Rhizofungus Aspergillus terreus Mitigates Heavy Metal Stress-Associated Damage in Triticum aestivum L.
Abstract
1. Introduction
2. Results
2.1. Rhizospheric Fungus Isolation and Screening against Heavy Metal Tolerance
2.2. Assay for Plant Growth Promoting Potential of the Isolated Fungi
2.3. Characterization of the Selected Isolate
2.4. Identification Based on ITS Sequences and Phylogenetic Analysis
2.5. Effect of Lead Acetate and Copper Sulfate on Fungal Growth
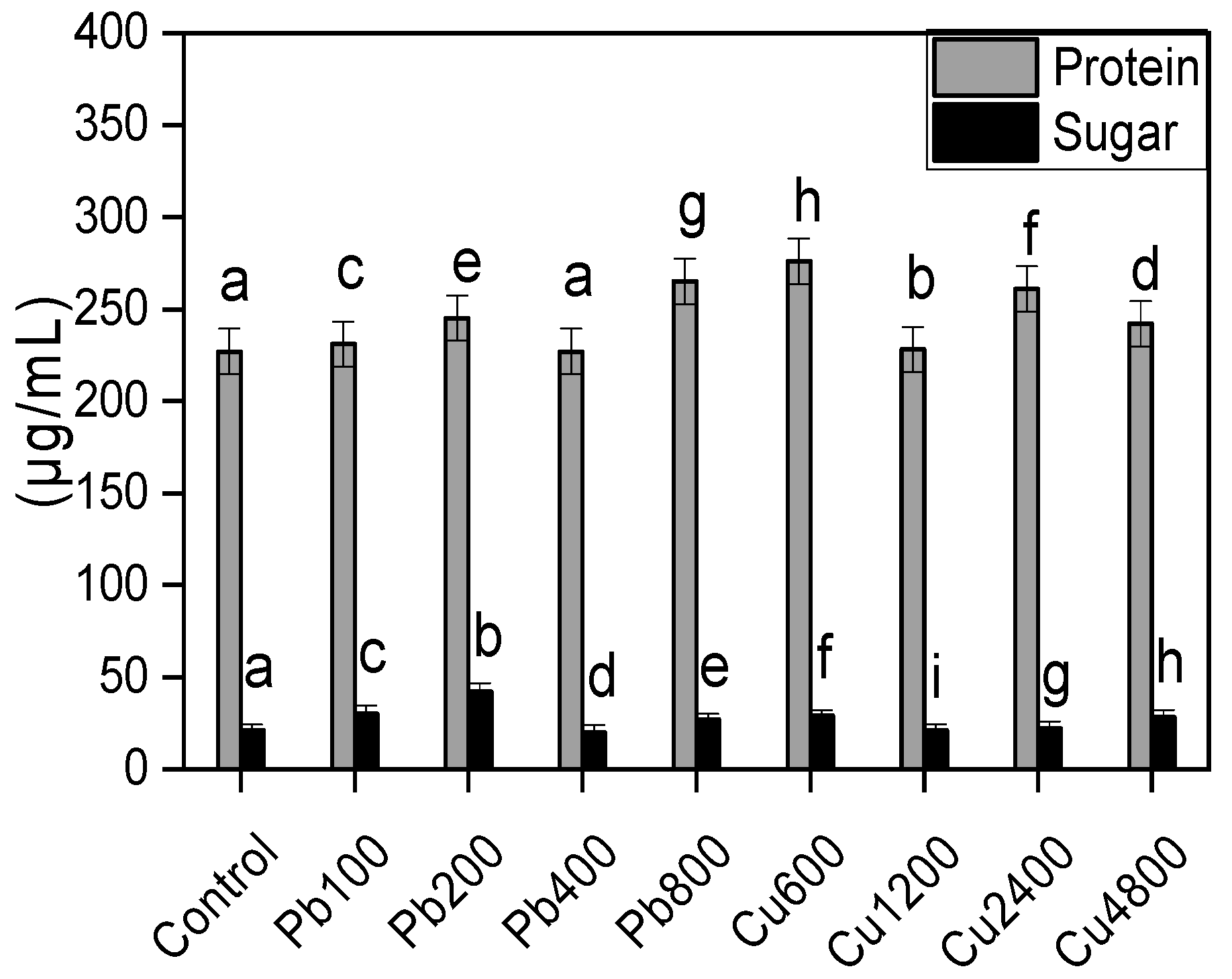
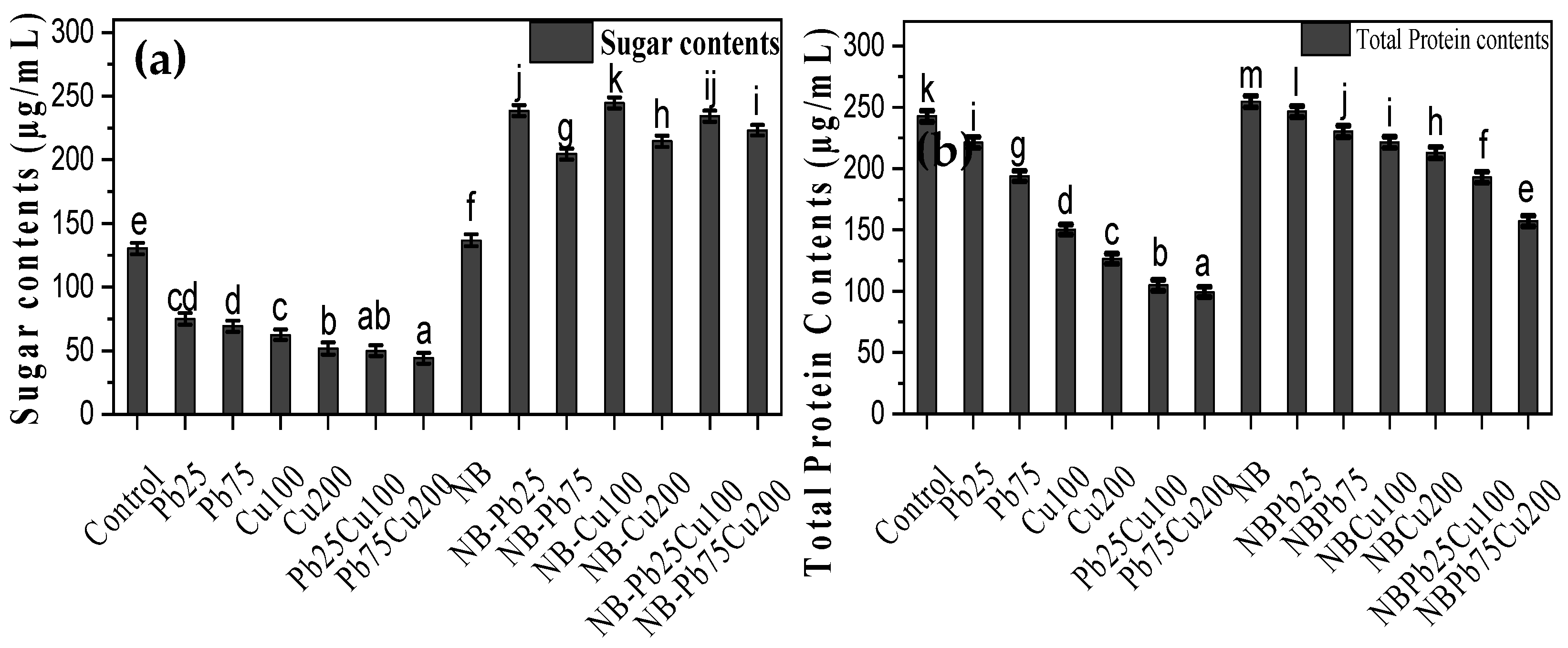
2.6. Release of Sugars and Proteins by the Rhizofungus
3. Growth of T. aestivum under Various Conditions
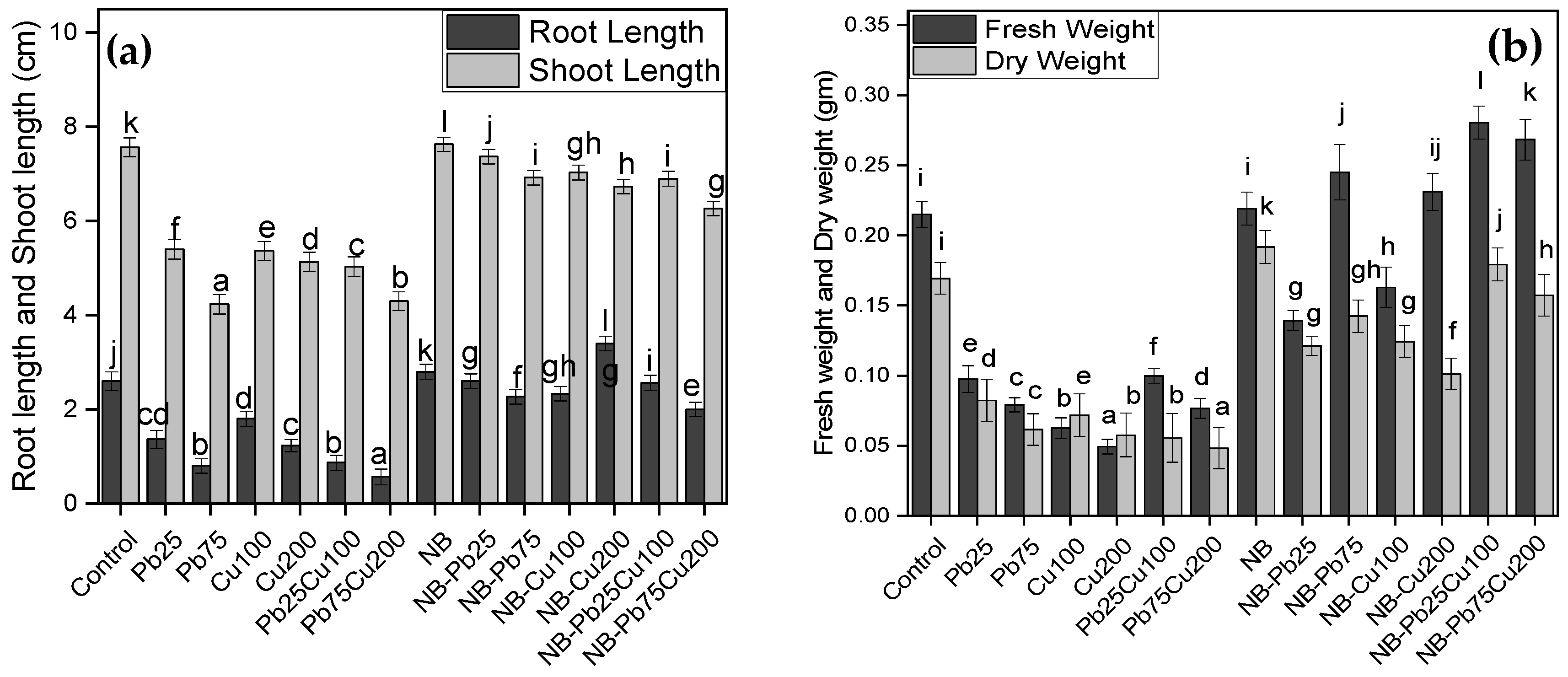
3.1. Shoot Length and Root Length
3.2. Fresh Weight and Dry Weight
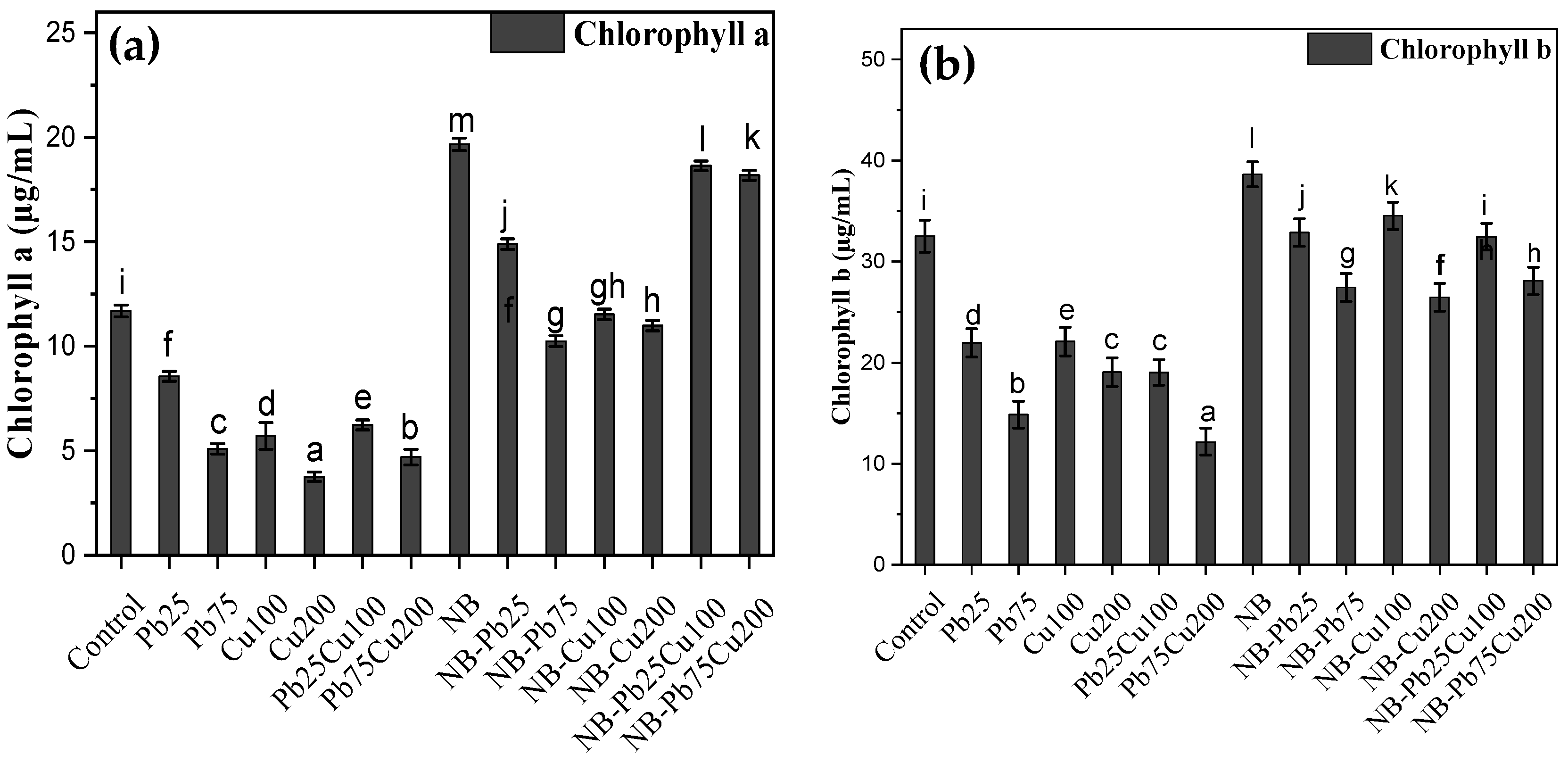
3.3. Chlorophyll a and b
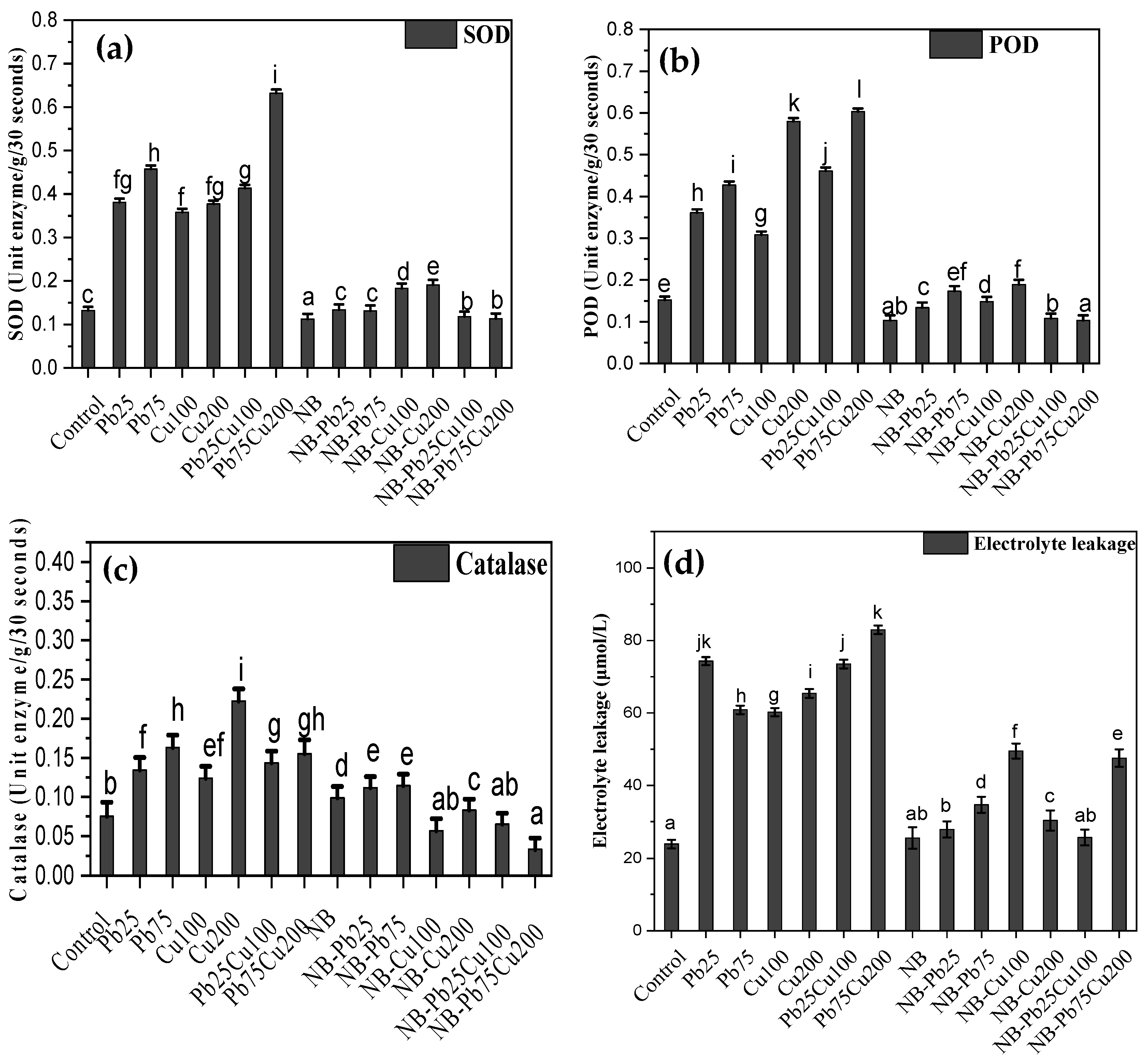
3.4. SOD, POD, CAT, and Electrolyte Leakage
3.5. H2O2 and MDA Content
3.6. Sugar and Protein Content in Triticum aestivum L.
3.7. Rhizofungal Colonization in Roots of T. aestivum L.
3.8. Expression of Phytochelatin and Metallothionein Genes under Heavy Metal Stress
3.9. Scanning Electron Microscopy of Triticum aestivum L roots.
3.10. Energy Dispersion Spectroscopy
4. Discussion
5. Materials and Methods
5.1. Isolation and Culturing of Rhizofungal Strains
5.2. Assessment of Fungal Isolates for Cu and Pb Tolerance
5.3. Molecular Identification of Rhizospheric Fungal Isolate
5.4. Experimental Design of Plant–Microbe Interaction Experiment
5.5. Growth Parameters of Wheat Seedlings
5.6. Estimation of Antioxidant Enzymes
5.7. Electrolyte Leakage
5.8. H2O2 Determination
5.9. The Malonaldehyde Content Analysis
5.10. Sugar and Protein Content
5.11. Root Colonization
5.12. Amplification of MT1 and PCS1 Genes
5.13. Expression Analysis of MT1 and PCS1 in T. aestivum L. under Lead and Copper Stress Condition
5.14. Scanning Electron Microscopy
5.15. Energy-Dispersion Spectroscopy (EDS)
5.16. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, N.; Khalaphallah, R. Root rot/wilt incidence of Phaseolus vulgaris and antagonistic effect of Trichoderma spp. and Aspergillus terreus isolated from rhizosphere soil. SVU Int. J. Agric. Sci. 2023, 5, 26–37. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Munir, H.M.S.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation techniques for elimination of heavy metal pollutants from soil. A review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef]
- Shah, N.; Irshad, M.; Hussain, A.; Qadir, M.; Murad, W.; Khan, A.; Ali, S. EDTA and IAA ameliorates phytoextraction potential and growth of sunflower by mitigating Cu-induced morphological and biochemical injuries. Life 2023, 13, 759. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, F.; Zhang, J.; Ao, W.; Zhou, X.; Yang, H.; Qiu, Y. Occurrence of Aflatoxin M1 in Three Types of Milk from Xinjiang, China, and the Risk of Exposure for Milk Consumers in Different Age-Sex Groups. Foods 2022, 11, 3922. [Google Scholar] [CrossRef]
- Zhou, H.; Ashworth, K.; Dodd, I.C. Exogenous monoterpenes mitigate H2O2-induced lipid damage but do not attenuate photosynthetic decline during water deficit in tomato. J. Exp. Bot. 2023, 74, 5327–5340. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Huang, J.; Wang, W.; Zeng, Y.; Li, X.; Zhao, F. Monitoring Low-Temperature Stress in Winter Wheat Using TROPOMI Solar-Induced Chlorophyll Fluorescence. IEEE Trans. Geosci. Remote Sens. 2024, 62, 1–11. [Google Scholar] [CrossRef]
- Hall, J.Á. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2015, 12, 55–61. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar]
- Waterlot, C.; Pruvot, C.; Marot, F.; Douay, F. Impact of a phosphate amendment on the environmental availability and phytoavailability of Cd and Pb in moderately and highly carbonated kitchen garden soils. Pedosphere 2017, 27, 588–605. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef]
- Larone, D.H. Medically Important Fungi: A Guide to Identification; Elsevier: New York, NY, USA, 1987. [Google Scholar]
- Smith, A.D.; Logeman, B.L.; Thiele, D.J. Copper Acquisition and Utilization in Fungi. Annu. Rev. Microbiol. 2017, 71, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Stolt, J.P.; Sneller, F.E.C.; Bryngelsson, T.; Lundborg, T.; Schat, H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003, 49, 21–28. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [PubMed]
- Cheng, P.; Wu, L.; Zhang, H.; Zhou, J. Inclusion of root water absorption and reinforcement in upper bound limit stability analysis of vegetated slopes. Comput. Geotech. 2024, 169, 106227. [Google Scholar] [CrossRef]
- Kibalnik, O.P.; Sazonova, I.A.; Bochkareva, Y.V.; Bychkova, V.V.; Semin, D.S. Influence of Abiotic Stresses on Morphophysiological Characteristics and Biological Value of Grain Sorghum bicolor (L.) Moench. Int. J. Plant Biol. 2023, 14, 150–161. [Google Scholar]
- Jiang, M.; Chen, S.; Lu, X.; Guo, H.; Chen, S.; Yin, X.; Liu, L. Integrating Genomics and Metabolomics for the Targeted Discovery of New Cyclopeptides with Antifungal Activity from a Marine-Derived Fungus Beauveria felina. J. Agric. Food Chem. 2023, 71, 9782–9795. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Guo, L.; Li, D.; Liu, A.; Bilal, M.; Wang, P. Antifreeze Polysaccharides from Wheat Bran: The Structural Characterization and Antifreeze Mechanism. Biomacromolecules. 2024, 25, 3877–3892. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xiao, L.; Liu, R. Response of arbuscular mycorrhizal fungi and phosphorus solubilizing bacteria to remediation abandoned solid waste of coal mine. Int. J. Coal Sci. Technol. 2019, 6, 603–610. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J. Copper bioavailability, uptake, toxicity, and tolerance in plants: A comprehensive review. Sci. Total Environ. 2019, 658, 618–629. [Google Scholar]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.E.; Duan, C. Effects of heavy metals on stomata in plants: A review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ullah, I.; Hasnain, S. Microbial manipulation of auxins and cytokinins in plants. In Auxins and Cytokinins in Plant Biology: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 61–72. [Google Scholar]
- Xiong, J.; Wen, D.; Zhou, H.; Chen, R.; Wang, H.; Wang, C.; Wu, L. Occurrence of aflatoxin M1 in yogurt and milk in central-eastern China and the risk of exposure in milk consumers. Food Control. 2022, 137, 108928. [Google Scholar] [CrossRef]
- Wijeratne, E.K.; Turbyville, T.J.; Zhang, Z.; Bigelow, D.; Pierson, L.S.; VanEtten, H.D.; Whitesell, L.; Canfield, L.M.; Gunatilaka, A.L. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the sonoran desert. J. Nat. Prod. 2003, 66, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of soluble sugars in metabolism and sensing under abiotic stress. Plant Growth Regulators: Signalling under Stress Conditions; Springer: Cham, Switzerland, 2021; pp. 305–334. [Google Scholar]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 2016, 6, 35393. [Google Scholar] [CrossRef]
- Akbar, M.; El-Sabrout, A.M.; Shokralla, S.; Mahmoud, E.A.; Elansary, H.O.; Akbar, F.; Din, B.U.; Haroon, U.; Ali, M.; Saleem, H.; et al. Preservation and recovery of metal-tolerant fungi from industrial soil and their application to improve germination and growth of wheat. Sustainability 2022, 14, 5531. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Qin, X.; Kaiduan, Z.; Fan, Y.; Fang, H.; Yong, N.; Wu, X. The Bacterial MtrAB Two-Component System Regulates the Cell Wall Homeostasis Responding to Environmental Alkaline Stress. Microbiol. Spectr. 2022, 10, e02311-22. [Google Scholar] [CrossRef]
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Soil fungi for bioremediation of pesticide toxicants: A perspective. Geomicrobiol. J. 2022, 39, 352–372. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Murad, W. Phytohormones producing rhizobacterium alleviates chromiumtoxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Waseem, A.; Arshad, J.; Iqbal, F.; Sajjad, A.; Mehmood, Z.; Murtaza, G. Pollution status of Pakistan: A retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Res. Int. 2014, 2014, 813206. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.-H.; Jung, Y.; Kim, M.; Kim, S.; Kim, B.K.; Lim, Y.-W. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007, 57, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Seema, N.; Hamayun, M.; Hussain, A.; Shah, M.; Irshad, M.; Qadir, M.; Ali, S. Endophytic Fusarium proliferatum Reprogrammed Phytohormone Production and Antioxidant System of Oryza sativa under Drought Stress. Agronomy 2023, 13, 873. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Juang, K.W.; Lo, Y.C.; Chen, T.H.; Chen, B.C. Effects of copper on root morphology, cations accumulation, and oxidative stress of grapevine seedlings. Bull. Environ. Contam. Toxicol. 2019, 102, 873–879. [Google Scholar] [CrossRef]
- Rahim, W.; Khan, M.; Al Azzawi, T.N.I.; Pande, A.; Methela, N.J.; Ali, S.; Imran, M.; Lee, D.S.; Lee, G.M.; Mun, B.G.; et al. Exogenously applied sodium nitroprusside mitigates lead toxicity in rice by regulating antioxidants and metal stress-related transcripts. Int. J. Mol. Sci. 2022, 23, 9729. [Google Scholar] [CrossRef]
- Torres, R.C.; Parcon, M.R.V.; Esmundo, H.J.N.; Canillo, D.C.P.; Ramil, C.C. Antioxidant activity and phytochemical constituents of Philippine Clitoria ternatea flowers as a potential therapeutic agent against infectious diseases. Issues Biol. Sci. Pharm. Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Debnath, C.; Vijayaraghavan, R.; Muthuraj, M. Trends in bioremediation of heavy metal contaminations. Environ. Eng. Res. 2023, 28, 220631. [Google Scholar] [CrossRef]
- Chi, M.H.; Park, S.Y.; Lee, Y.H. A quick and safe method for fungal DNA extraction. Plant Pathol. J. 2009, 25, 108–111. [Google Scholar] [CrossRef]
- Asim, S.; Hussain, A.; Murad, W.; Hamayun, M.; Iqbal, A.; Rehman, H.; Tawab, A.; Irshad, M.; Alataway, A.; Dewidar, A.Z.; et al. Endophytic Fusarium oxysporum GW controlling weed and an effective biostimulant for wheat growth. Front. Plant Sci. 2022, 13, 922343. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.; Dash, A.; Das, A.P. Detection of environmental microfiber pollutants through vibrational spectroscopic techniques: Recent advances of environmental monitoring and future prospects. Crit. Rev. Anal. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alici, E.H.; Arabaci, G. Determination of SOD, POD, PPO and cat enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Koren kov, V.D.; Bylina, N.N. Copper in soils and its bioavailability. Environ. Sci. Pollut. Res. 2009, 16, 579–585. [Google Scholar]
- Gao, H.; Guo, W.; Wang, Q.; Zhang, L.; Zhu, M.; Zhu, T.; Gu, Q.; Wang, W.; Li, D. Aspulvinones from a mangrove rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorganic Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizating the principle of protein dyes binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, H.; Guo, C.; Su, F.; Li, L. Effects of Cadmium on Superoxide Dismutase Activity in Reed Leaves. Nat. Environ. Pollut. Technol. 2022, 21, 1389–1393. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. A fruitful decade of bacterial ACC deaminase biotechnology: A pragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 34, 109–129. [Google Scholar] [CrossRef]







| Condition | Treatments | µg/mL |
|---|---|---|
| Normal condition | Control | Untreated |
| Copper and lead stress | Pb25 | 25 µg/mL |
| Pb75 | 75 µg/mL | |
| Cu100 | 100 µg/mL | |
| Cu200 | 200 µg/mL | |
| Pb25 + Cu100 | 25 µg/mL + 100 µg/mL | |
| Pb75 + Cu200 | 75 µg/mL + 200 µg/mL | |
| Fungal inoculation | NB (Rhizofungus) | 5 g |
| NB + Pb25 | 25 µg/mL | |
| NB + Pb75 | 75 µg/mL | |
| NB + Cu100 | 100 µg/mL | |
| NB + Cu200 | 200 µg/mL | |
| NB + Pb25 + Cu100 | 25 µg/mL + 100 µg/mL | |
| NB + Pb75 + Cu200 | 75 µg/mL + 200 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilawar, N.; Hamayun, M.; Iqbal, A.; Lee, B.; Ali, S.; Ahmad, A.; Alrefaei, A.F.; Faraj, T.K.; Kim, H.-Y.; Hussain, A. Rhizofungus Aspergillus terreus Mitigates Heavy Metal Stress-Associated Damage in Triticum aestivum L. Plants 2024, 13, 2643. https://doi.org/10.3390/plants13182643
Dilawar N, Hamayun M, Iqbal A, Lee B, Ali S, Ahmad A, Alrefaei AF, Faraj TK, Kim H-Y, Hussain A. Rhizofungus Aspergillus terreus Mitigates Heavy Metal Stress-Associated Damage in Triticum aestivum L. Plants. 2024; 13(18):2643. https://doi.org/10.3390/plants13182643
Chicago/Turabian StyleDilawar, Naveen, Muhammad Hamayun, Amjad Iqbal, Bokyung Lee, Sajid Ali, Ayaz Ahmad, Abdulwahed Fahad Alrefaei, Turki Kh. Faraj, Ho-Youn Kim, and Anwar Hussain. 2024. "Rhizofungus Aspergillus terreus Mitigates Heavy Metal Stress-Associated Damage in Triticum aestivum L." Plants 13, no. 18: 2643. https://doi.org/10.3390/plants13182643
APA StyleDilawar, N., Hamayun, M., Iqbal, A., Lee, B., Ali, S., Ahmad, A., Alrefaei, A. F., Faraj, T. K., Kim, H.-Y., & Hussain, A. (2024). Rhizofungus Aspergillus terreus Mitigates Heavy Metal Stress-Associated Damage in Triticum aestivum L. Plants, 13(18), 2643. https://doi.org/10.3390/plants13182643










