural practices. Adv. Agric. 2023, 2023, 8861216. [Google Scholar] [CrossRef]
- Ball, B.; Bingham, I.; Rees, R.; Watson, C.; Litterick, A. The role of crop rotations in determining soil structure and crop growth conditions. Can. J. Soil Sci. 2005, 85, 557–577. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Afshar, R.K.; Liu, X.; Herbert, S.J. Understanding intercropping to improve agricultural resiliency and environmental sustainability. Adv. Agron. 2020, 162, 199–256. [Google Scholar]
- Khan, M.H.; Liu, H.; Zhu, A.; Khan, M.H.; Hussain, S.; Cao, H. Conservation tillage practices affect soil microbial diversity and composition in experimental fields. Front. Microbiol. 2023, 14, 1227297. [Google Scholar] [CrossRef]
- Cappellari, L.d.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of Essential Oil Production in Mentha x Piperita by Plant Growth Promoting Bacteria Was Correlated with an Increase in Jasmonate and Salicylate Levels and a Higher Density of Glandular Trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 00148. [Google Scholar] [CrossRef]
- James, J.T.; Dubery, I.A. Pentacyclic Triterpenoids from the Medicinal Herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 57135. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 402666. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Naz, I.; Bano, A.; Ul-Hassan, T. Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr. J. Biotechnol. 2009, 8, 21. [Google Scholar]
- Riahi, L.; Cherif, H.; Miladi, S.; Neifar, M.; Bejaoui, B.; Chouchane, H.; Masmoudi, A.S.; Cherif, A. Use of Plant Growth Promoting Bacteria as an Efficient Biotechnological Tool to Enhance the Biomass and Secondary Metabolites Production of the Industrial Crop Pelargonium Graveolens L’Hér. under Semi-Controlled Conditions. Ind. Crops Prod. 2020, 154, 112721. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.C.M.V.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Singh, P.K.; Vyas, D. Biocontrol of plant diseases and sustainable agriculture. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2009, 79, 110–128. [Google Scholar]
- Vaishnav, A.; Kumar, R.; Singh, H.B.; Sarma, B.K. Extending the Benefits of PGPR to Bioremediation of Nitrile Pollution in Crop Lands for Enhancing Crop Productivity. Sci. Total Environ. 2022, 826, 154170. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Lim, K.; Ibal, J.C.; Kim, M.-C.; Kim, H.-B.; Baek, C.; Heo, Y.M.; Lee, H.; Kang, S.; Lee, D.-G.; et al. Growth Increase in the Herbaceous Plant Centella asiatica by the Plant Growth-Promoting Rhizobacteria Priestia Megaterium HyangYak-01. Plants 2023, 12, 2398. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Group Name | |
|---|---|---|
| Control | Tap water | Blank |
| R2A broth | Broth | |
| P. megaterium KACC 10482T | Culture medium | TM |
| Bacterial cells | TC | |
| Culture supernatant | TS | |
| P. megaterium HyangYak-01 | Culture medium | HM |
| Bacterial cells | HC | |
| Culture supernatant | HS | |
| Target | Forward | Reverse |
|---|---|---|
| Beta actin | 5′-CATGTACGTTGCTATCCAGGC-3′ | 5′-CTCCTTAATGTCACGCACGAT-3′ |
| AQP3 | 5′-AGACAGCCCCTTCAGGATTT-3′ | 5′-TCCCTTGCCCTGAATATCTG-3′ |
| HAS3 | 5′-CTTAAGGGTTGCTTGCTTGC-3′ | 5′-GTTCGTGGGAGATGAAGGAA-3′ |
| FLG | 5′-TGAAGCCTATGACACCACTGA-3′ | 5′-TCCCCTACGCTTTCTTGTCCT-3′ |
| CLDN1 | 5′-CTGTCATTGGGGGTGCGATA-3′ | 5′-CTGACCAAATTCGTACCTGGC-3′ |
| IL-1α | 5′-TGGCTCATTTTCCCTCAAAAGTTG-3′ | 5′-AGAAATCGTGAAATCCGAAGTCAAG-3′ |
| IL-1β | 5′-GTCATTCGCTCCCACATTCT-3′ | 5′-ACTTCTTGCCCCCTTTGAAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-C.; Jo, H.; Lim, K.; Kim, I.; Kim, H.-B.; Kim, S.; Nho, Y.; Kim, M.; Kim, H.; Baek, C.; et al. Metabolome Shift in Centella asiatica Leaves Induced by the Novel Plant Growth-Promoting Rhizobacterium, Priestia megaterium HyangYak-01. Plants 2024, 13, 2636. https://doi.org/10.3390/plants13182636
Kim M-C, Jo H, Lim K, Kim I, Kim H-B, Kim S, Nho Y, Kim M, Kim H, Baek C, et al. Metabolome Shift in Centella asiatica Leaves Induced by the Novel Plant Growth-Promoting Rhizobacterium, Priestia megaterium HyangYak-01. Plants. 2024; 13(18):2636. https://doi.org/10.3390/plants13182636
Chicago/Turabian StyleKim, Min-Chul, HyungWoo Jo, Kyeongmo Lim, Ikwhan Kim, Hye-Been Kim, Sol Kim, Younhwa Nho, Misun Kim, Hyeyoun Kim, Chaeyun Baek, and et al. 2024. "Metabolome Shift in Centella asiatica Leaves Induced by the Novel Plant Growth-Promoting Rhizobacterium, Priestia megaterium HyangYak-01" Plants 13, no. 18: 2636. https://doi.org/10.3390/plants13182636
APA StyleKim, M.-C., Jo, H., Lim, K., Kim, I., Kim, H.-B., Kim, S., Nho, Y., Kim, M., Kim, H., Baek, C., Heo, Y. M., Lee, H., Kang, S., Lee, D.-G., Han, K., & Shin, J.-H. (2024). Metabolome Shift in Centella asiatica Leaves Induced by the Novel Plant Growth-Promoting Rhizobacterium, Priestia megaterium HyangYak-01. Plants, 13(18), 2636. https://doi.org/10.3390/plants13182636







