Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant wcr (White-Core Rice)
Abstract
1. Introduction
2. Results
2.1. Analysis of Phenotypic and Crop Traits of the wcr Mutant
2.2. Physicochemical Characteristics of Mature Grains of wcr Mutant
2.3. Gelatinization Characteristics and Amylopectin Structure Analysis of wcr
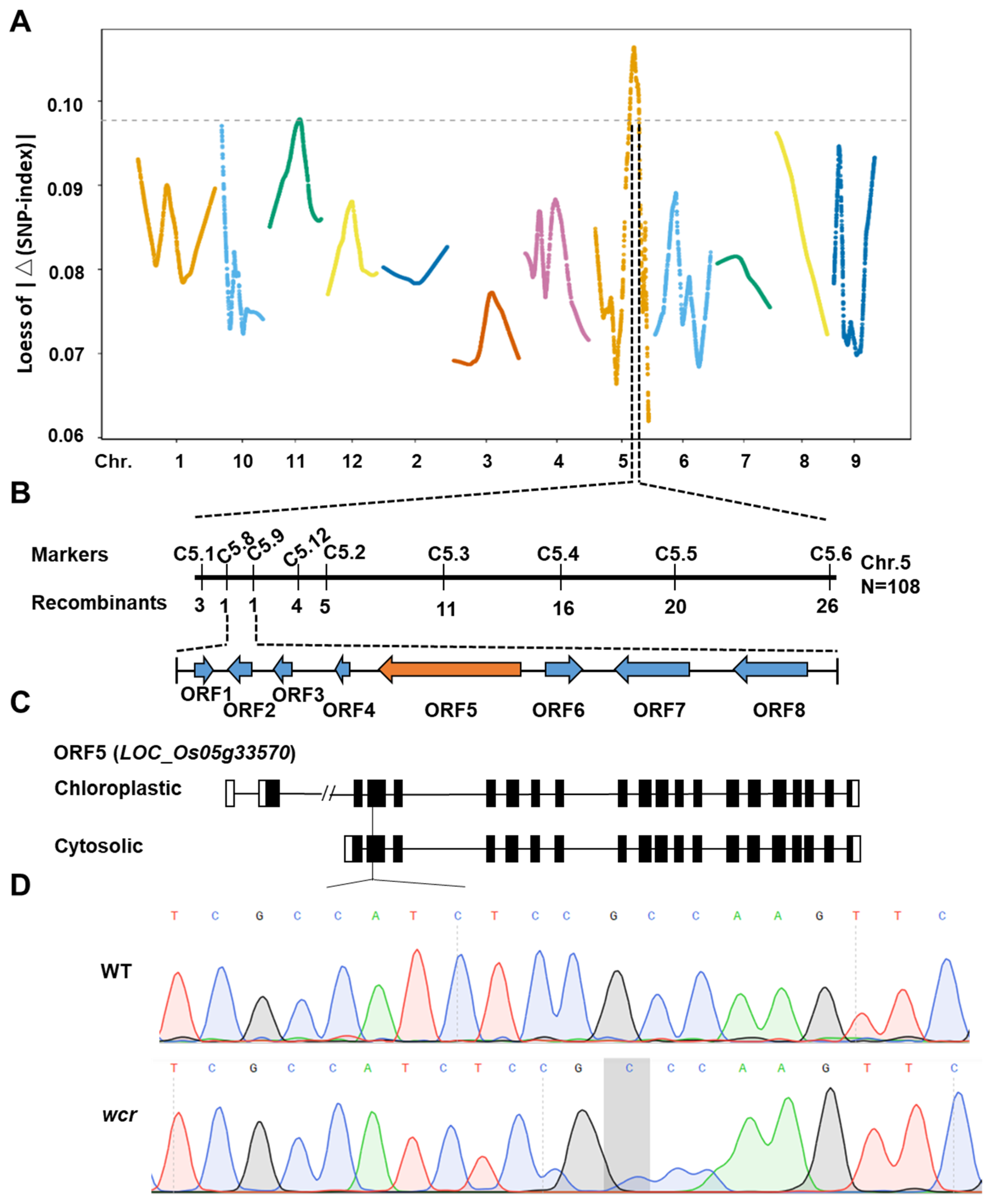
2.4. Genetic Analysis of the wcr Mutant
2.5. Fine Mapping of the wcr Gene
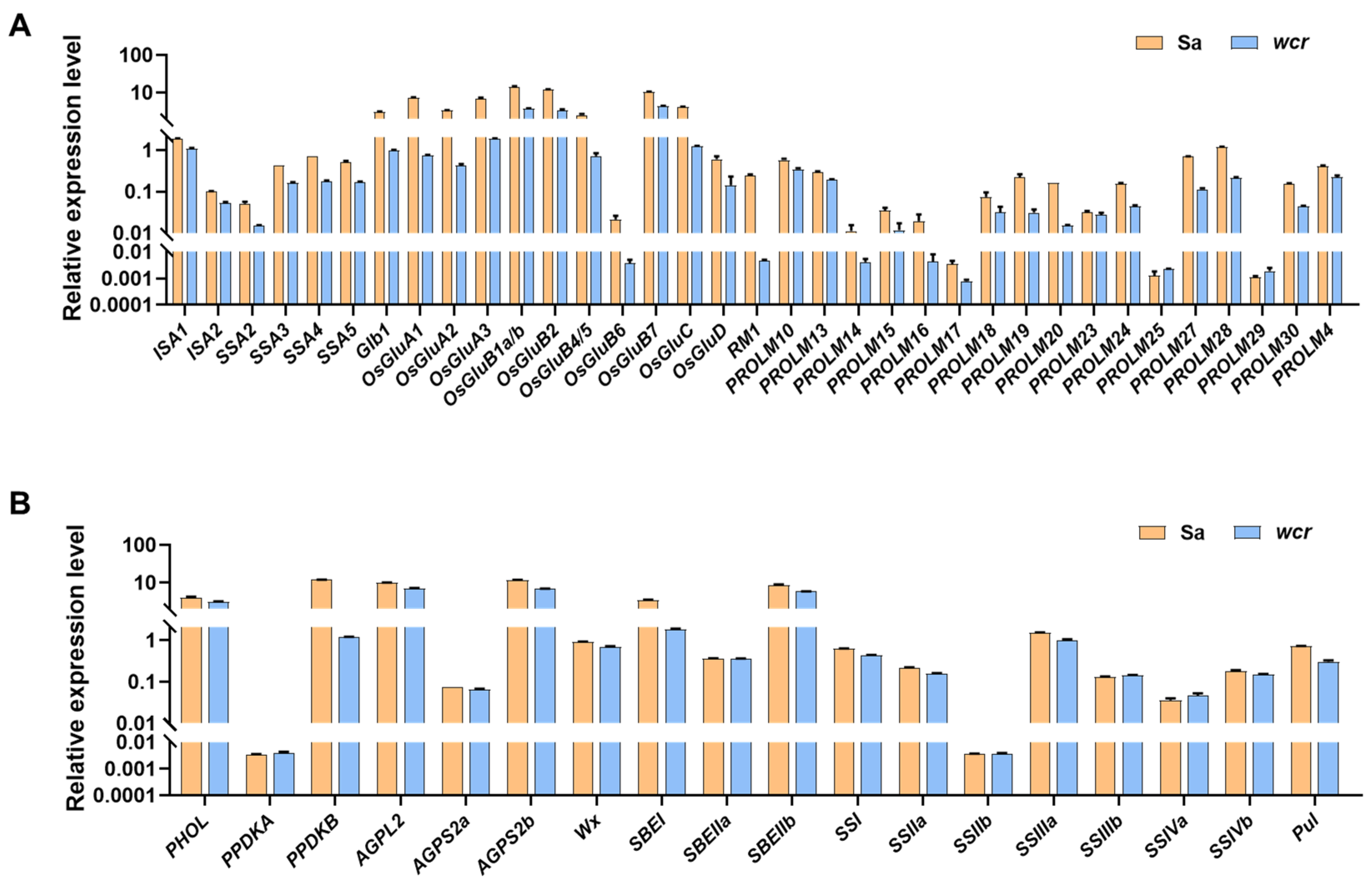
2.6. Gene Expression Analysis of Storage Substance-Related Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Field Design
4.2. Main Crop Characteristics of Rice Plants
4.3. Scanning Electron Microscopy Analysis
4.4. Protein Extraction and SDS-PAGE Analysis
4.5. Analysis of the Amylose and Total Starch Contents
4.6. Determination of Chain Length of Amylopectin
4.7. Genome Mapping of the wcr Locus
4.8. RNA Extraction, cDNA Preparation, and qRT-PCR
4.9. Data Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.M.; Wei, F.; Cheng, C.; Qian, Q. A historical review of hybrid rice breeding. J. Integr. Plant Biol. 2024, 66, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.Y.; Ding, C.Q.; Qian, Q. Molecular bases of rice grain size and quality for optimized productivity. Sci. Bull. 2023, 68, 314–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.S.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef] [PubMed]
- Long, W.H.; Dong, B.N.; Wang, Y.H.; Pan, P.Y.; Wang, Y.L.; Liu, L.L.; Chen, X.L.; Liu, X.; Liu, S.J.; Tian, Y.L.; et al. FLOURY ENDOSPERM 8, encoding the UDP-glucose pyro phosphorylase 1, affects the synthesis and structure of starch in rice endosperm. J. Plant Biol. 2017, 60, 513–522. [Google Scholar] [CrossRef]
- Tsuneo, K.; Ryutaro, M.; Shinjiro, O.; Yu, W.; Naohiro, A.; Akira, H. Evaluation of alleles at OsAGPS2, OsAGPL2, and OsSUT1 related to grain filling in rice in a common genetic background. Crop Sci. 2020, 61, 1154–1167. [Google Scholar]
- Bilal, C.; Shota, S.; Aytug, T.; Hiroaki, M.; Ryosuke, S.; Salvinder, S.; Naoko, C.; Yuko, H.; Naoko, F.; Seon-Kap, H.; et al. Analysis of the Rice ADP-Glucose Transporter (OsBT1) Indicates the Presence of Regulatory Processes in the Amyloplast Stroma That Control ADP-Glucose Flux into Starch. Plant Physiol. 2016, 170, 1271–1283. [Google Scholar]
- Yang, R.F.; Sun, C.L.; Bai, J.J.; Luo, Z.X.; Shi, B.; Zhang, J.M.; Yan, W.G.; Piao, Z.Z. A Putative Gene sbe3-rs for Resistant Starch Mutated from SBE3 for Starch Branching Enzyme in Rice (Oryza sativa L.). PLoS ONE 2012, 7, e43026. [Google Scholar] [CrossRef]
- Ryoo, N.; Yu, C.; Park, C.; Baik, M.; Park, I.M.; Cho, M.; Bhoo, S.H.; An, G.; Hahn, T.; Jeon, J. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 2007, 26, 1083–1095. [Google Scholar] [CrossRef]
- Satoh, H.; Shibahara, K.; Tokunaga, T.; Nishi, A.; Tasaki, M.; Hwang, S.; Okita, T.; Kaneko, N.; Fujita, N.; Yoshida, M.; et al. Mutation of the plastidial α-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 2008, 20, 1833–1849. [Google Scholar] [CrossRef]
- Sun, L.; Yang, D.L.; Kong, Y.; Chen, Y.; Li, X.Z.; Zeng, L.J.; Li, Q.; Wang, E.T.; He, Z.H. Sugar homeostasis mediated by cell wall invertase GRAIN INCOMPLETE FILLING 1 (GIF1) plays a role in pre-existing and induced defence in rice. Mol. Plant Pathol. 2014, 15, 161–173. [Google Scholar] [CrossRef]
- Huang, L.C.; Sreenivasulu, N.; Liu, Q.Q. Waxy editing: Old meets new. Trends Plant Sci. 2020, 25, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Ren, Y.L.; Dong, H.; Xie, C.; Zhao, L.; Wang, X.; Zhang, F.L.; Zhang, B.L.; Jiang, X.K.; Huang, Y.S.; et al. FLOURY ENDOSPERM24, a heat shock protein 101 (HSP101), is required for starch biosynthesis and endosperm development in rice. New Phytol. 2024, 242, 2635–2651. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, R.; Maekawa, M.; Kusano, M.; Kondo, H.; Fujita, N.; Kawagoe, Y.; Sakamoto, W. Amyloplast-Localized SUBSTANDARD STARCH GRAIN4 Protein Influences the Size of Starch Grains in Rice Endosperm. Plant Physiol. 2013, 164, 623–636. [Google Scholar] [CrossRef]
- Matsushima, R.; Maekawa, M.; Kusano, M.; Tomita, K.; Kondo, H.; Nishimura, H.; Crofts, N.; Fujita, N.; Sakamoto, W. Amyloplast Membrane Protein SUBSTANDARD STARCH GRAIN6 Controls Starch Grain Size in Rice Endosperm. Plant Physiol. 2016, 170, 1445–1459. [Google Scholar] [CrossRef]
- Yang, H.G.; Ren, Y.L.; Zhang, B.L.; Jin, J.; Du, F.L.; Shang, Z.Z.; Fu, Y.S.; Zhu, Y.; Wang, X.; Zhu, C.Y.; et al. SUBSTANDRAD STARCH GRAIN7 regulates starch grain size and endosperm development in rice. Plant Biotechnol. J. 2024, 1–15. [Google Scholar] [CrossRef]
- Long, W.H.; Wang, Y.L.; Zhu, S.S.; Jing, W.; Wang, Y.H.; Ren, Y.L.; Tian, Y.L.; Liu, S.J.; Liu, X.; Chen, L.M.; et al. FLOURY SHRUNKEN ENDOSPERM1 connects phospholipid metabolism and amyloplast development in rice. Plant Physiol. 2018, 177, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.T.; Tian, Y.L.; Zhu, J.P.; Wang, Y.L.; Jing, R.N.; Lei, J.; Sun, Y.L.; Yu, Y.F.; Li, J.F.; Chen, X.L.; et al. OsNDUFA9 encoding a mitochondrial complex I subunit is essential for embryo development and starch synthesis in rice. Plant Cell Rep. 2018, 37, 1667–1679. [Google Scholar] [CrossRef]
- Kim, S.R.; Yang, J.I.; Moon, S.; Ryu, C.H.; An, K.; Kim, K.M.; Yim, J.; An, G. Rice OGR1 encodes a pentatricopeptide repeat-DYW protein and is essential for RNA editing in mitochondria. Plant J. 2009, 59, 738–749. [Google Scholar] [CrossRef]
- Wu, M.M.; Ren, Y.L.; Cai, M.H.; Wang, Y.L.; Zhu, S.S.; Zhu, J.P.; Hao, Y.Y.; Teng, X.; Zhu, X.P.; Jing, R.N.; et al. Rice FLOURY ENDOSPERM10 encodes a pentatricopeptide repeat protein that is essential for the trans-splicing of mitochondrial nad1 intron 1 and endosperm development. New Phytol. 2019, 223, 736–750. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Wang, Y.L.; Wu, M.M.; Zhu, X.P.; Teng, X.; Sun, Y.L.; Zhu, J.P.; Zhang, Y.Y.; Jing, R.N.; Lei, J.; et al. The nuclear-localized PPR protein OsNPPR1 is important for mitochondrial function and endosperm development in rice. J. Exp. Bot. 2019, 70, 4705–4720. [Google Scholar] [CrossRef]
- Yu, M.Z.; Wu, M.M.; Ren, Y.L.; Wang, Y.H.; Li, J.F.; Lei, C.L.; Sun, Y.L.; Bao, X.H.; Wu, H.M.; Yang, H.; et al. Rice FLOURY ENDOSPERM 18 encodes a pentatricopeptide repeat protein required for 5’ processing of mitochondrial nad5 mRNA and endosperm development. J. Integr. Plant Biol. 2020, 63, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.L.; Tian, Y.L.; Teng, X.; Lv, Z.H.; Lei, J.; Duan, E.C.; Dong, H.; Yang, X.; Zhang, Y.Y.; et al. Rice FLOURY ENDOSPERM22, encoding a pentatricopeptide repeat protein, is involved in both mitochondrial RNA splicing and editing and is crucial for endosperm development. J. Integr. Plant Biol. 2022, 65, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, B.S.; Liu, X.X.; Ma, X.D.; Liu, Y.J.; Yao, H.Y.; Zhang, P.; Yin, J.L.; Wei, X.; Koh, H.J.; et al. Pyrophosphate-fructose 6-phosphate 1-phosphotransferase (PFP1) regulates starch biosynthesis and seed development via heterotetramer formation in rice (Oryza sativa L.). Plant Biotechnol. J. 2020, 18, 83–95. [Google Scholar] [CrossRef]
- Kang, H.G.; Park, S.; Matsuoka, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKb). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef]
- Cai, Y.C.; Li, S.F.; Jiao, G.A.; Sheng, Z.H.; Wu, Y.W.; Shao, G.N.; Xie, L.H.; Peng, C.; Xu, J.F.; Tang, S.Q.; et al. OsPK2 encodes a plastidic pyruvate kinase involved in rice endosperm starch synthesis, compound granule formation and grain filling. Plant Biotechnol. J. 2018, 16, 1878–1891. [Google Scholar] [CrossRef]
- Zhong, M.S.; Liu, X.; Liu, F.; Ren, Y.L.; Wang, Y.L.; Zhu, J.P.; Teng, X.; Duan, E.C.; Wang, F.; Zhang, H.; et al. FLOURY ENDOSPERM12 Encoding Alanine Aminotransferase 1 Regulates Carbon and Nitrogen Metabolism in Rice. J. Plant Biol. 2019, 62, 61–73. [Google Scholar] [CrossRef]
- You, X.M.; Zhang, W.W.; Hu, J.L.; Jing, R.N.; Cai, Y.; Feng, Z.M.; Kong, F.; Zhang, J.; Yan, H.G.; Chen, W.W.; et al. FLOURY ENDOSPERM15 encodes a glyoxalase I involved in compound granule formation and starch synthesis in rice endosperm. Plant Cell Rep. 2019, 38, 345–359. [Google Scholar] [CrossRef]
- Teng, X.; Zhong, M.S.; Zhu, X.P.; Wang, C.M.; Ren, Y.L.; Wang, Y.L.; Zhang, H.; Jiang, L.; Wang, D.; Hao, Y.Y.; et al. FLOURY ENDOSPERM16 encoding a NAD-dependent cytosolic malate dehydrogenase plays an important role in starch synthesis and seed development in rice. Plant Biotechnol. J. 2019, 17, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Ji, Y.; Zhao, W.Y.; Niu, H.Y.; Yang, X.; Jiang, X.K.L.; Zhang, Y.P.; Lei, J.; Yang, H.; Chen, R.B.; et al. Fructose-6-phosphate-2-kinase/fructose-2,6-bisphosphatase regulates energy metabolism and synthesis of storage products in developing rice endosperm. Plant Sci. 2023, 326, 111503. [Google Scholar] [CrossRef]
- Lei, J.; Teng, X.; Wang, Y.F.; Jiang, X.K.; Zhao, H.H.; Zheng, X.M.; Ren, Y.L.; Dong, H.; Wang, Y.L.; Duan, E.C.; et al. Plastidic pyruvate dehydrogenase complex E1 componentsubunit Alpha1 is involved in galactolipid biosynthesisrequired for amyloplast development in rice. Plant Biotechnol. J. 2022, 20, 437–453. [Google Scholar] [CrossRef]
- Lou, G.M.; Chen, P.L.; Zhou, H.; Li, P.B.; Xiong, J.W.; Wan, S.S.; Zheng, Y.Y.; Alam, M.; Liu, R.J.; Zhou, Y.; et al. FLOURY ENDOSPERM19 encoding a class I glutamine amidotransferase affects grain quality in rice. Mol. Breed. 2021, 41, 36. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Wang, Y.H.; Liu, X.; Jiang, L.; Ren, Y.L.; Liu, F.; Peng, C.; Li, J.J.; Jin, X.M.; Wu, F.P.; et al. The failure to express a protein disulphide isomerase-like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice. J. Exp. Bot. 2012, 63, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.L.; Wang, Y.H.; Liu, F.; Zhou, K.N.; Ding, Y.; Zhou, F.; Wang, Y.; Liu, K.; Gan, L.; Ma, W.W.; et al. GLUTELIN PRECURSOR ACCUMULATION3 encodes a regulator of post-Golgi vesicular traffic essential for vacuolar protein sorting in rice endosperm. Plant Cell 2014, 26, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ren, Y.L.; Wang, Y.H.; Peng, C.; Zhou, K.N.; Lv, J.; Guo, X.P.; Zhang, X.; Zhong, M.S.; Zhao, S.L.; et al. OsVPS9A Functions Cooperatively with OsRAB5A to Regulate Post-Golgi Dense Vesicle-Mediated Storage Protein Trafficking to the Protein Storage Vacuole in Rice Endosperm Cells. Mol. Plant 2013, 6, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Ren, Y.L.; Liu, X.; Jiang, L.; Chen, L.M.; Han, X.H.; Jin, M.N.; Liu, S.J.; Liu, F.; Lv, J.; et al. OsRab5a regulates endomembrane organization and storage protein trafficking in rice endosperm cells. Plant J. 2010, 64, 812–824. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, F.; Ren, Y.L.; Wang, Y.L.; Liu, X.; Long, W.H.; Wang, D.; Zhu, J.P.; Zhu, X.P.; Jing, R.N.; et al. GOLGI TRANSPORT 1B Regulates Protein Export from the Endoplasmic Reticulum in Rice Endosperm Cells. Plant Cell 2016, 28, 2850–2865. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, Y.H.; Pan, T.; Wang, Y.L.; Wang, Y.F.; Gan, L.; Wei, Z.Y.; Wang, F.; Wu, M.M.; Jing, R.N.; et al. GPA5 Encodes a Rab5a Effector Required for Post-Golgi Trafficking of Rice Storage Proteins. Plant Cell 2020, 32, 758–777. [Google Scholar] [CrossRef]
- Zhu, J.P.; Ren, Y.L.; Wang, Y.L.; Liu, F.; Teng, X.; Zhang, Y.Y.; Duan, E.C.; Wu, M.M.; Zhong, M.S.; Hao, Y.Y.; et al. OsNHX5-mediated pH homeostasis is required for post-Golgi trafficking of seed storage proteins in rice endosperm cells. BMC Plant Biol. 2019, 19, 295. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.H.; Jing, R.N.; Wang, Y.F.; Wei, Z.Y.; Zhang, B.L.; Lei, C.L.; Qi, Y.Z.; Wang, F.; Bao, X.H.; et al. Post-Golgi trafficking of rice storage proteins requires the small GTPase Rab7 activation complex MON1–CCZ1. Plant Physiol. 2021, 187, 2174–2191. [Google Scholar] [CrossRef]
- Zhu, J.P.; Ren, Y.L.; Zhang, Y.Y.; Yang, J.; Duan, E.C.; Wang, Y.L.; Liu, F.; Wu, M.M.; Pan, T.; Wang, Y.F.; et al. Subunit E isoform 1 of vacuolar H+-ATPase OsVHA enables post-Golgi trafficking of rice seed storage proteins. Plant Physiol. 2021, 187, 2192–2208. [Google Scholar] [CrossRef]
- Wang, R.Q.; Ren, Y.L.; Yan, H.G.; Teng, X.; Zhu, X.P.; Wang, Y.P.; Zhang, X.; Guo, X.P.; Lin, Q.B.; Cheng, Z.J.; et al. ENLARGED STARCH GRAIN1 affects amyloplast development and starch biosynthesis in rice endosperm. Plant Sci. 2021, 305, 110831. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Wu, X.B.; Yao, X.F.; Yu, R.; Larkin, P.; Liu, C.M. DNA demethylation by ROS1a in rice vegetative cells promotes methylation in sperm. Proc. Natl. Acad. Sci. USA 2018, 115, 11327–11332. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Pan, T.; Zhu, Y.; Jiang, X.; Yu, M.; Wang, R.; Zhang, F.; Luo, S.; Bao, X.; Chen, Y.; et al. FLOURY ENDOSPERM20 encoding SHMT4 is required for rice endosperm development. Plant Biotechnol. J. 2022, 20, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef]
- Fu, F.F.; Xue, H.W. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef]
- Bello, B.K.; Hou, Y.X.; Zhao, J.; Jiao, G.A.; Wu, Y.W.; Li, Z.Y.; Wang, Y.F.; Tong, X.H.; Wang, W.; Yuan, W.Y.; et al. NF-YB1-YC12-bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.). Plant Biotechnol. J. 2019, 17, 1222–1235. [Google Scholar] [CrossRef]
- Xiong, Y.F.; Ren, Y.; Li, W.; Wu, F.S.; Yang, W.J.; Huang, X.L.; Yao, J.L. NF-YC12 is a key multi-functional regulator of accumulation of seed storage substances in rice. J. Exp. Bot. 2019, 70, 3765–3780. [Google Scholar] [CrossRef]
- She, K.C.; Kusano, H.; Koizumi, K.; Yamakawa, H.; Hakata, M.; Imamura, T.; Fukuda, M.; Naito, N.; Tsurumaki, Y.; Yaeshima, M.; et al. A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell 2010, 22, 3280–3294. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, J.; Zhao, L.; Wei, C. The CBM48 domain-containing protein FLO6 regulates starch synthesis by interacting with SSIVb and GBSS in rice. Plant Mol. Biol. 2022, 108, 343–361. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.L.; Lu, B.Y.; Wan, J.M. FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice. J. Exp. Bot. 2016, 67, 633–647. [Google Scholar] [CrossRef]
- Zhu, X.P.; Teng, X.; Wang, Y.L.; Hao, Y.Y.; Jing, R.N.; Wang, Y.F.; Liu, Y.; Zhu, J.P.; Wu, M.M.; Zhong, M.S.; et al. FLOURY ENDOSPERM11 encoding a plastid heat shock protein 70 is essential for amyloplast development in rice. Plant Sci. 2018, 277, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.G.; Zhang, W.W.; Wang, Y.H.; Jin, J.; Xu, H.C.; Fu, Y.S.; Shan, Z.Z.; Wang, X.; Teng, X.; Li, X.; et al. LIKE EARLY STARVATION1 cooperates with FLOURY ENDOSPERM6 to modulate starch biosynthesis and endosperm development. Plant Cell 2024, 36, 1892–1912. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Tan, H.Y.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H. The oxygen status of the developing seed. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Rolletschek, H.; Koch, K.; Wobus, U.; Borisjuk, L. Positional cues for the starch/lipid balance in maize kernels and resource partitioning to the embryo. Plant J. 2005, 42, 69–83. [Google Scholar] [CrossRef]
- Hu, L.; Tu, B.; Yang, W.; Yuan, H.; Li, J.L.; Guo, L.N.; Zheng, L.; Chen, W.L.; Zhu, X.B.; Wang, Y.P.; et al. Mitochondria-Associated Pyruvate Kinase Complexes Regulate Grain Filling in Rice. Plant Physiol. 2020, 183, 1073–1087. [Google Scholar] [CrossRef]
- Lee, S.K.; Jeon, J.S. Review: Crucial Role of Inorganic Pyrophosphate in Integrating Carbon Metabolism from Sucrose Breakdown to Starch Synthesis in Rice Endosperm. Plant Sci. 2020, 298, 110572. [Google Scholar] [CrossRef]
- Imaizumi, N.; Ku, M.S.; Ishihara, K.; Samejima, M.; Kaneko, S.; Matsuoka, M. Characterization of the gene for pyruvate, orthophosphate dikinase from rice, a C3 plant, and a comparison of structure and expression between C3 and C4 genes for this protein. Plant Mol. Biol. 1997, 34, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Podestá, F.E. The functional organization and control of plant respiration. Crit. Rev. Plant Sci. 2006, 25, 159–198. [Google Scholar] [CrossRef]
- Lappe, R.R.; Baier, J.W.; Boehlein, S.K.; Huffman, R.; Lin, Q.H.; Wattebled, F.; Settles, A.M.; Hannah, L.C.; Borisjuk, L.; Rolletschek, H.; et al. Functions of maize genes encoding pyruvate phosphate dikinase in developing endosperm. Proc. Natl. Acad. Sci. USA 2018, 115, 24–33. [Google Scholar] [CrossRef]
- Chastain, C.J.; Heck, J.W.; Colquhoun, T.A.; Voge, D.G.; Gu, X.-Y. Posttranslational regulation of pyruvate, orthophosphate dikinase in developing rice (Oryza sativa) seeds. Planta 2006, 224, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Wang, L.J.; Liu, G.F.; Meng, X.B.; Jing, Y.H.; Shu, X.L.; Kong, X.L.; Sun, J.; Yu, H.; Smith, S.M.; et al. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 12844–12849. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, S.; Maruyama-Funatsuki, W.; Umemoto, T.; Kato, H.; Kuroki, M.; Yokogami, N.; Ikegaya, T.; Shimizu, H.; Iriki, N. The Induced Mutant Allele flo4-303 Confers Floury Characteristics on the Japonica Rice Cultivar ‘Hoshinoko’. Breed. Sci. 2022, 72, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mo, Y.; Im, D.E.; Jang, S.G.; Ham, T.H.; Lee, J.; Jeung, J.U.; Kwon, S.W. A New SNP in CyOsPPDK Gene is Associated with Floury Endosperm in Suweon 542. Mol. Genet. Genom. 2018, 293, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ham, T.H.; Im, D.E.; Lar, S.M.; Jang, S.G.; Lee, J.; Mo, Y.; Jeung, J.U.; Kim, S.T.; Kwon, S.W. A New SNP in Rice Gene Encoding Pyruvate Phosphate Dikinase (PPDK) Associated with Floury Endosperm. Genes 2020, 11, 465. [Google Scholar] [CrossRef]
- Ha, S.K.; Lee, H.S.; Lee, S.Y.; Lee, C.M.; Mo, Y.J.; Jeung, J.U. Characterization of flo4-6, a novel cyOsPPDKB allele conferring floury endosperm characteristics suitable for dry-milled rice flour production. Agronomy 2023, 13, 1306. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.L.; Lin, L.S.; Zhao, L.X.; Liu, Q.Q.; Wei, C.X. A Novel Mutation of OsPPDKB, Encoding Pyruvate Orthophosphate Dikinase, Affects Metabolism and Structure of Starch in the Rice Endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef]
- Muroyama, R.; Ito, H.; Takahashi, S.; Kang, D.J.; Hamada, S. Biochemical Analysis of a Novel Allele of the OsPPDKB Gene Associated with Floury Endosperm. J. Cereal Sci. 2022, 107, 103529. [Google Scholar] [CrossRef]
- Ando, T.; Yamamoto, T.; Shimizu, T.; Ma, X.F.; Shomura, A.; Takeuchi, Y.; Lin, S.Y.; Yano, M. Genetic dissection and pyramiding of quantitative traits for panicle architecture by using chromosomal segment substitution lines in rice. Theor. Appl. Genet. 2008, 116, 881–890. [Google Scholar] [CrossRef]
- Liu, J.X.; Wu, M.W.; Liu, C.M. Cereal Endosperms: Development and Storage Product Accumulation. Annu. Rev. Plant Biol. 2022, 73, 255–291. [Google Scholar] [CrossRef]
- Mo, Y.; Jeung, J.-U. The Use of Floury Endosperm Mutants to Develop Rice Cultivars Suitable for Dry Milling. Plant Biotechnol. Rep. 2020, 14, 185–191. [Google Scholar] [CrossRef]
- Mo, Y.; Jeung, J.-U.; Shin, Y.-S.; Park, C.S.; Kang, K.-H.; Kim, B.-K. Agronomic and Genetic Analysis of Suweon 542, a Rice Floury Mutant Line Suitable for Dry Milling. Rice 2013, 6, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Won, Y.-J.; Ahn, E.-K.; Jeong, E.-G.; Chang, J.-K.; Lee, J.-H.; Jung, K.-H.; Hyun, U.-J.; Cho, Y.-C.; Oh, S.-K.; Yoon, M.-R.; et al. An Opaque Endosperm Rice Cultivar, ‘Hangaru’, Suitable for Exclusive Dry-Milling Rice Flour Production. Korean J. Breed. Sci. 2019, 51, 134–139. [Google Scholar] [CrossRef]
- Cho, Y.; Baek, M.; Park, H.; Cho, J.; Ahn, E.; Suh, J.; Jeung, J. ‘Shingil (Milyang317)’, Tongil-Type Variety Specialized for Rice Flour. Korean J. Breed. Sci. 2020, 72, 58–72. [Google Scholar] [CrossRef]
- Takahashi, K.; Kohno, H.; Okuda, M. Spatial Distribution and Characteristics of Protein Content and Composition in Japonica Rice Grains: Implications for Sake Quality. Rice 2024, 17, 26. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhang, Y.; Sun, Z.X.; Shen, Z.Y.; Li, Y.G.; Guo, Y.F.; Feng, Y.T.; Sun, S.Y.; Guo, M.; Hu, Z.; et al. Knocking out OsAAP11 to improve rice grain quality using CRISPR/Cas9 system. Int. J. Mol. Sci. 2023, 24, 14360. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Shigemitsu, T.; Yamasaki, R.; Sasou, A.; Goto, F.; Kishida, K.; Kuroda, M.; Tanaka, K.; Morita, S.; Satoh, S.; et al. Formation mechanism of the internal structure of type I protein bodies in rice endosperm: Relationship between the localization of prolamin species and the expression of individual genes. Plant J. 2012, 70, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Zhang, X.Q.; Rasmussen, S.K.; Jiang, X.T.; Song, W.J.; Wu, D.X.; Shu, X.L. Dependence of physiochemical, functional and textural properties of high–resistant starch rice on endogenous nonstarch polysaccharides. Inte. J. Food Sci. Technol. 2018, 53, 1079–1086. [Google Scholar] [CrossRef]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Mitsuoka, C.; Tamiru, M.; et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]




| Classification | Name | Gene ID | Annotation |
|---|---|---|---|
| Starch synthesis and sucrose metabolism | FLO8 | Os09g0553200 | UTP-glucose-1-phosphate uridylyltransferase [4] |
| OsAGPL2 | Os01g0633100 | Glucose-1-phosphate adenylyltransferase large subunit [5] | |
| OsAGPS | Os09g0298200 | Glucose-1-phosphate adenylyltransferase large subunit [5] | |
| OsBT1 | Os02g0202400 | ADP-Glucose Transporter [6] | |
| OsBEIIb | Os02g0528200 | 1,4-alpha-glucan-branching enzyme [7] | |
| FLO5 | Os08g0191433 | Starch synthase III [8] | |
| Pho1 | Os03g0758100 | Alpha-glucan phosphorylase isozyme [9] | |
| GIF1 | Os04g0413500 | Cell wall invertase [10] | |
| Wx | Os06g0133000 | Starch synthase [11] | |
| FLO24 | Os03g0426900 | Heat shock protein 101 [12] | |
| Amyloplast development | SSG4 | Os01g0179400 | Protein containing a DUF490 domain [13] |
| SSG6 | Os06g0130400 | Aminotransferase [14] | |
| SSG7 | Os11g0524300 | Plant-specific DUF1001 domain-containing protein [15] | |
| FSE1 | Os08g0110700 | Phospholipase-like protein [16] | |
| Energy supply | FLO13 | Os02g0816800 | Mitochondrial complex I subunit [17] |
| OGR1 | Os12g0270200 | Pentatricopeptide repeat–DYW protein [18] | |
| FLO10 | Os03g0168400 | Pentatricopeptide repeat protein [19] | |
| OsNPPR1 | Os08g0290000 | Pentatricopeptide repeat protein [20] | |
| FLO18 | Os07g0688100 | Pentatricopeptide repeat protein [21] | |
| FLO22 | Os07g0179000 | P-type pentatricopeptide repeat (PPR) protein [22] | |
| Glycolytic metabolism | PFPβ | Os06g0247500 | Pyrophosphate-fructose 6-phosphate 1-phosphotransferase [23] |
| FLO4 | Os05g0405000 | Pyruvate, phosphate dikinase [24] | |
| OsPK2 | Os07g0181000 | Plastidic pyruvate kinase [25] | |
| FLO12 | Os10g0390500 | Aminotransferase [26] | |
| FLO15 | Os05g0230900 | Glyoxalase family protein [27] | |
| FLO16 | Os10g0478200 | Lactate/malate dehydrogenase [28] | |
| FLO23 | Os03g0294200 | Fructose-6-phosphate-2-kinase/fructose-2, 6-bisphosphatase [29] | |
| FLO19 | Os04g0119400 | Pyruvate dehydrogenase complex E1 component subunit α1 [30] | |
| FLO19 | Os03g0685300 | Class I glutamine amidotransferase [31] | |
| Protein processing and transport | PDIL1-1 | Os11g0199200 | Protein disulfide isomerase-like enzyme [32] |
| GPA1 | Os12g0631100 | Small GTPase [33] | |
| GPA2 | Os03g0262900 | Guanine nucleotide exchange factor [34] | |
| GPA3 | Os03g0835800 | Regulator of post-Golgi vesicular traffic [35] | |
| GPA4 | Os03g0209400 | Golgi Transport 1 [36] | |
| GPA5 | Os06g0643000 | Rab5a Effector [37] | |
| GPA6 | Os09g0286400 | Vacuolar Na+/H+ antiporter [38] | |
| GPA7 | Os08g0427300 | Homolog of Arabidopsis CCZ1a and CCZ1b [39] | |
| GPA8 | Os01g0659200 | Subunit E isoform 1 of vacuolar H+-ATPase [40] | |
| Lipid transport | ESG1 | Os04g0553000 | Bacterial MlaE lipid transfer protein [41] |
| Epigenetics | OsROS1 | Os01g0218032 | DNA demethylase [42] |
| FLO20-1 | Os01g0874900 | Serine hydroxymethyltransferase [43] | |
| Transcriptional regulation and protein interaction | RISBZ1 | Os07g0182000 | bZIP transcription factor [44] |
| RSR1 | Os05g0121600 | Transcription factor of the AP2/EREBP family [45] | |
| NF-YB1 | Os02g0725900 | Nuclear transcription factor Y subunit B [46] | |
| NF-YC12 | Os05g0304800 | CCAAT-box-binding transcription factor [46,47] | |
| bHLH144 | Os04g0429400 | Helix-loop-helix DNA-binding domain containing protein [46] | |
| FLO2 | Os04g0645100 | Tetratricopeptide repeat domain-containing protein [48] | |
| FLO6 | Os03g0686900 | CBM48 domain-containing protein [49] | |
| FLO7 | Os10g0463800 | DUF1388 domain protein [50] | |
| FLO11 | Os12g0244100 | Plastid heat shock protein 70 [51] | |
| FLO9 | Os11g0586300 | Homologous to Arabidopsis LIKE EARLY STARVATION1 [52] |
| Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| C5.5 | CTATGCAGTGCAGTGTGCAC | AGCCGAAGGAGGTGTGAATC |
| C5.4 | GCTCAAGCAAGGTCCATTCC | CAGCTACTAGGCCCCATTTG |
| C5.3 | CCTGGCGTCAAACACATCTG | CTGAGGGTGTTCTTTTGGGC |
| C5.2 | ATGGGAGAAGTGTCCAGCAG | GTGTGGACTGTGGATTGTGG |
| C5.1 | AGAACGGAGGGAGTAGGATC | TCGCGGCTCTGAATTACCAG |
| C5.8 | GTCCACCCGTTTCTTGCATG | CCACCCGTTTCTTGCATACC |
| C5.9 | CCGGATTGTAGCTGTAGCTC | GGGTCACAGCATCAAAGCAG |
| C5.12 | GTGCTGGAAACTCCATGTCG | ATGGCTCTATCGGTGTCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yang, X.; Wu, L.; Sun, Z.; Zhang, Y.; Shen, Z.; Zhou, J.; Guo, M.; Yan, C. Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant wcr (White-Core Rice). Plants 2024, 13, 2653. https://doi.org/10.3390/plants13182653
Yang Y, Yang X, Wu L, Sun Z, Zhang Y, Shen Z, Zhou J, Guo M, Yan C. Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant wcr (White-Core Rice). Plants. 2024; 13(18):2653. https://doi.org/10.3390/plants13182653
Chicago/Turabian StyleYang, Yihao, Xiaoyi Yang, Lingjun Wu, Zixing Sun, Yi Zhang, Ziyan Shen, Juan Zhou, Min Guo, and Changjie Yan. 2024. "Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant wcr (White-Core Rice)" Plants 13, no. 18: 2653. https://doi.org/10.3390/plants13182653
APA StyleYang, Y., Yang, X., Wu, L., Sun, Z., Zhang, Y., Shen, Z., Zhou, J., Guo, M., & Yan, C. (2024). Phenotypic Analysis and Gene Cloning of Rice Floury Endosperm Mutant wcr (White-Core Rice). Plants, 13(18), 2653. https://doi.org/10.3390/plants13182653





