Effects of Acmella radicans Invasion on Soil Seed Bank Community Characteristics in Different Habitats
Abstract
1. Introduction
2. Results
2.1. Plant Species and Seed Density
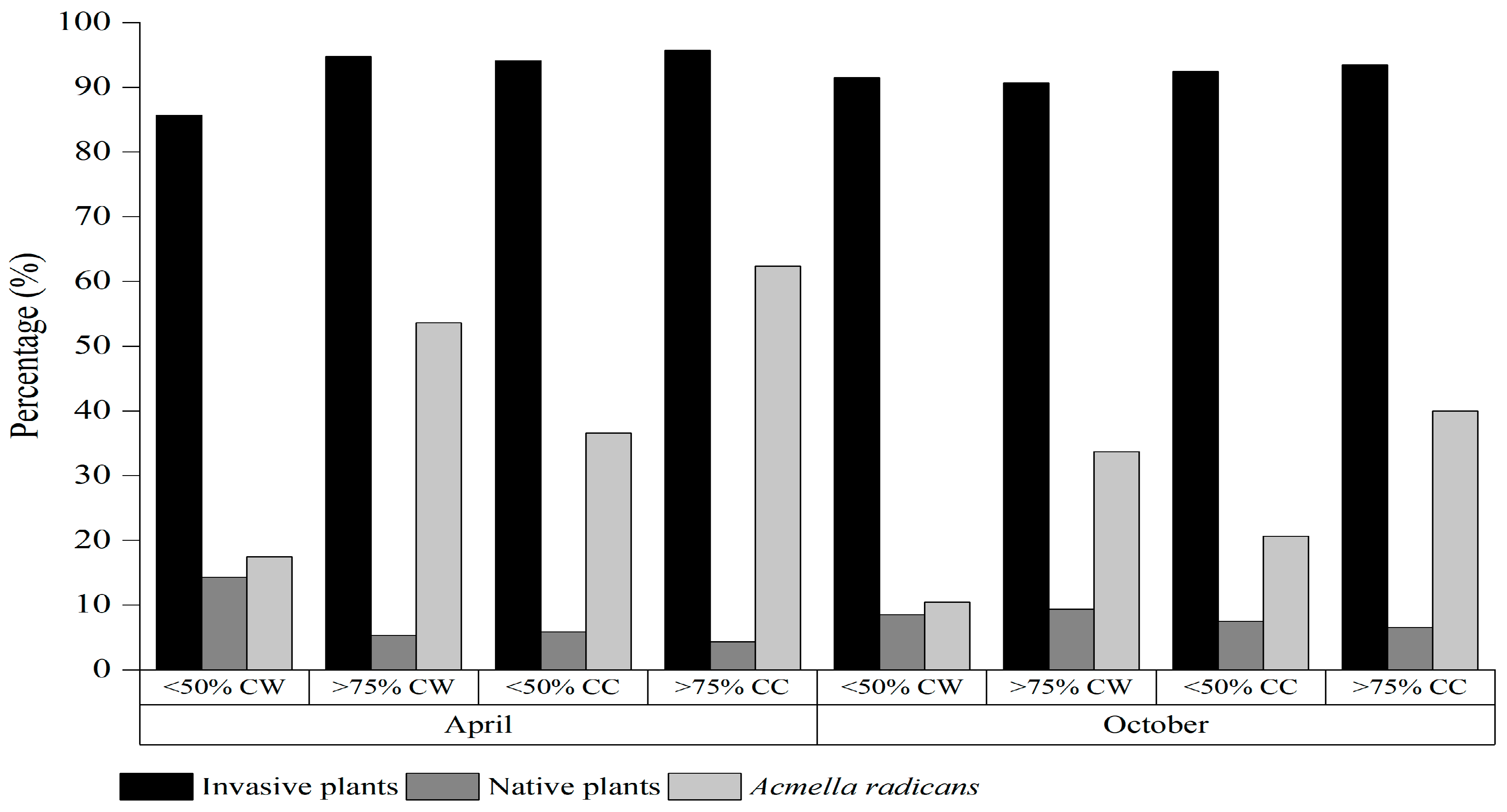
2.2. Soil Seed Germination and Distribution of Acmella radicans
2.3. Effects of Acmella radicans on Soil Plant Species Diversity
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Soil Collection
4.3. Germination Tests
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, S.; Jiang, G. The research development of soil seed bank and several hot topics. Acta Phytoecol. Sin. 2003, 27, 552–560. [Google Scholar] [CrossRef]
- Yu, S.; Chen, H.; Lang, N. The classification systems of soil seed banks and seed persistence in soil. Acta Ecol. Sin. 2007, 27, 2099–2108. [Google Scholar]
- Liu, R.; Zhan, J.; Shi, Z.; Chen, L. Soil seed bank and its correlations with aboveground vegetation and environmental factors in water level fluctuating zone of Danjiangkou Reservoir, Central China. Chin. J. Appl. Ecol. 2013, 24, 801–808. [Google Scholar]
- Chu, H.; Zhang, C.; Dong, Q.; Shang, Z.; Degen, A.; Yang, X.; Yu, Y.; Yang, Z.; Zhang, Y. The effect of grazing intensity and season on the soil seed bank and its relation with above-ground vegetation on the alpine steppe. Agr. Ecosyst. Environ. 2019, 285, 106622. [Google Scholar] [CrossRef]
- Hou, Z.; Xie, Y.; Yu, X.; Li, F. Characteristics of soil seed banks in different water level areas after returning farmland into lake in Qingshanyuan of Dongting Lake. Chin. J. Appl. Ecol. 2009, 20, 1323–1328. [Google Scholar]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W.; et al. A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Gioria, M.; Le Roux, J.J.; Hirsch, H.; Moravcová, L.; Pyšek, P. Characteristics of the soil seed bank of invasive and non-invasive plants in their native and alien distribution range. Biol. Invasions 2019, 21, 2313–2332. [Google Scholar] [CrossRef]
- Marques, A.R.; Costa, C.F.; Atman, A.P.F.; Garcia, Q.S. Germination characteristics and seedbank of the alien species Leucaena leucocephala (Fabaceae) in Brazilian forest: Ecological implications. Weed Res. 2014, 54, 576–583. [Google Scholar] [CrossRef]
- Bagga, J.; Deshmukh, U.B. Acmella radicans (Jacquin) R.K. Jansen (Asteraceae)—A new distributional plant record for Jharkhand State (India). J. New Biol. Rep. 2018, 7, 24–27. [Google Scholar]
- Panyadee, P.; Inta, A. Taxonomy and ethnobotany of Acmella (Asteraceae) in Thailand. Biodiversitas 2022, 23, 2177–2186. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.A.; Hossain, G.M.; Jakaria, M.; Rahim, M.A. Acmella radicans (Jacq.) R.K. Jansen (Asteraceae)—A new angiosperm record. J. Biol. Sci. 2016, 5, 87–93. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Li, H.; Ma, J. Acmella radicans var. debilis (Kunth) R.K. Jansen (Asteraceae), a newly naturalized plant in China. J. Trop. Subtrop. Bot. 2015, 23, 643–646. [Google Scholar] [CrossRef]
- Yang, K.; Wu, X.; Zheng, F.; Fan, Z.; Wu, R.; Xu, G.; Yang, Y.; Zhang, F.; Yang, S.; Shen, S. Allelopathic effects of aqueous extracts of the invasive plant Acmella radicans on seed germination and seedling growth of four weeds. Acta Agrestia Sin. 2023, 31, 3757–3765. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Wu, X.; Zheng, F.; Xu, G.; Yang, S.; Jin, G.; Clements, D.R.; Shen, S.; Zhang, F. Allelopathic potential and chemical composition of essential oil from the invasive plant Acmella radicans. Agronomy 2024, 14, 342. [Google Scholar] [CrossRef]
- Shen, S.; Xu, G.; Zhang, F.; Li, T.; Jin, G.; Zhang, Y. Characteristics of the seed banks and seedling banks of Mikania micrantha-invaded soils different in type of habitat. J. Eco. Rural Environ. 2013, 29, 483–488. [Google Scholar]
- Nan, K.; Wu, Q.; Hu, R.; Chen, S.; Ding, B. Seasonal dynamics of species composition of community and soil seed bank invaded by Oenothera laciniata Hill in Wenzhou, Zhejiang. J. Trop. Subtrop. Bot. 2009, 17, 535–542. [Google Scholar]
- Wang, S.; Gao, X.; Wang, J.; Dang, W. Characteristics of soil seed banks of crofton weed and their effects on seedlings. Chin. J. Plant Ecol. 2009, 33, 380–386. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Hao, G.; Kang, B.; Gao, Y.; Li, H. Effects of Amaranthus palmeri on soil seed bank community characteristics in abandoned cultivated land. J. Ecol. Rural Environ. 2023, 39, 1188–1195. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P. The legacy of plant invasions: Changes in the soil seed bank of invaded plant communities. BioScience 2016, 66, 40–53. [Google Scholar] [CrossRef]
- Wang, R.; Dong, H.; Liu, T.; Zhao, W.; Wang, H.; Ma, Q.; Liu, Y. Characteristics of soil seed banks and their contribution to aboveground population of invasive weeds Ambrosia artemisiifolia and A. trifid. J. Shihezi Univ. 2021, 39, 72–79. [Google Scholar] [CrossRef]
- Dang, W.; Gao, X.; Wang, J.; Li, A. Soil seed bank traits in an area invaded by Eupatorium adenophorum. Biodivers. Sci. 2008, 16, 126–132. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W. Persistent soil seed bank of Eupatorium adenophorum. Chin. J. Plant Ecol. 2004, 28, 768–772. [Google Scholar] [CrossRef]
- Salomé-Díaz, J.; Golubov, J.; Eguiarte, L.E.; Búrquez, A. Difference in germination traits between congeneric native and exotic species may affect invasion. Plants 2024, 13, 478. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, W.; Li, B.; Chen, J. Phenotypic plasticity and invasiveness of alien plants. Biodivers. Sci. 2004, 12, 447–455. [Google Scholar] [CrossRef]
- Zenni, R.D.; da Cunha, W.L.; Musso, C.; de Souza, J.V.; Nardoto, G.B.; Miranda, H.S. Synergistic impacts of co-occurring invasive grasses cause persistent effects in the soil-plant system after selective removal. Funct. Ecol. 2020, 34, 1102–1112. [Google Scholar] [CrossRef]
- Zheng, S.; Dai, L.; Lin, P.; Chen, X.; Ding, B. Species composition of community invaded by Spermacoce latifolia Aubl. and seasonal dynamics of its soil seed bank. J. Zhejiang Univ. (Agric. Life Sci.) 2009, 35, 677–685. [Google Scholar] [CrossRef]
- Robertson, S.G.; Hickman, K.R. Aboveground plant community and seed bank composition along an invasion gradient. Plant Ecol. 2012, 213, 1461–1475. [Google Scholar] [CrossRef]
- Shen, S.; Xu, G.; Clements, D.R.; Jin, G.; Liu, S.; Zhang, F.; Yang, Y.; Chen, A.; Kato-Noguchi, H. Effects of invasive plant Mikania micrantha on plant community and diversity in farming systems. Asian J. Plant Sci. 2015, 14, 27–33. [Google Scholar] [CrossRef]
- Lembrechtsa, J.J.; Pauchard, A.; Lenoird, J.; Nuñeze, M.A.; Geronf, C.; Vena, A.; Bravo-Monasteriob, P.; Tenebg, E.; Nijsa, I.; Ann Milbau, A. Disturbance is the key to plant invasions in cold environments. Proc. Natl. Acad. Sci. USA 2016, 113, 14061–14066. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lu, B.; Lu, X.; Huang, S. On reproductive strategies of invasive plants and their impacts on native plants. Biodivers. Sci. 2018, 26, 457–467. [Google Scholar] [CrossRef]
- Angert, A.L.; Huxman, T.E.; Chesson, P.; Venable, D.L. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl. Acad. Sci. USA 2009, 106, 11641–11645. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, Z.; Hatier, J.H.B.; Liu, B. The vertical distribution of soil seed bank and its restoration implication in an active sand dune of northeastern Inner Mongolia, China. Land Degrad. Dev. 2016, 27, 305–315. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Smagacz, J.; Kwiatkowski, C.A.; Harasim, E.; Woźniak, A. Weed flora and soil seed bank composition as affected by tillage system in three-year crop rotation. Agriculture 2020, 10, 186. [Google Scholar] [CrossRef]
- Skuodienė, R.; Matyžiūtė, V.; Aleinikovienė, J.; Frercks, B.; Repšienė, R. Seed bank community under different-intensity agrophytocenoses on Hilly Terrain in Lithuania. Plants 2023, 12, 1084. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ma, K.; Liu, Y. Measurement of biotic community diversity I α diversity (Part 2). Biodivers. Sci. 1994, 2, 231–239. [Google Scholar] [CrossRef]

| Family | Scientific Name | Life Form | Origin |
|---|---|---|---|
| Amaranthaceae | Chenopodium ficifolium L. | Annual herb | China |
| Asteraceae | Acmella radicans (Jacquin) R.K. Jansen | Annual herb | Central America and Mexico |
| Ageratina adenophora (Spreng.) R. M. King and H. Rob | Perennial herb | Mexico and Costa Rica | |
| Ageratum conyzoides L. | Annual herb | Tropical America | |
| Bidens pilosa L. | Annual herb | Tropical America | |
| Crassocephalum crepidioides (Benth.) S. Moore | Annual herb | Tropical Africa | |
| Erigeron canadensis L. | Annual herb | North America | |
| Galinsoga quadriradiata Ruiz et Pav. | Annual herb | Tropical America | |
| Gamochaeta pensylvanica (Willd.) Cabrera | Annual herb | North America | |
| Laggera crispata (Vahl) Hepper & J. R. I. Wood | Perennial herb | China | |
| Brassicaceae | Cardamine occulta Hornem. | Annual herb | China |
| Caryophyllaceae | Stellaria media (L.) Vill. | Annual herb | Europe |
| Commelinaceae | Commelina benghalensis L. | Perennial herb | China |
| Cyperaceae | Cyperus iria L. | Annual herb | China |
| Linderniaceae | Lindernia procumbens (Krock.) Borbás | Annual herb | China |
| Onagraceae | Oenothera rosea L’Hér. ex Aiton. | Perennial herb | South and Tropical America |
| Oxalidaceae | Oxalis corniculata L. | Annual/Perennial herb | China |
| Phyllanthaceae | Phyllanthus urinaria L. | Annual herb | China |
| Poaceae | Chloris virgata Sw. | Annual herb | China |
| Digitaria sanguinalis (L.) Scop. | Annual herb | China | |
| Eleusine indica (L.) Gaertn. | Annual herb | North America | |
| Oplismenus undulatifolius (Ard.) Roemer & Schuit. | Perennial herb | China | |
| Poa annua L. | Annual herb | China | |
| Polypogon fugax Nees ex Steud. | Annual herb | China | |
| Polygonaceae | Polygonum aviculare L. | Annual herb | China |
| Ranunculaceae | Ranunculus japonicus Thunb. | Perennial herb | China |
| Rubiaceae | Spermacoce alata Aubl. | Perennial herb | South and Tropical America |
| Solanaceae | Solanum nigrum L. | Annual herb | China |
| Scientific Name | Wasteland | Cultivated Land | ||
|---|---|---|---|---|
| <50% Cover | >75% Cover | <50% Cover | >75% Cover | |
| Acmella radicans (Jacquin) R.K. Jansen | 7750.0 | 22,325.0 | 18,275.0 | 38,775.0 |
| Ageratina adenophora (Spreng.) R. M. King and H. Rob | 50.0 | 0.0 | 0.0 | 0.0 |
| Ageratum conyzoides L. | 11,900.0 | 8575.0 | 16,650.0 | 12,100.0 |
| Bidens pilosa L. | 2350.0 | 925.0 | 1150.0 | 825.0 |
| Cardamine occulta Hornem. | 125.0 | 50.0 | 100.0 | 200.0 |
| Chenopodium ficifolium L. | 125.0 | 25.0 | 50.0 | 50.0 |
| Chloris virgata Sw. | 1175.0 | 525.0 | 225.0 | 0.0 |
| Commelina benghalensis L. | 150.0 | 25.0 | 175.0 | 275.0 |
| Crassocephalum crepidioides (Benth.) S. Moore | 250.0 | 25.0 | 75.0 | 50.0 |
| Cyperus iria L. | 450.0 | 50.0 | 375.0 | 225.0 |
| Digitaria sanguinalis (L.) Scop. | 725.0 | 400.0 | 75.0 | 75.0 |
| Eleusine indica (L.) Gaertn. | 1650.0 | 1100.0 | 1275.0 | 975.0 |
| Erigeron canadensis L. | 275.0 | 25.0 | 125.0 | 150.0 |
| Galinsoga quadriradiata Ruiz et Pav. | 775.0 | 300.0 | 625.0 | 500.0 |
| Gamochaeta pensylvanica (Willd.) Cabrera | 11,750.0 | 5750.0 | 7450.0 | 4925.0 |
| Laggera crispata (Vahl) Hepper & J. R. I. Wood | 300.0 | 50.0 | 100.0 | 50.0 |
| Lindernia procumbens (Krock.) Borbás | 100.0 | 175.0 | 100.0 | 75.0 |
| Oenothera rosea L’Hér. ex Aiton. | 100.0 | 0.0 | 0.0 | 0.0 |
| Oplismenus undulatifolius (Ard.) Roemer & Schuit. | 625.0 | 125.0 | 300.0 | 400.0 |
| Oxalis corniculata L. | 550.0 | 375.0 | 650.0 | 625.0 |
| Phyllanthus urinaria L. | 275.0 | 75.0 | 75.0 | 75.0 |
| Poa annua L. | 700.0 | 100.0 | 275.0 | 125.0 |
| Polypogon fugax Nees ex Steud. | 600.0 | 125.0 | 200.0 | 200.0 |
| Polygonum aviculare L. | 75.0 | 25.0 | 100.0 | 125.0 |
| Ranunculus japonicus Thunb. | 50.0 | 0.0 | 0.0 | 0.0 |
| Solanum nigrum L. | 275.0 | 75.0 | 150.0 | 200.0 |
| Spermacoce alata Aubl. | 675.0 | 400.0 | 1375.0 | 1125.0 |
| Stellaria media (L.) Vill. | 225.0 | 25.0 | 125.0 | 100.0 |
| Scientific Name | Wasteland | Cultivated Land | ||
|---|---|---|---|---|
| <50% Cover | >75% Cover | <50% Cover | >75% Cover | |
| Acmella radicans (Jacquin) R.K. Jansen | 800.0 | 1550.0 | 1850.0 | 2750.0 |
| Ageratina adenophora (Spreng.) R. M. King and H. Rob | 50.0 | 0.0 | 0.0 | 0.0 |
| Ageratum conyzoides L. | 2800.0 | 650.0 | 3425.0 | 1950.0 |
| Bidens pilosa L. | 0.0 | 0.0 | 0.0 | 0.0 |
| Cardamine occulta Hornem. | 25.0 | 0.0 | 50.0 | 25.0 |
| Chenopodium ficifolium L. | 0.0 | 25.0 | 0.0 | 0.0 |
| Chloris virgata Sw. | 75.0 | 100.0 | 0.0 | 0.0 |
| Commelina benghalensis L. | 0.0 | 0.0 | 0.0 | 0.0 |
| Crassocephalum crepidioides (Benth.) S. Moore | 50.0 | 75.0 | 50.0 | 100.0 |
| Cyperus iria L. | 75.0 | 25.0 | 50.0 | 50.0 |
| Digitaria sanguinalis (L.) Scop. | 25.0 | 50.0 | 25.0 | 75.0 |
| Eleusine indica (L.) Gaertn. | 400.0 | 300.0 | 250.0 | 200.0 |
| Erigeron canadensis L. | 50.0 | 0.0 | 100.0 | 50.0 |
| Galinsoga quadriradiata Ruiz et Pav. | 150.0 | 50.0 | 125.0 | 125.0 |
| Gamochaeta pensylvanica (Willd.) Cabrera | 2525.0 | 1575.0 | 2300.0 | 1150.0 |
| Laggera crispata (Vahl) Hepper & J. R. I. Wood | 25.0 | 0.0 | 50.0 | 0.0 |
| Lindernia procumbens (Krock.) Borbás | 100.0 | 75.0 | 100.0 | 75.0 |
| Oenothera rosea L’Hér. ex Aiton. | 50.0 | 75.0 | 0.0 | 0.0 |
| Oplismenus undulatifolius (Ard.) Roemer & Schuit. | 150.0 | 25.0 | 100.0 | 0.0 |
| Oxalis corniculata L. | 25.0 | 75.0 | 100.0 | 100.0 |
| Phyllanthus urinaria L. | 0.0 | 0.0 | 0.0 | 0.0 |
| Poa annua L. | 100.0 | 50.0 | 150.0 | 50.0 |
| Polypogon fugax Nees ex Steud. | 0.0 | 0.0 | 0.0 | 0.0 |
| Polygonum aviculare L. | 0.0 | 25.0 | 50.0 | 25.0 |
| Ranunculus japonicus Thunb. | 50.0 | 0.0 | 0.0 | 0.0 |
| Solanum nigrum L. | 0.0 | 0.0 | 0.0 | 50.0 |
| Spermacoce alata Aubl. | 75.0 | 75.0 | 175.0 | 125.0 |
| Stellaria media (L.) Vill. | 50.0 | 0.0 | 25.0 | 0.0 |
| April | October | ||||
|---|---|---|---|---|---|
| Group | N | Mean rank of seed density in invasive plants | Group | N | Mean rank of seed density in invasive plants |
| <50% cover of wasteland | 4 | 2.63 | <50% cover of wasteland | 4 | 10.00 |
| >75% cover of wasteland | 4 | 6.38 | >75% cover of wasteland | 4 | 2.50 |
| <50% cover of cultivated land | 4 | 10.5 | <50% cover of cultivated land | 4 | 14.50 |
| >75% cover of cultivated land | 4 | 14.5 | >75% cover of cultivated land | 4 | 7.00 |
| Kruskal–Wallis test | 13.988 | Kruskal–Wallis test | 13.500 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.004 | (Chi-square approximation) |
| Group | N | Mean rank of seed density in native plants | Group | N | Mean rank of seed density in native plants |
| <50% cover of wasteland | 4 | 14.50 | <50% cover of wasteland | 4 | 11.00 |
| >75% cover of wasteland | 4 | 4.25 | >75% cover of wasteland | 4 | 6.50 |
| <50% cover of cultivated land | 4 | 8.25 | <50% cover of cultivated land | 4 | 9.63 |
| >75% cover of cultivated land | 4 | 7.00 | >75% cover of cultivated land | 4 | 6.88 |
| Kruskal–Wallis test | 9.963 | Kruskal–Wallis test | 2.558 | ||
| p | 0.019 | (Chi-square approximation) | p | 0.465 | (Chi-square approximation) |
| Group | N | Mean rank of seed density in Acmella radicans | Group | N | Mean rank of seed density in Acmella radicans |
| <50% cover of wasteland | 4 | 2.50 | <50% cover of wasteland | 4 | 2.50 |
| >75% cover of wasteland | 4 | 10.5 | >75% cover of wasteland | 4 | 6.50 |
| <50% cover of cultivated land | 4 | 6.50 | <50% cover of cultivated land | 4 | 10.50 |
| >75% cover of cultivated land | 4 | 14.50 | >75% cover of cultivated land | 4 | 14.50 |
| Kruskal–Wallis test | 14.159 | Kruskal–Wallis test | 14.264 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.003 | (Chi-square approximation) |
| April | October | ||||
|---|---|---|---|---|---|
| Group | N | Mean rank of seed density in 0–5 cm layer | Group | N | Mean rank of seed density in 0–5 cm layer |
| <50% cover of wasteland | 4 | 2.50 | <50% cover of wasteland | 4 | 2.50 |
| >75% cover of wasteland | 4 | 10.50 | >75% cover of wasteland | 4 | 9.50 |
| <50% cover of cultivated land | 4 | 6.50 | <50% cover of cultivated land | 4 | 7.50 |
| >75% cover of cultivated land | 4 | 14.50 | >75% cover of cultivated land | 4 | 14.50 |
| Kruskal–Wallis test | 14.118 | Kruskal–Wallis test | 13.234 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.004 | (Chi-square approximation) |
| Group | N | Mean rank of seed density in 5–10 cm layer | Group | N | Mean rank of seed density in native plants |
| <50% cover of wasteland | 4 | 2.50 | <50% cover of wasteland | 4 | 3.13 |
| >75% cover of wasteland | 4 | 6.50 | >75% cover of wasteland | 4 | 5.88 |
| <50% cover of cultivated land | 4 | 10.50 | <50% cover of cultivated land | 4 | 10.50 |
| >75% cover of cultivated land | 4 | 14.50 | >75% cover of cultivated land | 4 | 14.50 |
| Kruskal–Wallis test | 14.138 | Kruskal–Wallis test | 13.532 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.004 | (Chi-square approximation) |
| Group | N | Mean rank of seed density in 10–20 cm layer | Group | N | Mean rank of seed density in 10–20 cm layer |
| <50% cover of wasteland | 4 | 2.63 | <50% cover of wasteland | 4 | 4.50 |
| >75% cover of wasteland | 4 | 6.38 | >75% cover of wasteland | 4 | 4.50 |
| <50% cover of cultivated land | 4 | 10.50 | <50% cover of cultivated land | 4 | 11.50 |
| >75% cover of cultivated land | 4 | 14.50 | >75% cover of cultivated land | 4 | 13.50 |
| Kruskal–Wallis test | 13.988 | Kruskal–Wallis test | 13.492 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.004 | (Chi-square approximation) |
| April | October | ||||
|---|---|---|---|---|---|
| Group | N | Mean rank of seed species richness (S) | Group | N | Mean rank of species richness (S) |
| <50% cover of wasteland | 4 | 14.38 | <50% cover of wasteland | 4 | 12.63 |
| >75% cover of wasteland | 4 | 4.25 | >75% cover of wasteland | 4 | 7.5 |
| <50% cover of cultivated land | 4 | 8.38 | <50% cover of cultivated land | 4 | 8.75 |
| >75% cover of cultivated land | 4 | 7.00 | >75% cover of cultivated land | 4 | 5.13 |
| Kruskal–Wallis test | 10.017 | Kruskal–Wallis test | 5.407 | ||
| p | 0.018 | (Chi-square approximation) | p | 0.144 | (Chi-square approximation) |
| Group | N | Mean rank of Simpson index (D) | Group | N | Mean rank of Simpson index (D) |
| <50% cover of wasteland | 4 | 14.5 | <50% cover of wasteland | 4 | 8.25 |
| >75% cover of wasteland | 4 | 6.5 | >75% cover of wasteland | 4 | 12.00 |
| <50% cover of cultivated land | 4 | 10.5 | <50% cover of cultivated land | 4 | 9.00 |
| >75% cover of cultivated land | 4 | 2.5 | >75% cover of cultivated land | 4 | 4.75 |
| Kruskal–Wallis test | 14.118 | Kruskal–Wallis test | 4.699 | ||
| p | 0.03 | (Chi-square approximation) | p | 0.195 | (Chi-square approximation) |
| Group | N | Mean rank of Shannon–Wiener index (H) | Group | N | Mean rank of Shannon–Wiener index (H) |
| <50% cover of wasteland | 4 | 14.5 | <50% cover of wasteland | 4 | 10.75 |
| >75% cover of wasteland | 4 | 6.25 | >75% cover of wasteland | 4 | 11.00 |
| <50% cover of cultivated land | 4 | 10.5 | <50% cover of cultivated land | 4 | 7.50 |
| >75% cover of cultivated land | 4 | 2.75 | >75% cover of cultivated land | 4 | 4.75 |
| Kruskal–Wallis test | 13.787 | Kruskal–Wallis test | 4.654 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.199 | (Chi-square approximation) |
| Group | N | Mean rank of Pielou index (J) | Group | N | Mean rank of Pielou index (J) |
| <50% cover of wasteland | 4 | 14.5 | <50% cover of wasteland | 4 | 5.50 |
| >75% cover of wasteland | 4 | 6.5 | >75% cover of wasteland | 4 | 13.25 |
| <50% cover of cultivated land | 4 | 10.5 | <50% cover of cultivated land | 4 | 6.75 |
| >75% cover of cultivated land | 4 | 2.5 | >75% cover of cultivated land | 4 | 8.5 |
| Kruskal–Wallis test | 14.118 | Kruskal–Wallis test | 6.110 | ||
| p | 0.003 | (Chi-square approximation) | p | 0.106 | (Chi-square approximation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, K.; Zheng, F.; Xu, G.; Fan, Z.; Clements, D.R.; Yang, Y.; Yang, S.; Jin, G.; Zhang, F.; et al. Effects of Acmella radicans Invasion on Soil Seed Bank Community Characteristics in Different Habitats. Plants 2024, 13, 2644. https://doi.org/10.3390/plants13182644
Wu X, Yang K, Zheng F, Xu G, Fan Z, Clements DR, Yang Y, Yang S, Jin G, Zhang F, et al. Effects of Acmella radicans Invasion on Soil Seed Bank Community Characteristics in Different Habitats. Plants. 2024; 13(18):2644. https://doi.org/10.3390/plants13182644
Chicago/Turabian StyleWu, Xiaohan, Kexin Yang, Fengping Zheng, Gaofeng Xu, Zewen Fan, David Roy Clements, Yunhai Yang, Shaosong Yang, Guimei Jin, Fudou Zhang, and et al. 2024. "Effects of Acmella radicans Invasion on Soil Seed Bank Community Characteristics in Different Habitats" Plants 13, no. 18: 2644. https://doi.org/10.3390/plants13182644
APA StyleWu, X., Yang, K., Zheng, F., Xu, G., Fan, Z., Clements, D. R., Yang, Y., Yang, S., Jin, G., Zhang, F., & Shen, S. (2024). Effects of Acmella radicans Invasion on Soil Seed Bank Community Characteristics in Different Habitats. Plants, 13(18), 2644. https://doi.org/10.3390/plants13182644









