Genome-Wide Association for Morphological and Agronomic Traits in Phaseolus vulgaris L. Accessions
Abstract
1. Introduction
2. Results
2.1. Phenotypic Data
2.2. Genome-Wide Association Study (GWAS)
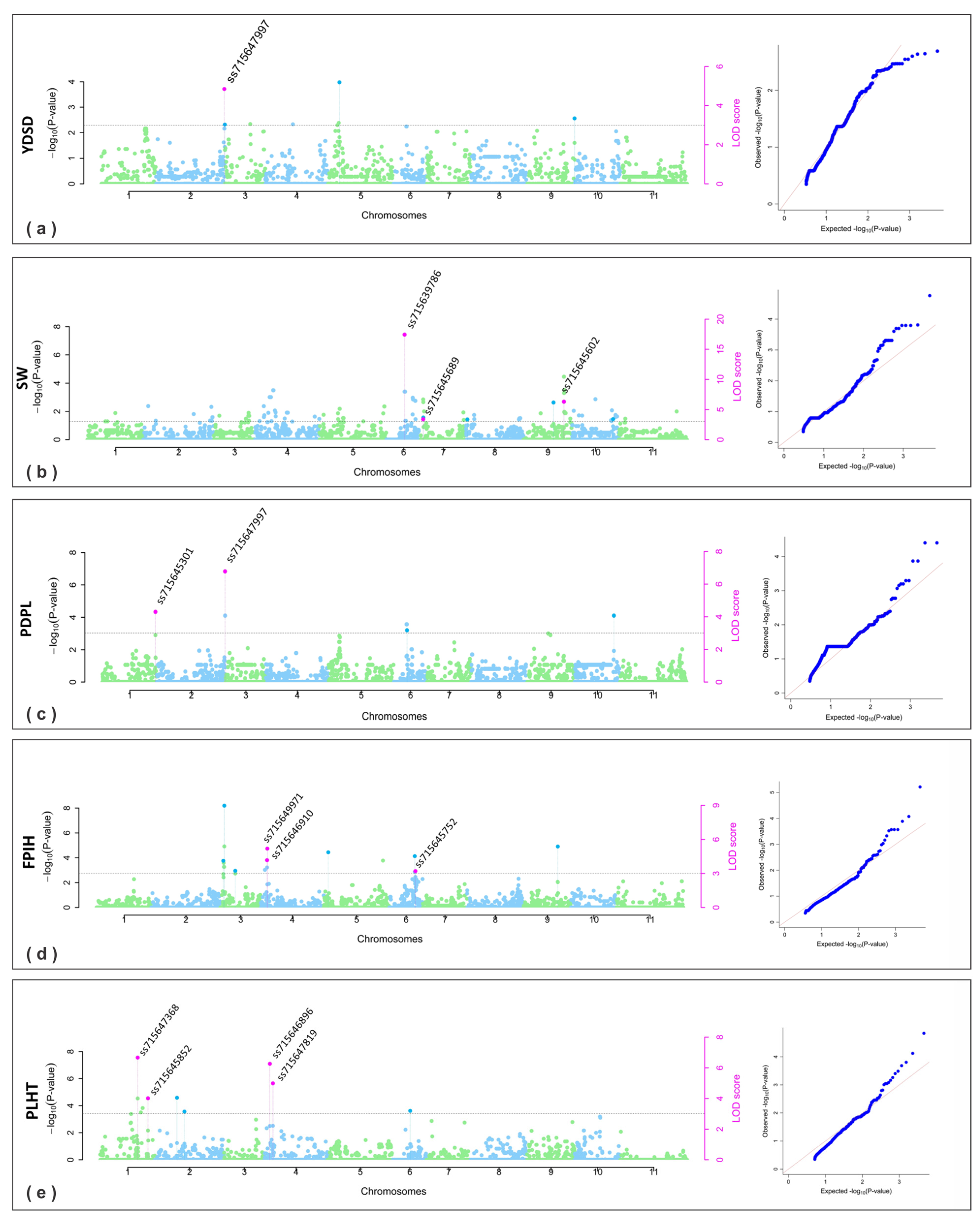
2.2.1. SNPs for Yield and Its Components
2.2.2. SNPs for Plant Architecture
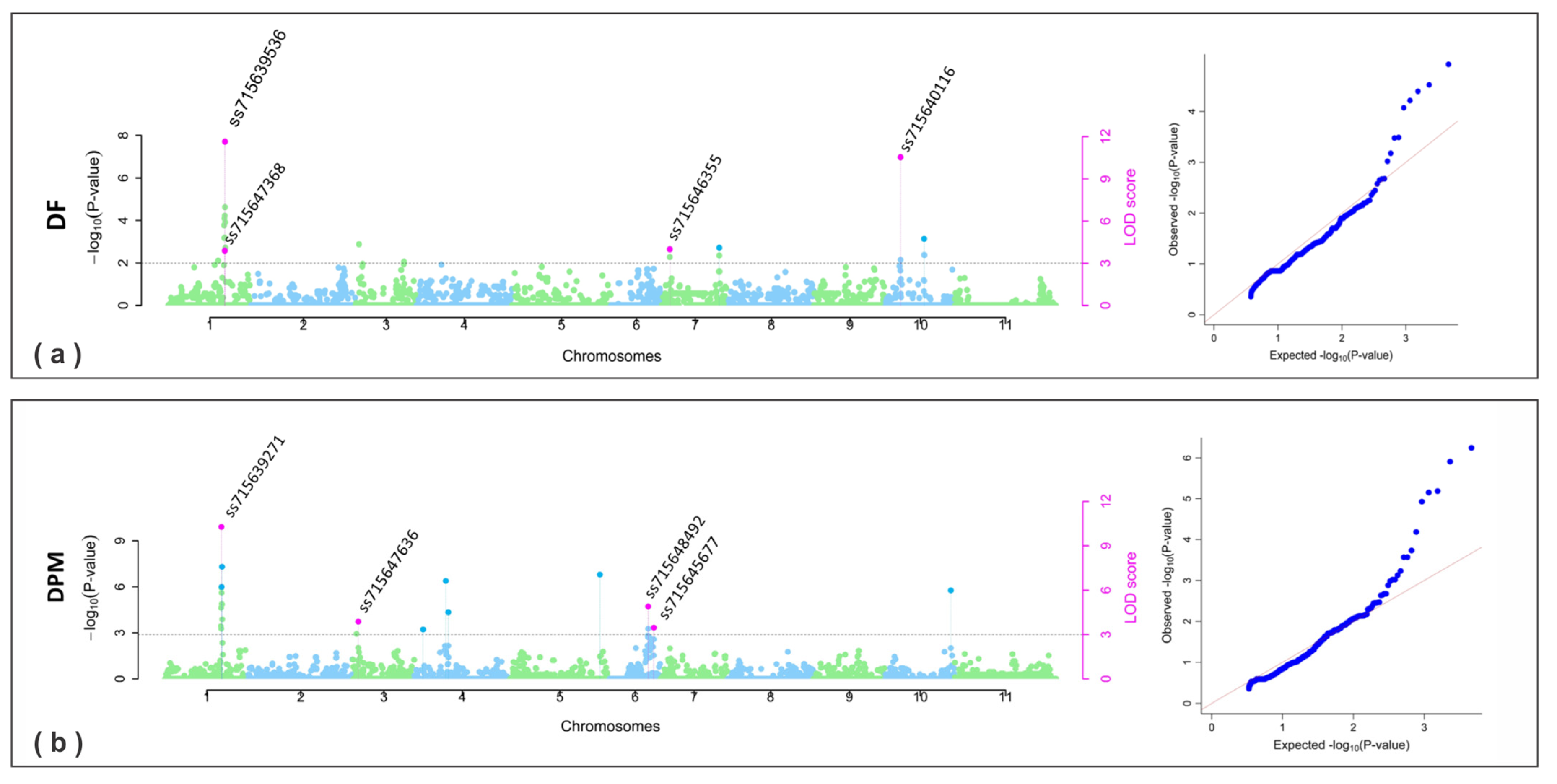
2.2.3. SNPs for Plant Phenology
3. Discussion
3.1. Genotype × Environment Interaction
3.2. Common Bean Genomic Regions Associated with Morphological and Agronomic Traits
4. Materials and Methods
4.1. Plant Material
4.2. Phenotyping Evaluation
4.3. Phenotypic Data Analysis
4.4. Genotyping of the Common Bean Accessions Using BARCBean6K_3 BeadChip
4.5. Genome-Wide Association Study
4.6. Candidate Gene Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Statistical Database, Food and Agriculture. Crops: Beans, Dry. 2022. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 30 May 2024).
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B.J.R.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Jeuffroy, M.H.; Baranger, E.; Carrouée, B.; De Chezelles, E.; Gosme, M.; Hénault, C.; Schneider, A.; Cellier, P. Nitrous oxide emissions from crop rotations including wheat, oilseed rape and dry peas. Biogeosciences 2013, 10, 1787–1797. [Google Scholar] [CrossRef]
- Burle, M.L.; Fonseca, J.R.; Kami, J.A.; Gepts, P. Microsatellite diversity and genetic structure among common bean (Phaseolus vulgaris L.) landraces in Brazil, a secondary center of diversity. Theor. Appl. Genet. 2010, 121, 801–813. [Google Scholar] [CrossRef]
- Blair, M.W.; Brondani, R.V.P.; Diaz, L.M.; Del Peloso, M.J. Diversity and population structure of common bean from Brazil. Crop Sci. 2013, 53, 1983–1993. [Google Scholar] [CrossRef]
- Valdisser, P.A.M.R.; Pereira, W.J.; Almeida Filho, J.E.; Müller, B.S.F.; Coelho, G.R.C.; De Menezes, I.P.P.; Vianna, J.P.G.; Zucchi, M.I.; Lanna, A.C.; Coelho, A.S.G.; et al. In-depth genome characterization of a Brazilian common bean core collection using DArTseq high-density SNP genotyping. BMC Genom. 2017, 18, 423. [Google Scholar] [CrossRef]
- Valentini, G.; Gonçalves-Vidigal, M.C.; Elias, J.C.F.; Moiana, L.D.; Mindo, N.N.A. Population Structure and Genetic Diversity of Common Bean Accessions from Brazil. Plant Mol. Biol. Rep. 2018, 36, 897–906. [Google Scholar] [CrossRef]
- de Carvalho Paulino, J.F.; de Almeida, C.P.; Song, J.; Carbonell, S.A.M.; Chioroto, A.F.; Benchimol-Reis, L.L. Genetic diversity and inter-gene pool introgression of Mesoamerican Diversity Panel in common beans. J. Appl. Genet. 2021, 62, 585–600. [Google Scholar] [CrossRef]
- Gepts, P.; Papa, R. Evolution during Domestication. Encycl. Sci. 2003. [Google Scholar] [CrossRef]
- Schmutz, J.; Mcclean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C.; et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus spp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Kamfwa, K.; Cichy, K.A.; Kelly, J.D. Genome-wide association study of agronomic traits in common bean. Plant Genome 2015, 8, plantgenome2014-09. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.T.; De Resende, M.D.V.; Azevedo, C.F.; Fonseca e Silva, F.; Melo, L.C.; Pereira, H.S.; Souza, T.L.P.O.; Valdisser, P.A.M.R.; Brondani, C.; Vianello, R.P. Genome-wide association and regional heritability mapping of plant architecture, lodging and productivity in Phaseolus vulgaris. G3 Genes|Genomes|Genet. 2018, 8, 2841–2854. [Google Scholar] [CrossRef]
- Delfini, J.; Moda-Cirino, V.; Dos Santos Neto, J.; Zeffa, D.M.; Nogueira, A.F.; Ribeiro, L.A.B.; Ruas, P.M.; Gepts, P.; Gonçalves, L.S.A. Genome-Wide Association Study Identifies Genomic Regions for Important Morpho-Agronomic Traits in Mesoamerican Common Bean. Front. Plant Sci. 2021, 12, 748829. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Han, X.; Zuo, J.-F.; Song, J.; Han, C.-Y.; Zhang, Y.-W.; Zhang, Y.-M. Identification of QTNs and their candidate genes for 100-seed weight in soybean (Glycine max L.) using multi-locus genome-wide association studies. Genes 2020, 11, 714. [Google Scholar] [CrossRef]
- Pérez-de-Castro, A.M.; Vilanova, S.; Cañizares, J.; Pascual, L.; Blanca, J.M.; Diez, M.J.; Prohens, J.; Pico, B. Application of genomic tools in plant breeding. Curr. Genom. 2012, 13, 179–195. [Google Scholar] [CrossRef]
- Korte, A.; Farlow, A. The advantages and limitations of trait analysis with GWAS: A review. Plant Methods 2013, 9, 29. [Google Scholar] [CrossRef]
- Burghardt, L.T.; Young, N.D.; Tiffin, P. A guide to genome-wide association mapping in plants. Curr. Protoc. Plant Biol. 2017, 2, 22–38. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, N.; Jia, T.; Leach, L.; Cockram, J.; Waugh, R.; Ramsay, L.; Thomas, B.; Luo, Z. Genome-wide association mapping of agronomic and morphologic traits in highly structured populations of barley cultivars. Theor. Appl. Genet. 2012, 124, 233–246. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, J.Y.; Ni, Y.L.; Wen, Y.J.; Niu, Y.; Tamba, C.L.; Yue, C.; Song, Q.; Zhang, Y.-M. pLARmEB: Integration of least angle regression with empirical Bayes for multilocus genome-wide association studies. Heredity 2017, 118, 517–524. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, F.; Zhou, Y. The application of multi-locus GWAS for the detection of salt-tolerance loci in rice. Front. Plant Sci. 2018, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ma, Q.; Mei, L.; Hao, F.; Wang, C. Multi-Locus Genome-Wide Association Studies of Fiber-Quality Related Traits in Chinese Early-Maturity Upland Cotton. Front. Plant Sci. 2018, 9, 1169. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.P.; Brown-Guedira, G.; Kolb, F.L.; Van Sanford, D.A.; Tyagi, P.; Sneller, C.H.; Griffey, C.A. Genome-wide association studies for yield-related traits in soft red winter wheat grown in Virginia. PLoS ONE 2019, 14, e0208217. [Google Scholar] [CrossRef]
- Han, X.; Xu, Z.-R.; Zhou, L.; Han, C.-Y.; Zhang, Y.-M. Identification of QTNs and their candidate genes for flowering time and plant height in soybean using multi-locus genome-wide association studies. Mol. Breed. 2021, 41, 39. [Google Scholar] [CrossRef]
- Nemli, S.; Asciogul, T.K.; Kaya, H.B.; Kahraman, A.; Eşiyok, D.; Tanyolac, B. Association mapping for five agronomic traits in the common bean (Phaseolus vulgaris L.). J. Sci. Food Agric. 2014, 94, 3141–3151. [Google Scholar] [CrossRef]
- Moghaddam, S.M.; Mamidi, S.; Osorno, J.M.; Lee, R.; Brick, M.; Kelly, J.; Miklas, P.; Urrea, C.; Song, Q.; Cregan, P.; et al. Genome-Wide Association Study Identifies Candidate Loci Underlying Agronomic Traits in A Middle American Diversity Panel of Common Bean. Plant Genome 2016, 9, plantgenome2016-02. [Google Scholar] [CrossRef]
- Almeida, C.P.D.E.; Paulino, J.F.D.E.C.; Carbonell, S.A.M.; Chiorato, A.F.; Song, Q.; Di Vittori, V.; Rodriguez, M.; Papa, R.; Benchimol-Reis, L.L. Genetic Diversity, Population Structure, and Andean Introgression in Brazilian Common Bean Cultivars after Half a Century of Genetic Breeding. Genes 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mutari, B.; Sibiya, J.; Shayanowako, A.; Chidzanga, C.; Matova, P.M.; Gasura, E. Identification of genomic regions of dry bean (Phaseolus vulgaris L.) associated with agronomic and physiological traits under drought stressed and well-watered conditions using genome-wide association study. BioRxiv 2022, 20, 2022-11. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic. Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Elias, J.C.F.; Gonçalves-Vidigal, M.C.; Vaz Bisneta, M.; Valentini, G.; Vidigal Filho, P.S.; Gilio, T.A.S.; Moda-Cirino, V.; Song, Q. Genetic Mapping for Agronomic Traits in IAPAR 81/LP97-28 Population of Common Bean (Phaseolus vulgaris L.) under Drought Conditions. Plants 2021, 10, 1568. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Jia, Z.; Dunwell, J.M. The applications of new multi-locus GWAS methodologies in the genetic dissection of complex traits. Front. Plant Sci. 2019, 10, 100. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Y.; Zhang, X.; Lu, S.; Zhao, Z.; Wei, D.; Chen, L.; Hu, Y.-G. Multi-locus GWAS of quality traits in bread wheat: Mining more candidate genes and possible regulatory network. Front. Plant Sci. 2020, 11, 1091. [Google Scholar] [CrossRef]
- Koinange, E.M.K.; Singh, S.P.; Gepts, P. Genetic control of the domestication syndrome in common bean. Crop Sci. 1996, 36, 1037–1045. [Google Scholar] [CrossRef]
- Blair, M.W.; Iriarte, G.; Beebe, S. QTL analysis of yield traits in an advanced backcross population derived from a cultivated Andean x wild common bean (Phaseolus vulgaris L.) cross. Theor. Appl. Genet. 2006, 112, 1149–1163. [Google Scholar] [CrossRef]
- Blair, M.W.; Díaz, L.M.; Buendía, H.F.; Duque, M.C. Genetic Diversity, seed size associations and population structure of a core collection of common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 955–972. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Veja, E.; Pañeda, A.; Rodríguez-Suárez, C.; Campa, A.; Giraldez, R.; Ferreira, J. Mapping of QTLs for morpho-agronomic and seed quality traits in a RIL population of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2010, 120, 1367–1380. [Google Scholar] [CrossRef]
- González, A.M.; De La Fuente, M.; De Ron, A.M.; Santalla, M. Protein markers and seed size variation in common bean segregating populations. Mol. Breed. 2010, 25, 723–740. [Google Scholar] [CrossRef]
- Roberts, A.W.; Bushoven, J.T. The cellulose synthase (CESA) gene superfamily of the moss Physcomitrella patens. BMC Plant Biol. 2007, 63, 207–2019. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Int. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Melis, R.; Chirwa, R.; Mzengeza, T.; Mathew, I.; Shayanowako, A. Genome-wide association analysis of bean fly resistance and agro-morphological traits in common bean. PLoS ONE 2021, 16, e0250729. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Sang, T.; Lin, Z.; Li, R.; Ren, W.; Shen, X.; Zhao, B.; Wang, X.; Zhang, X.; et al. Cell type-specific proteomics uncovers a RAF15-SnRK2.6/OST1 Kinase cascade in guard cells. J. Int. Plant Biol. 2023, 65, 2122–2137. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, A.H.; White, J.W.; Lee, R.; Osorno, J.M.; Schmutz, J.; Miklas, P.N.; Myers, J.I.M.; McClean, P.; Thomas, E. Genetic associations in four decades of multi-environment trials reveal agronomic trait evolution in common bean. Genetics 2020, 215, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.D.V. Software Selegen-REML/BLUP: A useful tool for plant breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Song, Q.; Jia, G.; Hyten, D.L.; Jenkins, J.; Hwang, E.Y.; Schroeder, S.G.; Osorno, J.M.; Schumtz, J.; Jackson, S.A.; Mcclean, P.E.; et al. SNP Assay Development for Linkage Map Construction, Anchoring Whole Genome Sequence and Other Genetic and Genomic Applications in Common Bean. G3 Genes|Genomes|Genet. 2015, 5, 2285–2290. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistics Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 30 May 2022).
- Zhang, Y.-W.; Tamba, C.L.; Wen, Y.-J.; Pei, L.; Ren, W.-L.; Ni, Y.-L.; Gao, J.; Zhang, Y.-M. mrMLM v4.0.2: An R platform for multi-locus genome-wide association studies. Genom. Proteom. Bioinform. 2020, 18, 481–487. [Google Scholar] [CrossRef]
- Wang, S.B.; Feng, J.Y.; Ren, W.L.; Huang, B.; Zhou, L.; Wen, Y.J.; Zhang, J.; Dunwell, J.M.; Xu, S.; Zhang, Y.-M. Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci. Rep. 2016, 6, 19444. [Google Scholar] [CrossRef]
- Tamba, C.L.; Zhang, Y.M. A FAST mrMLM algorithm for multi-locus genome-wide association studies. BioRxiv 2018, 1–34. [Google Scholar] [CrossRef]
- Wen, Y.J.; Zhang, H.; Ni, Y.L.; Huang, B.; Zhang, J.; Feng, J.Y.; Wang, S.-B.; Dunwell, J.M.; Zhang, Y.-M.; Wu, R. Methodological implementation of mixed linear models in multi-locus genome-wide association studies. Brief Bioinform. 2018, 19, 700–712. [Google Scholar] [CrossRef]
- Tamba, C.L.; Ni, Y.L.; Zhang, Y.M. Iterative sure Independence screening EM-Bayesian LASSO algorithm for multi-locus genome-wide association studies. PLoS Comput. Biol. 2017, 13, e1005357. [Google Scholar] [CrossRef] [PubMed]
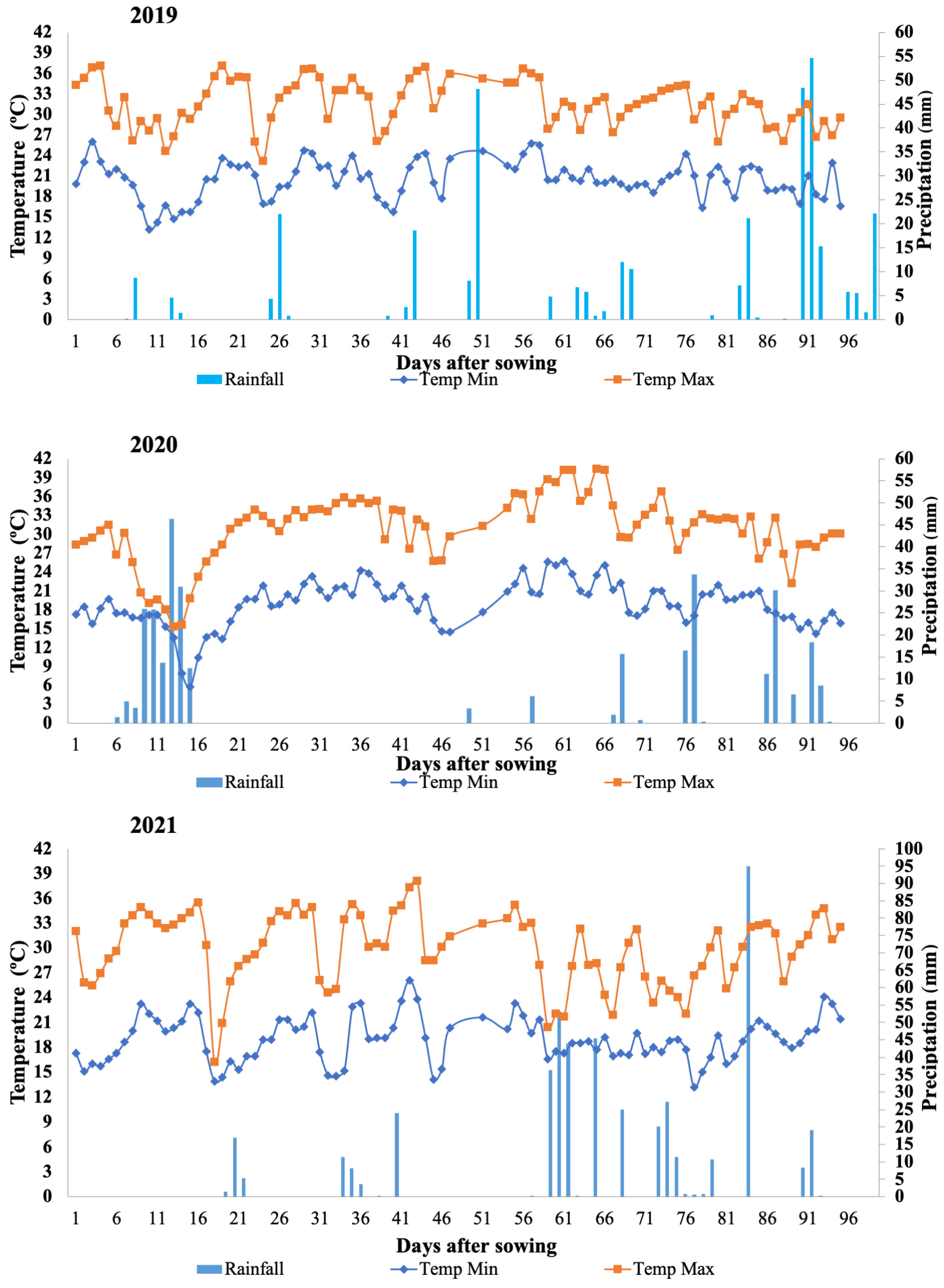

| Traits | Year | Accessions | § Gê | PEV | ||
|---|---|---|---|---|---|---|
| Average | Interval | LRT (x2) | ||||
| Yield and its Components | ||||||
| Grain yield (kg ha−1) | 2019 | 990.44 | 191.63–1798.61 | 215.24 ** | ** | 10,221.53 |
| 2020 | 1736.26 | 335.15–3723.92 | 193.41 ** | 47,059.12 | ||
| 2021 | 1093.57 | 292.21–2469.66 | 149.20 ** | 30,490.65 | ||
| 100-seed weight (g) | 2019 | 28.00 | 15.22–49.73 | 611.54 ** | ** | 0.99 |
| 2020 | 27.92 | 16.21–54.63 | 565.88 ** | 1.27 | ||
| 2021 | 28.10 | 14.15–44.50 | 421.76 ** | 2.32 | ||
| Number of seeds per pod | 2019 | 3.21 | 2.12–4.54 | 135.70 ** | ** | 0.05 |
| 2020 | 3.60 | 1.91–5.99 | 154.82 ** | 0.09 | ||
| 2021 | 2.73 | 2.05–3.52 | 38.85 ** | 0.05 | ||
| Number of pods per plant | 2019 | 10.04 | 4.34–15.14 | 119.59 ** | ** | 1.37 |
| 2020 | 9.67 | 3.50–17.35 | 119.90 ** | 1.32 | ||
| 2021 | 10.42 | 5.45–21.00 | 60.21 ** | 3.81 | ||
| Architecture | ||||||
| First pod insertion height (cm) | 2019 | 13.34 | 11.32–18.14 | 150.65 ** | ** | 0.30 |
| 2020 | 13.53 | 11.21–21.71 | 152.08 ** | 0.56 | ||
| 2021 | 13.14 | 11.19–16.01 | 51.08 ** | 0.44 | ||
| Plant height (cm) | 2019 | 49.00 | 37.56–58.63 | 309.44 ** | ** | 1.17 |
| 2020 | 50.12 | 36.17–60.80 | 492.35 ** | 0.68 | ||
| 2021 | 47.88 | 37.77–58.08 | 134.13 ** | 3.33 | ||
| Phenology | ||||||
| Number of days for flowering | 2019 | 42.70 | 30.99–60.99 | 1208.73 ** | ** | 0.06 |
| 2020 | 42.26 | 31.73–60.96 | 925.23 ** | 0.22 | ||
| 2021 | 42.71 | 30.00–60.00 | 1281.12 ** | 0.0009 | ||
| Number of days for maturity | 2019 | 81.46 | 73.65–89.59 | 360.75 ** | ** | 1.03 |
| 2020 | 81.49 | 73.57–91.30 | 292.75 ** | 1.32 | ||
| 2021 | 80.89 | 73.28–87.69 | 263.45 ** | 2.35 | ||
| § Traits | Year | Chr | Position (pb) | SNP | # LOD Score | −Log10 P | ¥ SNP Effect | ¶ R2 (%) | Methods |
|---|---|---|---|---|---|---|---|---|---|
| YDSD | 2019 | 2 | 48,720,860 | ss715647997 | 4.55 | 5.33 | −147.00~−124.64 | 12.43~17.29 | 1, 5 |
| 2020 | 4 | 25,245,150 | ss715650918 | 4.72 | 5.50 | −513.05~−361.05 | 9.81~16.55 | 1, 2 | |
| 7 | 39,295,293 | ss715640487 | 5.35~6.10 | 6.16~6.94 | −303.83~−235.68 | 11.69~17.70 | 1, 2, 4, 5 | ||
| 8 | 55,278,106 | ss715639359 | 3.99 | 4.74 | 148.49~238.74 | 5.73~12.37 | 1, 2 | ||
| 2021 | 5 | 5,399,253 | ss715648672 | 4.63 | 5.41 | −299.91~−150.73 | 12.70~12.96 | 3, 4 | |
| C * | 2 | 48,720,860 | ss715647997 | 4.85 | 5.64 | −203.83~−175.25 | 13.38~18.32 | 1, 4 | |
| SW | 2019 | 7 | 819,750 | ss715645689 | 3.21~9.19 | 3.92~10.11 | −7.55~−2.99 | 13.48~21.51 | 2, 3, 4 |
| 10 | 24,598,176 | ss715641543 | 3.46~4.95 | 4.18~5.74 | −6.90~−3.79 | 10.13~21.71 | 3, 4 | ||
| 2020 | 2 | 3,118,774 | ss715639502 | 3.41~9.10 | 4.13~10.01 | −6.48~−3.38 | 2.08~29.97 | 1, 3, 4 | |
| 5 | 40,128,137 | ss715646697 | 5.01~5.90 | 5.81~6.73 | 2.75~6.47 | 11.28~15.64 | 2, 3 | ||
| 7 | 819,750 | ss715645689 | 5.75~10.15 | 6.57~11.09 | −7.30~−3.45 | 17.83~25.23 | 2, 3, 4, 5 | ||
| 2021 | 9 | 33,444,042 | ss715645602 | 6.41~7.89 | 7.26~8.78 | −3.03~−2.03 | 5.30~11.75 | 1, 4, 5 | |
| C * | 6 | 22,116,966 | ss715639786 | 4.49~30.63 | 5.26~31.54 | −6.21~−3.52 | 18.43~57.29 | 4, 5 | |
| 7 | 819,750 | ss715645689 | 3.10~3.38 | 3.80~4.10 | −9.49~−2.34 | 8.15~33.45 | 2, 3, 4 | ||
| 9 | 33,444,042 | ss715645602 | 5.17~7.42 | 5.98~8.29 | −2.13~−1.96 | 4.83~5.74 | 4, 5 | ||
| SDPD | 2020 | 6 | 27,760,992 | ss715645673 | 3.77~4.00 | 4.51~4.76 | −0.35~−0.32 | 4.92~8.24 | 1, 5 |
| PDPL | 2019 | 1 | 51,819,821 | ss715645301 | 3.22~15.14 | 3.93~16.16 | 0.80~1.77 | 10.11~43.39 | 1, 2, 3, 4, 5 |
| 2 | 48,720,860 | ss715647997 | 4.80~8.15 | 5.59~9.04 | −0.97~−0.88 | 8.83~10.74 | 1, 4, 5 | ||
| 10 | 39,797,018 | ss715646330 | 3.18~4.09 | 3.89~4.85 | −1.03~−0.61 | 2.92~8.13 | 1, 2 | ||
| 2021 | 10 | 39,797,018 | ss715646330 | 3.13~5.64 | 3.83~6.46 | −2.38~−0.95 | 5.07~15.24 | 1, 2, 3, 4, 5 | |
| 11 | 48,780,038 | ss715650748 | 6.69~6.86 | 7.54~7.72 | −1.59~−1.12 | 9.46~19.15 | 1, 4 | ||
| PDPL | C * | 1 | 51,819,821 | ss715645301 | 3,54~14,89 | 4.27~15.91 | 0.95~1.92 | 12.22~43.10 | 1, 2, 3, 4, 5 |
| 2 | 48,720,860 | ss715647997 | 4.56~6.89 | 5.34~7.76 | −1.04~−0.99 | 8.61~9.36 | 1, 4, 5 | ||
| FPIH | 2019 | 3 | 4,083,079 | ss715647965 | 4.86~7.70 | 5.65~8.59 | −0.46~−0.30 | 4.32~12.85 | 4, 5 |
| 4 | 1,627,690 | ss715649971 | 4.03~9.59 | 4.78~10.52 | 0.36~0.63 | 7.00~21.68 | 1, 2, 4, 5 | ||
| 6 | 25,791,849 | ss715645752 | 3.09~6.38 | 3.79~7.23 | −0.83~−0.56 | 9.98~21.74 | 1, 2 | ||
| 9 | 31,832,898 | ss715647626 | 4.23~5.39 | 4.99~6.21 | 0.42~0.50 | 7.12~10.12 | 2, 4, 5 | ||
| 2020 | 5 | 40,141,917 | ss715646699 | 3.53~4.09 | 4.25~4.85 | −0.95~−0.88 | 7.20~8.47 | 1, 2 | |
| 6 | 25,791,849 | ss715645752 | 4.45~5.82 | 5.22~6.65 | −2.31~−0.94 | 14.47~19.87 | 1, 2, 3, 5 | ||
| 2021 | 4 | 1,627,690 | ss715649971 | 3.33~4.37 | 4.05~5.14 | 0.29~0.73 | 8.98~23.46 | 1, 2, 3, 4 | |
| C * | 4 | 1,503,482 | ss715646910 | 3.93~4.41 | 4.67~5.18 | −1.05~−0.59 | 9.25~10.92 | 3, 4 | |
| 4 | 1,627,690 | ss715649971 | 4.02~6.35 | 4.77~7.20 | 0.39~0.52 | 7.02~12.51 | 1, 2 | ||
| 6 | 25,791,849 | ss715645752 | 3.04~6.30 | 3.74~7.15 | −0.89~−0.59 | 6.28~21.51 | 1, 2, 4 | ||
| PLHT | 2019 | 1 | 45,894,030 | ss715647368 | 5.24~15.56 | 6.05~16.59 | −6.00~−2.04 | 18.04~43.65 | 1, 2, 3, 4, 5 |
| 1 | 49,657,760 | ss715645852 | 3.90~4.06 | 4.65~4.82 | −1.25~−1.58 | 7.43~10.31 | 1, 2, 5 | ||
| 4 | 1,224,240 | ss715646896 | 6.17 | 7.00 | −1.69~−2.25 | 14.20~21.93 | 1, 2 | ||
| 4 | 1,982,297 | ss715647819 | 4.95 | 5.75 | 1.53~2.02 | 9.91~15.00 | 1, 2 | ||
| 2020 | 1 | 45,894,030 | ss715647368 | 4.49~12.03 | 5.27~13.01 | −6.18~−3.09 | 29.53~33.98 | 2, 3, 4, 5 | |
| 2021 | 1 | 45,894,030 | ss715647368 | 4.58~8.20 | 5.35~9.10 | −5.17~−1.94 | 20.00~30.53 | 1, 2, 3, 4, 5 | |
| 2 | 45,319,921 | ss715645964 | 4.73~5.02 | 5.51~5.82 | −1.82~−1.28 | 7.57~15.23 | 1, 2, 4 | ||
| 7 | 3,648,568 | ss715646353 | 3.25 | 3.96 | −1.26~−0.82 | 3.45~8.13 | 1, 2 | ||
| C * | 1 | 45,894,030 | ss715647368 | 5.29~15.61 | 6.10~16.64 | −6.16~−2.11 | 18.12~38.65 | 1, 2, 3, 4, 5 | |
| 1 | 49,657,760 | ss715645852 | 3.81~4.01 | 4.55~4.76 | −1.62~−1.26 | 7.10~10.16 | 1, 2, 5 | ||
| 4 | 1,224,240 | ss715646896 | 5.11~6.26 | 5.91~7.10 | −2.33~−1.37 | 8.59~22.18 | 1, 2, 4 | ||
| 4 | 1,982,297 | ss715647819 | 3.74~4.99 | 4.48~5.78 | 1.14~2.08 | 5.04~15.05 | 1, 2, 4 | ||
| DF | 2019 | 1 | 45,894,030 | ss715647368 | 3.83 | 4.57 | −3.75~−2.56 | 12.80~27.40 | 1, 2 |
| 1 | 46,027,600 | ss715639536 | 11.42~11.94 | 12.38~12.91 | −3.68~−3.53 | 24.52~26.65 | 4, 5 | ||
| 7 | 3,770,008 | ss715646355 | 3.02~3.98 | 3.71~4.73 | −2.19~−1.13 | 1.49~5.60 | 1, 2, 5 | ||
| 10 | 7,867,881 | ss715640116 | 10.39~12.48 | 11.35~13.46 | −3.35~−3.98 | 23.79~33.55 | 1, 2, 5 | ||
| 2020 | 1 | 45,894,030 | ss715647368 | 3.51 | 4.24 | −3.90~−2.53 | 10.82~25.80 | 1, 2 | |
| 1 | 46,027,600 | ss715639536 | 7.80~11.41 | 8.69~12.37 | −3.41~−3.22 | 17.46~19.89 | 4, 5 | ||
| 7 | 3,770,008 | ss715646355 | 4.13 | 4.88 | −2.37~−1.74 | 3.07~5.72 | 1, 2 | ||
| 10 | 7,867,881 | ss715640116 | 11.04 | 12.00 | 3.69~4.39 | 25.10~35.50 | 1, 2 | ||
| 2021 | 1 | 46,027,600 | ss715639536 | 10.57~16.56 | 11.52~17.60 | −3.93~−3.30 | 23.26~33.10 | 4, 5 | |
| 7 | 3,770,008 | ss715646355 | 3.48~3.69 | 4.20~4.42 | −1.33~−1.28 | 1.93~2.27 | 1, 2 | ||
| 7 | 48,520,131 | ss715648580 | 4.10~5.64 | 4.85~6.47 | 2.50~3.32 | 3.01~5.30 | 4, 5 | ||
| 10 | 7,867,881 | ss715640116 | 7.94~9.19 | 8.83~10.11 | 2.50~5.17 | 14.42~56.84 | 1, 2, 5 | ||
| C * | 1 | 45,894,030 | ss715647368 | 3.89 | 4.63 | −3.79~−2.57 | 12.71~27.53 | 1, 2 | |
| 1 | 46,027,600 | ss715639536 | 11.14~12.15 | 12.11~13.13 | −3.59~−3.57 | 24.72~24.97 | 4, 5 | ||
| 7 | 3,770,008 | ss715646355 | 3.02~3.99 | 3.72~4.75 | −2.19~−1.13 | 1.48~5.33 | 1, 2, 5 | ||
| 10 | 7,867,881 | ss715640116 | 10.54~12.65 | 11.48~13.64 | 3.57~4.02 | 23.78~33.67 | 1, 2, 5 | ||
| DPM | 2019 | 1 | 45,746,595 | ss715639271 | 7.39~7.70 | 8.27~8.58 | −2.80~−2.22 | 21.53~22.62 | 1, 2 |
| 1 | 46,027,600 | ss715639536 | 3.42~7.59 | 4.14~8.47 | −2.31~−2.24 | 22.66~24.25 | 4, 5 | ||
| DPM | 2019 | 3 | 3,963,582 | ss715647636 | 3.03~4.84 | 3.73~5.63 | 0.83~1.39 | 2.44~6.83 | 4, 5 |
| 4 | 4,497,307 | ss715649528 | 4.93~7.78 | 5.73~8.67 | 2.52~2.79 | 29.33~32.58 | 1, 2 | ||
| 6 | 25,619,371 | ss715648492 | 5.99~6.07 | 6.82~6.90 | −1.99~−1.64 | 12.34~16.31 | 1, 2 | ||
| 6 | 27,370,471 | ss715645677 | 3.27~5.77 | 3.98~5.60 | 1.05~2.15 | 5.01~5.22 | 3, 4 | ||
| 2020 | 1 | 46,027,600 | ss715639536 | 3.75~7.15 | 4.49~8.01 | −2.46~−1.58 | 13.04~22.07 | 1, 2, 5 | |
| 3 | 4,083,079 | ss715647965 | 4.33~5.07 | 5.09~5.87 | −2.65~−1.84 | 15.28~24.25 | 1, 2 | ||
| 10 | 39,577,266 | ss715646318 | 4.35~5.16 | 5.12~5.96 | 2.12~2.81 | 20.14~26.89 | 1, 2 | ||
| 2021 | 1 | 45,894,030 | ss715647368 | 4.48~6.05 | 5.25~6.88 | −3.47~−1.74 | 8.44~10.71 | 1, 2, 3, 4 | |
| 4 | 4,497,307 | ss715649528 | 4.90~16.14 | 5.69~17.18 | 2.97~5.95 | 30.35~51.06 | 1, 2, 3, 4 | ||
| 6 | 25,619,371 | ss715648492 | 3.27~3.80 | 3.99~4.54 | −1.83~−1.31 | 5.50~9.45 | 1, 2 | ||
| C * | 1 | 45,746,595 | ss715639271 | 8.22~12.34 | 9.11~13.33 | −3.18~−2.26 | 22.92~45.08 | 1, 2 | |
| 3 | 3,963,582 | ss715647636 | 3.31~4.33 | 4.02~5.20 | 1.18~1.95 | 4.69~12.81 | 1, 5 | ||
| 6 | 25,619,371 | ss715648492 | 3.51~6.28 | 4.23~7.13 | −1.63~−1.12 | 5.51~11.86 | 1, 2 | ||
| 6 | 27,370,471 | ss715645677 | 3.22~3.70 | 3.92~4.43 | 0.93~2.19 | 3.70~5.15 | 3, 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, S.M.; Lacanallo, G.F.; Gonçalves-Vidigal, M.C.; Vaz Bisneta, M.; Vidigal Rosenberg, A.G.; Vidigal Filho, P.S. Genome-Wide Association for Morphological and Agronomic Traits in Phaseolus vulgaris L. Accessions. Plants 2024, 13, 2638. https://doi.org/10.3390/plants13182638
Alves SM, Lacanallo GF, Gonçalves-Vidigal MC, Vaz Bisneta M, Vidigal Rosenberg AG, Vidigal Filho PS. Genome-Wide Association for Morphological and Agronomic Traits in Phaseolus vulgaris L. Accessions. Plants. 2024; 13(18):2638. https://doi.org/10.3390/plants13182638
Chicago/Turabian StyleAlves, Stephanie Mariel, Giselly Figueiredo Lacanallo, Maria Celeste Gonçalves-Vidigal, Mariana Vaz Bisneta, Andressa Gonçalves Vidigal Rosenberg, and Pedro Soares Vidigal Filho. 2024. "Genome-Wide Association for Morphological and Agronomic Traits in Phaseolus vulgaris L. Accessions" Plants 13, no. 18: 2638. https://doi.org/10.3390/plants13182638
APA StyleAlves, S. M., Lacanallo, G. F., Gonçalves-Vidigal, M. C., Vaz Bisneta, M., Vidigal Rosenberg, A. G., & Vidigal Filho, P. S. (2024). Genome-Wide Association for Morphological and Agronomic Traits in Phaseolus vulgaris L. Accessions. Plants, 13(18), 2638. https://doi.org/10.3390/plants13182638






