Evaluation of the Spike Diversity of Seven Hexaploid Wheat Species and an Artificial Amphidiploid Using a Quadrangle Model Obtained from 2D Images
Abstract
:1. Introduction
- Fusiform (the middle part of the spike is the widest, narrowing toward the apex and partially toward the base);
- Elliptical (spikes of an elongated oval shape);
- Prismatic (spikes of nearly equal width along the entire length, with the exception of the apical and basal parts);
- Cone-shaped (spikes narrowing to the apex from the base);
- Square-headed (spikes expanding toward the apex);
- Cylindrical (spikes having the same cross-section radius along the entire length).
- The spike size and shape diversity in seven hexaploid wheat species and one amphidiploid (190 plants) are evaluated using a simplified (symmetrized) quadrangle model;
- Digital estimates of the spike characteristics are in agreement with the manually measured parameters of the same biological meaning;
- Digitally estimated spike characteristics make it possible to classify spikes both by species and by type with high accuracy through linear discriminant analysis (LDA), where the classification performance increases when manually estimated spike parameters are added;
- This method makes it possible to identify characteristics whose values differ not only between representatives of different species but also between different accessions of the same species.
2. Results
2.1. Spike Traits and Correlations between Them
2.2. Diversity of Wheat Spikes in Size and Shape
2.3. Linear Discriminant Analysis of Spikes Based on Shape Characteristics
2.4. Comparison of the Spike Characteristics of Different Accessions of the Same Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Spike Imaging
4.3. Simplified Representation of the Spike Contour Model
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igrejas, G.; Branlard, G. The Importance of Wheat. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–7. ISBN 978-3-030-34162-6. [Google Scholar]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Gaju, O.; Reynolds, M.P.; Sparkes, D.L.; Foulkes, M.J. Relationships between Large-Spike Phenotype, Grain Number, and Yield Potential in Spring Wheat. Crop Sci. 2009, 49, 961–973. [Google Scholar] [CrossRef]
- Farooq, S.; Shahid, M.; Khan, M.B.; Hussain, M.; Farooq, M. Improving the Productivity of Bread Wheat by Good Management Practices under Terminal Drought. J. Agron. Crop Sci. 2015, 201, 173–188. [Google Scholar] [CrossRef]
- Triticum, L. The International COMECON List of Descriptors for the Genus; Institute of Plant Industry: Leningrad, Russia, 1989; p. 43. [Google Scholar]
- Spagnoletti Zeuli, P.L.; De Pace, C.; Porceddu, E. Variation in Durum Wheat Populations from Three Geographical Origins. I. Material and Spike Characteristics. Euphytica 1984, 33, 563–575. [Google Scholar] [CrossRef]
- Zecevic, V.; Knezevic, D.; Kraljevic-Balalic, M.; Micanovic, D. Genetic and Phenotypic Variability of Yield Components in Wheat, Triticum aestivum L. Genetika 2004, 36, 151–159. [Google Scholar] [CrossRef]
- Revised Descriptor List for Wheat (Triticum spp.); International Board for Plant Genetic Resources: Rome, Italy, 1985; pp. 1–14.
- Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Longin, C.F.H. Phenotypic and Genetic Analysis of Spike and Kernel Characteristics in Wheat Reveals Long-Term Genetic Trends of Grain Yield Components. Theor. Appl. Genet. 2018, 131, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Flaksberger, R.A. Pshenitsi—Rod Triticum L. pr. p. (wheats—Genus Triticum L. pr. p.). In Cultivated Flora of the USSR, Volume 1. Bread Cereals—Wheat; Wulff, E.V., Ed.; Gosselkhozgiz: Moscow, Russia, 1935; pp. 19–434. [Google Scholar]
- Clark, J.A.; Ball, C.R.; Martin, J.H. Classification of American Wheat Varieties; USDA Bulletin; US Department of Agriculture: Washington, DC, USA, 1922.
- Dorofeev, V.F.; Filatenko, A.A.; Migushova, E.F.; Udachin, R.A.; Jakubziner, M.M. Flora of Cultivated Plants; Kolos: Leningrad, Russia, 1979. [Google Scholar]
- Feldman, M.; Levy, A.A. Triticum L. In Wheat Evolution and Domestication; Springer International Publishing: Cham, Switzerland, 2023; pp. 365–526. ISBN 978-3-031-30174-2. [Google Scholar]
- Johnson, E.B.; Nalam, V.J.; Zemetra, R.S.; Riera-Lizarazu, O. Mapping the Compactum Locus in Wheat (Triticum aestivum L.) and Its Relationship to Other Spike Morphology Genes of the Triticeae. Euphytica 2008, 163, 193–201. [Google Scholar] [CrossRef]
- Sormacheva, I.; Golovnina, K.; Vavilova, V.; Kosuge, K.; Watanabe, N.; Blinov, A.; Goncharov, N.P. Q Gene Variability in Wheat Species with Different Spike Morphology. Genet. Resour. Crop Evol. 2015, 62, 837–852. [Google Scholar] [CrossRef]
- Konopatskaia, I.; Vavilova, V.; Blinov, A.; Goncharov, N.P. Spike Morphology Genes in Wheat Species (Triticum L.). Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2016, 70, 345–355. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, H.; Guo, H.; Li, Y.; Xie, X.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; et al. Identification of the Q Gene Playing a Role in Spike Morphology Variation in Wheat Mutants and Its Regulatory Network. Front. Plant Sci. 2022, 12, 807731. [Google Scholar] [CrossRef] [PubMed]
- Garland-Campbell, K.A. Club Wheat—A Review of Club Wheat History, Improvement, and Spike Characteristics in Wheat. In Plant Breeding Reviews; Goldman, I., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 421–465. ISBN 978-1-119-87412-6. [Google Scholar]
- Clark, J.A.; Bayles, B.B. Classification of Wheat Varieties Grown in the United States in 1939; USDA Technical Bulletin; US Department of Agriculture: Washington, DC, USA, 1942.
- Spagnoletti Zeuli, P.L.S.; Qualset, C.O. Geographical Diversity for Quantitative Spike Characters in a World Collection of Durum Wheat. Crop Sci. 1987, 27, 235–241. [Google Scholar] [CrossRef]
- Börner, A.; Schäfer, M.; Schmidt, A.; Grau, M.; Vorwald, J. Associations between Geographical Origin and Morphological Characters in Bread Wheat (Triticum aestivum L.). Plant Genet. Resour. 2005, 3, 360–372. [Google Scholar] [CrossRef]
- Phogat, B.S.; Kumar, S.; Kumari, J.; Kumar, N.; Pandey, A.C.; Singh, T.P.; Kumar, S.; Tyagi, R.K.; Jacob, S.R.; Singh, A.K.; et al. Characterization of Wheat Germplasm Conserved in the Indian National Genebank and Establishment of a Composite Core Collection. Crop Sci. 2021, 61, 604–620. [Google Scholar] [CrossRef]
- Spanic, V.; Lalic, Z.; Berakovic, I.; Jukic, G.; Varnica, I. Morphological Characterization of 1322 Winter Wheat (Triticum aestivum L.) Varieties from EU Referent Collection. Agriculture 2024, 14, 551. [Google Scholar] [CrossRef]
- Solimani, F.; Cardellicchio, A.; Nitti, M.; Lako, A.; Dimauro, G.; Renò, V. A Systematic Review of Effective Hardware and Software Factors Affecting High-Throughput Plant Phenotyping. Information 2023, 14, 214. [Google Scholar] [CrossRef]
- Abebe, A.M.; Kim, Y.; Kim, J.; Kim, S.L.; Baek, J. Image-Based High-Throughput Phenotyping in Horticultural Crops. Plants 2023, 12, 2061. [Google Scholar] [CrossRef] [PubMed]
- Kolhar, S.; Jagtap, J. Plant Trait Estimation and Classification Studies in Plant Phenotyping Using Machine Vision—A Review. Inf. Process. Agric. 2023, 10, 114–135. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Strange, H.; Zwiggelaar, R.; Sturrock, C.; Mooney, S.J.; Doonan, J.H. Automatic Estimation of Wheat Grain Morphometry from Computed Tomography Data. Funct. Plant Biol. 2015, 42, 452. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, B.; Xiong, S.; Lin, C.; Yuan, X. 3D Morphological Processing for Wheat Spike Phenotypes Using Computed Tomography Images. Remote Sens. 2019, 11, 1110. [Google Scholar] [CrossRef]
- Hughes, A.; Oliveira, H.R.; Fradgley, N.; Corke, F.M.K.; Cockram, J.; Doonan, J.H.; Nibau, C. μ CT Trait Analysis Reveals Morphometric Differences between Domesticated Temperate Small Grain Cereals and Their Wild Relatives. Plant J. 2019, 99, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Liang, N.; He, Y.; Xu, C.; Sun, S.; Zhou, Z.; Qiu, Z. A Novel Method for Wheat Spike Phenotyping Based on Instance Segmentation and Classification. Appl. Sci. 2024, 14, 6031. [Google Scholar] [CrossRef]
- Hammers, M.; Winn, Z.J.; Ben-Hur, A.; Larkin, D.; Murry, J.; Mason, R.E. Phenotyping and Predicting Wheat Spike Characteristics Using Image Analysis and Machine Learning. Plant Phenome J. 2023, 6, e20087. [Google Scholar] [CrossRef]
- Qiu, R.; He, Y.; Zhang, M. Automatic Detection and Counting of Wheat Spikelet Using Semi-Automatic Labeling and Deep Learning. Front. Plant Sci. 2022, 13, 872555. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Jiang, P.; Lei, L. Non-destructive measurement of wheat spike characteristics based on morphological image processing. Trans. CSAE 2010, 26, 212–216. [Google Scholar]
- Li, Y.; Du, S.; Zhong, H.; Chen, Y.; Liu, Y.; He, R.; Ding, Q. A Grain Number Counting Method Based on Image Characteristic Parameters of Wheat Spikes. Agriculture 2024, 14, 982. [Google Scholar] [CrossRef]
- Artemenko, N.V.; Genaev, M.A.; Epifanov, R.U.; Komyshev, E.G.; Kruchinina, Y.V.; Koval, V.S.; Goncharov, N.P.; Afonnikov, D.A. Image-Based Classification of Wheat Spikes by Glume Pubescence Using Convolutional Neural Networks. Front. Plant Sci. 2024, 14, 1336192. [Google Scholar] [CrossRef] [PubMed]
- Genaev, M.A.; Komyshev, E.G.; Smirnov, N.V.; Kruchinina, Y.V.; Goncharov, N.P.; Afonnikov, D.A. Morphometry of the Wheat Spike by Analyzing 2D Images. Agronomy 2019, 9, 390. [Google Scholar] [CrossRef]
- Cope, J.S.; Corney, D.; Clark, J.Y.; Remagnino, P.; Wilkin, P. Plant Species Identification Using Digital Morphometrics: A Review. Expert Syst. Appl. 2012, 39, 7562–7573. [Google Scholar] [CrossRef]
- Noshita, K.; Murata, H.; Kirie, S. Model-Based Plant Phenomics on Morphological Traits Using Morphometric Descriptors. Breed. Sci. 2022, 72, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chuanromanee, T.S.; Cohen, J.I.; Ryan, G.L. Morphological Analysis of Size and Shape (MASS): An Integrative Software Program for Morphometric Analyses of Leaves. Appl. Plant Sci. 2019, 7, e11288. [Google Scholar] [CrossRef] [PubMed]
- Bodor-Pesti, P.; Taranyi, D.; Deák, T.; Nyitrainé Sárdy, D.Á.; Varga, Z. A Review of Ampelometry: Morphometric Characterization of the Grape (Vitis spp.) Leaf. Plants 2023, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H. Quantitative Genetic Analyses of Crop Organ Shape Based on Principal Components of Elliptic Fourier Descriptors. In Proceedings of the Biological Shape Analysis, Tsukuba, Japan, 3–6 June 2011; World Scientific: Singapore, 2011; pp. 50–66. [Google Scholar]
- Søgaard, H.T. Weed Classification by Active Shape Models. Biosyst. Eng. 2005, 91, 271–281. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín, J.J.; Saadaoui, E. Updated Methods for Seed Shape Analysis. Scientifica 2016, 2016, 5691825. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, E.; Martín Gómez, J. Seed Shape Description and Quantification by Comparison with Geometric Models. Horticulturae 2019, 5, 60. [Google Scholar] [CrossRef]
- Kumar, N.; Belhumeur, P.N.; Biswas, A.; Jacobs, D.W.; Kress, W.J.; Lopez, I.C.; Soares, J.V.B. Leafsnap: A Computer Vision System for Automatic Plant Species Identification. In Computer Vision—ECCV 2012; Fitzgibbon, A., Lazebnik, S., Perona, P., Sato, Y., Schmid, C., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7573, pp. 502–516. ISBN 978-3-642-33708-6. [Google Scholar]
- Kupe, M.; Sayıncı, B.; Demir, B.; Ercisli, S.; Baron, M.; Sochor, J. Morphological Characteristics of Grapevine Cultivars and Closed Contour Analysis with Elliptic Fourier Descriptors. Plants 2021, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Ling, X.; Wang, L. From Shallow Feature Learning to Deep Learning: Benefits from the Width and Depth of Deep Architectures. WIREs Data Min. Knowl. Discov. 2019, 9, e1255. [Google Scholar] [CrossRef]
- Bi, K.; Huang, F.F.; Wang, C. Quick Acqusition of Wheat Ear Morphology Parameter Based on Imaging Processing. In Computer Science for Environmental Engineering and Ecoinformatics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 300–307. [Google Scholar]
- Chen, Y.; Huang, Y.; Zhang, Z.; Wang, Z.; Liu, B.; Liu, C.; Huang, C.; Dong, S.; Pu, X.; Wan, F.; et al. Plant Image Recognition with Deep Learning: A Review. Comput. Electron. Agric. 2023, 212, 108072. [Google Scholar] [CrossRef]
- Xiong, J.; Yu, D.; Liu, S.; Shu, L.; Wang, X.; Liu, Z. A Review of Plant Phenotypic Image Recognition Technology Based on Deep Learning. Electronics 2021, 10, 81. [Google Scholar] [CrossRef]
- Conejo-Rodríguez, D.F.; Gonzalez-Guzman, J.J.; Ramirez-Gil, J.G.; Wenzl, P.; Urban, M.O. Digital Descriptors Sharpen Classical Descriptors, for Improving Genebank Accession Management: A Case Study on Arachis spp. and Phaseolus spp. PLoS ONE 2024, 19, e0302158. [Google Scholar] [CrossRef]
- Díez, M.J.; De La Rosa, L.; Martín, I.; Guasch, L.; Cartea, M.E.; Mallor, C.; Casals, J.; Simó, J.; Rivera, A.; Anastasio, G.; et al. Plant Genebanks: Present Situation and Proposals for Their Improvement. The Case of the Spanish Network. Front. Plant Sci. 2018, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y. The Vulnerability of Plant Genetic Resources Conserved Ex Situ. Crop Sci. 2017, 57, 2314–2328. [Google Scholar] [CrossRef]
- Ghamkhar, K.; Hay, F.R.; Engbers, M.; Dempewolf, H.; Schurr, U. Realizing the Potential of Plant Genetic Resources: The Use of Phenomics for Genebanks. Plants People Planet 2024, 1. [Google Scholar] [CrossRef]
- Joseph Fernando, E.A.; Selvaraj, M.; Ghamkhar, K. The Power of Phenomics: Improving Genebank Value and Utility. Mol. Plant 2023, 16, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Z.; Meng, Y.; Fu, Z.Q.; Wang, D.; Zhang, K. A New Phase of Treasure Hunting in Plant Genebanks. Mol. Plant 2023, 16, 503–505. [Google Scholar] [CrossRef]
- Varshney, R.K.; Sinha, P.; Singh, V.K.; Kumar, A.; Zhang, Q.; Bennetzen, J.L. 5Gs for Crop Genetic Improvement. Curr. Opin. Plant Biol. 2020, 56, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Huang, F.; Wang, C.; Li, L.; Huang, D. Automatic Acquisition Characteristic Parameters of Wheat Ear Based on Machine Vision. In Proceedings of the 2011 International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; pp. 148–154. [Google Scholar]
- Wolde, G.M.; Trautewig, C.; Mascher, M.; Schnurbusch, T. Genetic Insights into Morphometric Inflorescence Traits of Wheat. Theor. Appl. Genet. 2019, 132, 1661–1676. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.; Pont, C.; Sibout, R.; Martinek, P.; Badaeva, E.; Murat, F.; Chosson, A.; Watanabe, N.; Prat, E.; Gautier, N.; et al. FRIZZY PANICLE Drives Supernumerary Spikelets in Bread Wheat. Plant Physiol. 2014, 167, 189–199. [Google Scholar] [CrossRef]
- Goncharov, N.P. Genus Triticum L. Taxonomy: The Present and the Future. Plant Syst. Evol. 2011, 295, 1–11. [Google Scholar] [CrossRef]
- Mac Key, J. Wheat: Its Concept, Evolution and Taxonomy. In Durum Wheat, Current Approaches, Future Strategies; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, pp. 3–61. ISBN 978-0-429-18029-3. [Google Scholar]
- Bernhardt, N. Taxonomic Treatments of Triticeae and the Wheat Genus Triticum. In Alien Introgression in Wheat; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–19. [Google Scholar]
- Babenko, L.M.; Hospodarenko, H.M.; Rozhkov, R.V.; Pariy, Y.F.; Pariy, M.F.; Babenko, A.V.; Kosakivska, I.V. Triticum spelta: Origin, biological characteristics and perspectives for use in breeding and agriculture. Regul. Mech. Biosyst. 2018, 9, 250–257. [Google Scholar] [CrossRef]
- Barisashvili, M.A.; Gorgidze, A.D. O Proiskhozhdenii Pshenitsy T. Macha Dek. et. Men. (On the Origin of T. Macha Dek. et Men. Wheat). Soobshcheniya Akad Nauk Gruz SSR 1979, 95, 409–412. [Google Scholar]
- Golovnina, K.A.; Kondratenko, E.; Blinov, A.G.; Goncharov, N.P. Molecular Characterization of Vernalization Loci VRN1 in Wild and Cultivated Wheats. BMC Plant Biol. 2010, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Heer, O. Die Pflanzen Der Pfahlbauten. Neujahrsbl. Naturforschenden Ges. Zrich Fr Jahr 1866 1865, 68, 1–54. [Google Scholar]
- Goncharov, N.P.; Gaidalenok, R.F. Localization of Genes Controlling Spherical Grain and Compact Ear in Triticum antiquorum Heer Ex Udacz. Russ. J. Genet. 2005, 41, 1262–1267. [Google Scholar] [CrossRef]
- Kihara, H.; Yamashita, H.; Tanaka, M. Morphological, Physiological, Genetical, and Cytological Studies in Aegilops and Triticum Collected in Pakistan, Afghanistan, Iran. Results of the Kyoto University Scientific Expedition to the Korakoram and Hidukush in 1955. In Cultivated Plants and Their Relatives; Yamashita, K., Ed.; Kyoto University: Kyoto, Japan, 1965. [Google Scholar]
- Badaeva, E.D. Genome Evolution in Wheats and Their Wild Relatives: A Molecular Cytogenetic Study. Ph.D. Thesis, Institute of Molecular Biology, Moscow, Russia, 2000. [Google Scholar]
- Dong, Y.-S.; Zheng, D.-S.; Qiao, D.-Y. Expedition and Investigation of Yunnan Wheat (Triticum aestivum ssp. Yunnanense King). Acta Agron. Sin. 1981, 7, 145–152. [Google Scholar]
- Fu, H.; Goncharov, N.P. Endemic wheats of China as resources for breeding. Genet. Resur. Rosl. Plant Genet. Resour. 2019, 25, 11–25. [Google Scholar] [CrossRef]
- Vavilova, V.; Konopatskaia, I.; Blinov, A.; Kondratenko, E.Y.; Kruchinina, Y.V.; Goncharov, N.P. Genetic Variability of Spelt Factor Gene in Triticum and Aegilops Species. BMC Plant Biol. 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Genaev, M.A.; Komyshev, E.G.; Hao, F.; Koval, V.S.; Goncharov, N.P.; Afonnikov, D.A. SpikeDroidDB: An information system for annotation of morphometric characteristics of wheat spike. Vavilov J. Genet. Breed. 2018, 22, 132–140. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistical Software Package for Education and Data Analysis. Palaeontol. Electron. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
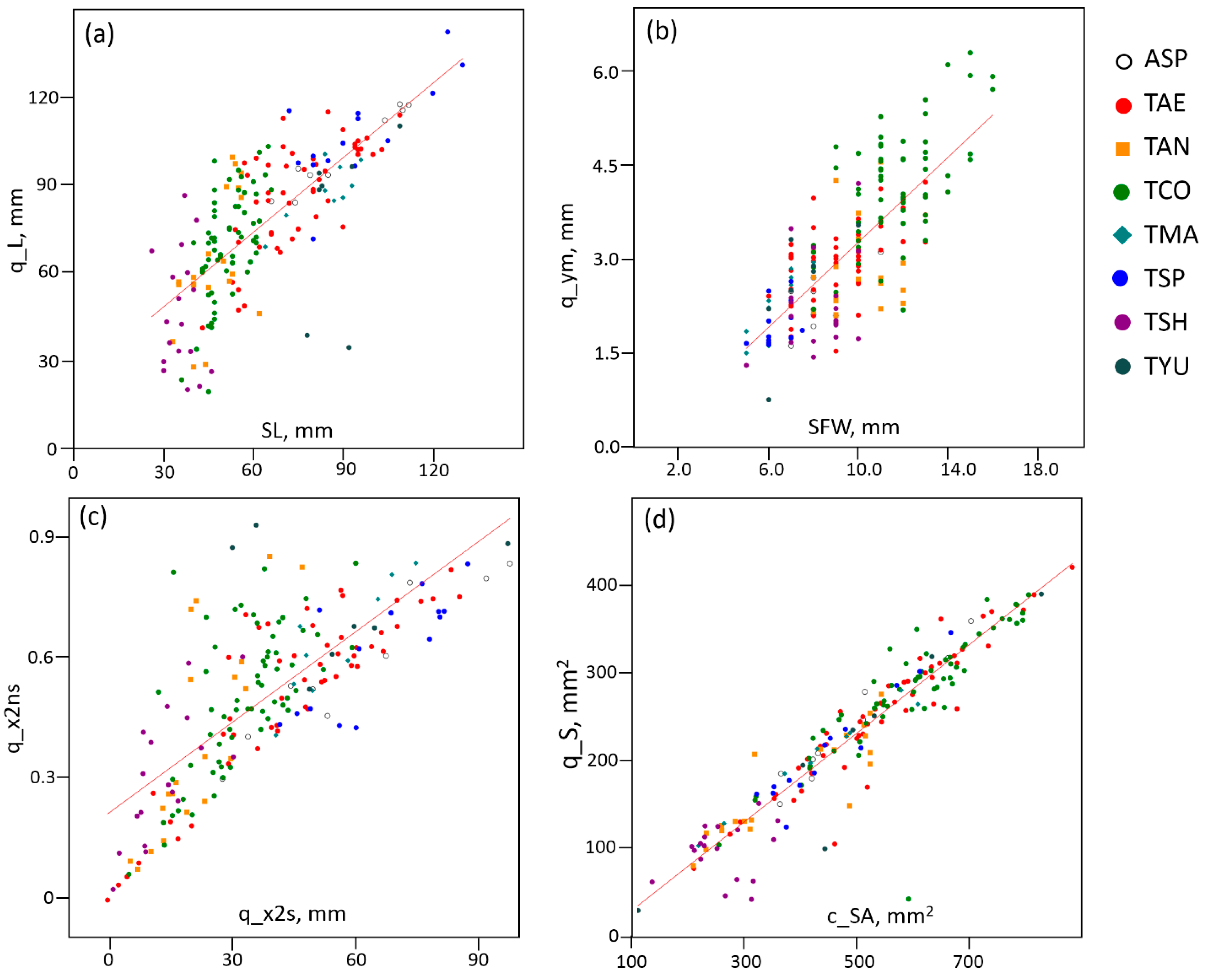
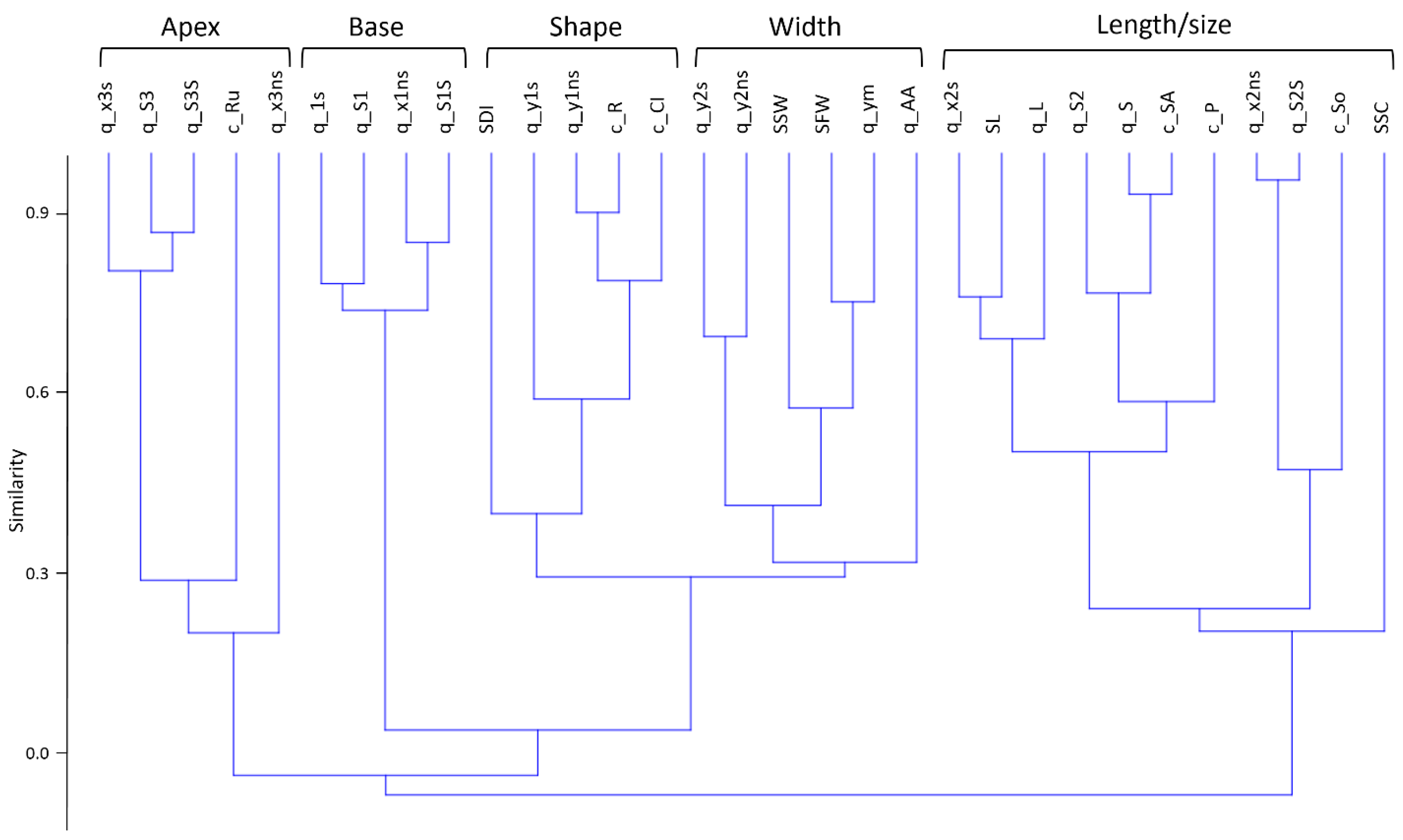
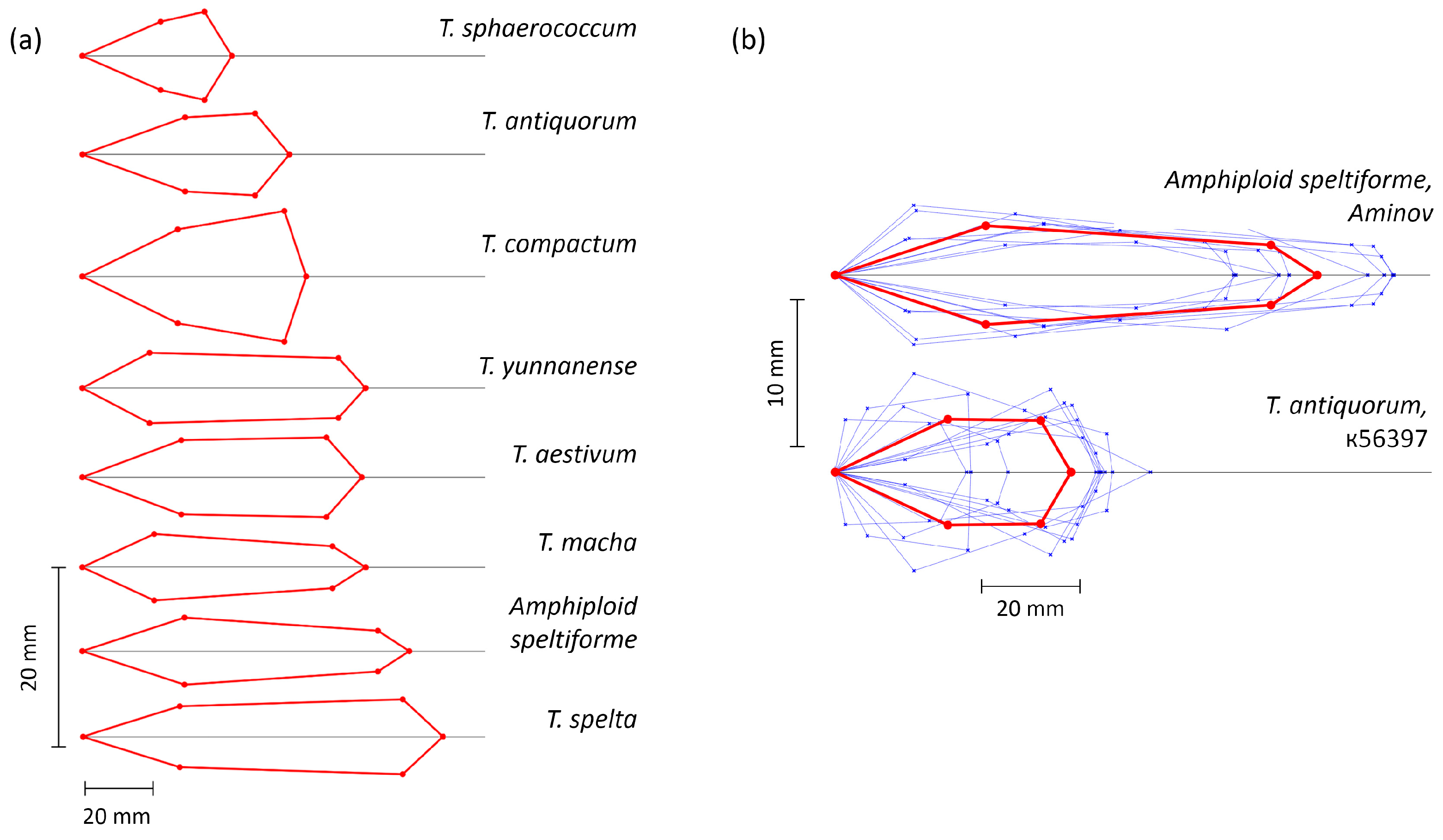
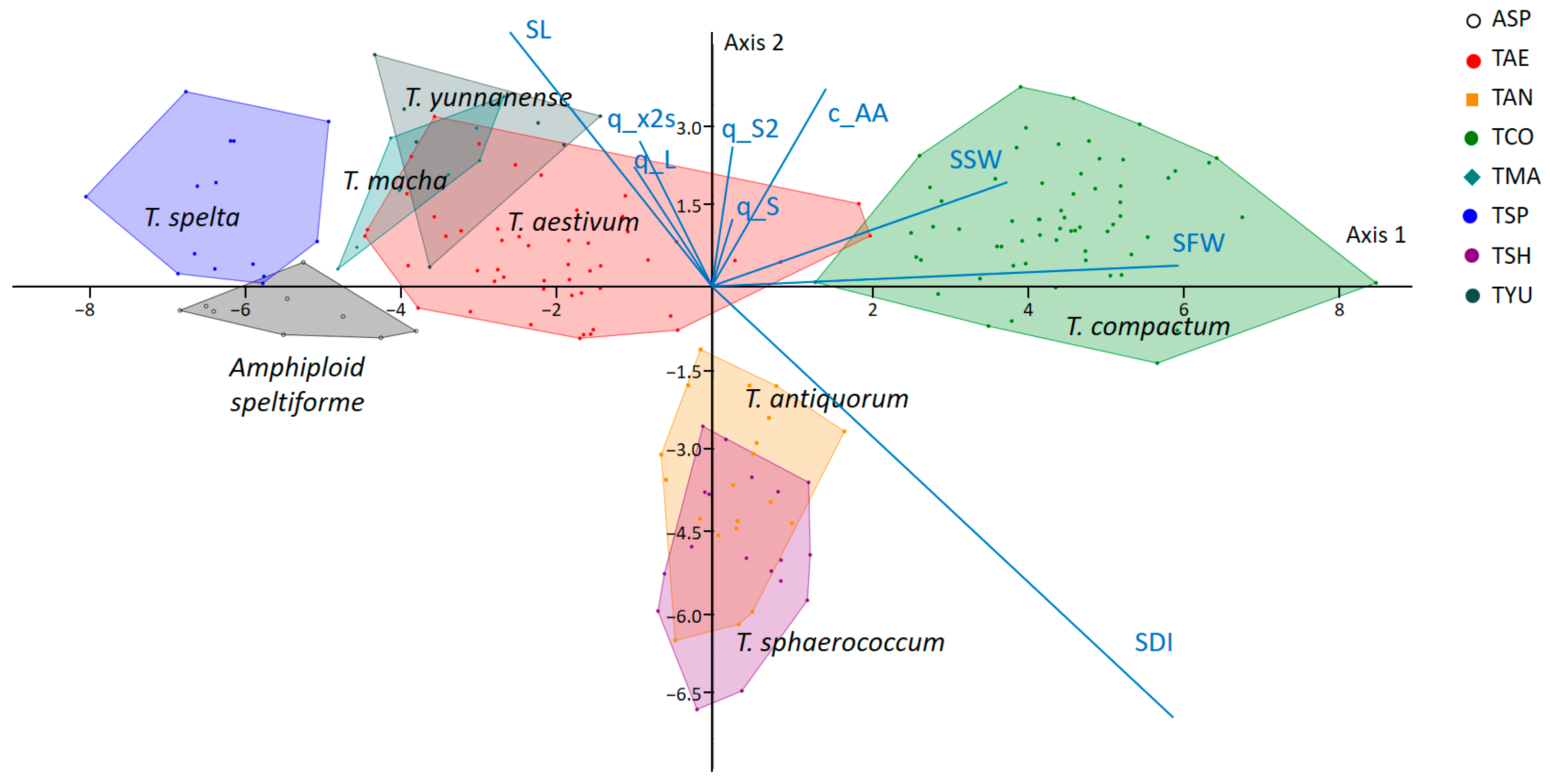

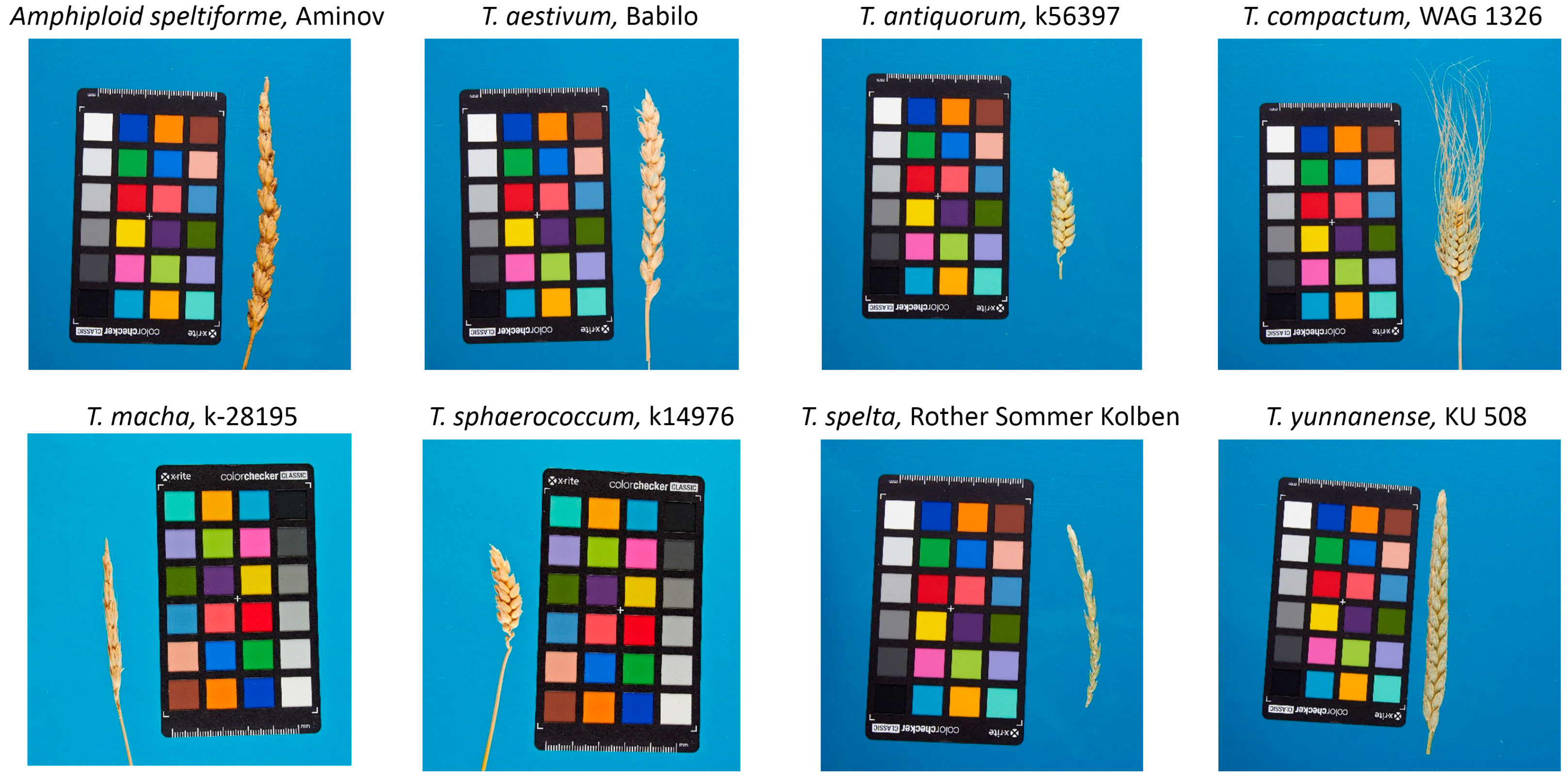
| Trait | ASP | TAE | TAN | TCO | TMA | TSP | TSH | TYU |
|---|---|---|---|---|---|---|---|---|
| N | 9 | 50 | 10 | 63 | 9 | 14 | 18 | 8 |
| SL | 90.44 ± 6.06 | 74.64 ± 2.25 | 39.70 ± 1.35 | 51.56 ± 0.89 | 84.22 ± 3.46 | 94.71 ± 5.02 | 35.83 ± 1.17 | 87.00 ± 3.76 |
| SFW | 8.22 ± 0.40 | 9.12 ± 0.25 | 8.70 ± 0.15 | 11.65 ± 0.24 | 6.33 ± 0.33 | 6.46 ± 0.19 | 8.22 ± 0.32 | 7.38 ± 0.46 |
| SSW | 6.33 ± 0.33 | 7.52 ± 0.24 | 6.90 ± 0.18 | 8.29 ± 0.20 | 6.22 ± 0.36 | 5.96 ± 0.13 | 6.61 ± 0.24 | 7.00 ± 0.46 |
| SSC | 19.22 ± 1.19 | 15.60 ± 0.41 | 17.60 ± 0.37 | 16.51 ± 0.41 | 18.78 ± 0.66 | 15.36 ± 0.39 | 14.89 ± 0.27 | 17.13 ± 1.06 |
| SDI | 20.25 ± 0.71 | 19.88 ± 0.51 | 42.61 ± 2.11 | 30.48 ± 0.92 | 21.29 ± 0.90 | 15.58 ± 0.75 | 39.30 ± 1.19 | 18.46 ± 0.55 |
| q_x1s | 31.70 ± 5.46 | 30.72 ± 2.07 | 23.70 ± 5.08 | 29.60 ± 2.21 | 22.29 ± 4.70 | 27.42 ± 4.33 | 24.31 ± 3.02 | 16.27 ± 4.76 |
| q_x2s | 59.96 ± 8.20 | 45.00 ± 2.99 | 19.55 ± 2.57 | 33.07 ± 1.52 | 55.31 ± 4.03 | 65.68 ± 4.11 | 13.60 ± 2.05 | 61.96 ± 9.47 |
| q_x3s | 9.75 ± 3.85 | 10.98 ± 1.51 | 6.36 ± 2.46 | 6.78 ± 0.61 | 10.28 ± 2.48 | 14.48 ± 3.36 | 8.46 ± 1.84 | 6.17 ± 2.59 |
| q_y1s | 3.36 ± 0.31 | 3.71 ± 0.23 | 3.60 ± 0.51 | 4.69 ± 0.26 | 3.32 ± 0.29 | 2.80 ± 0.55 | 3.42 ± 0.61 | 3.06 ± 0.51 |
| q_y2s | 2.81 ± 0.80 | 3.98 ± 0.22 | 3.51 ± 0.46 | 6.53 ± 0.25 | 2.10 ± 0.18 | 2.72 ± 0.63 | 4.41 ± 0.60 | 3.17 ± 0.59 |
| q_L | 101.41 ± 4.70 | 86.71 ± 2.58 | 49.61 ± 4.28 | 69.46 ± 2.26 | 87.88 ± 3.32 | 107.58 ± 4.62 | 46.37 ± 4.75 | 84.40 ± 11.30 |
| q_S1 | 55.54 ± 9.96 | 60.98 ± 5.66 | 45.44 ± 11.10 | 78.29 ± 7.19 | 37.20 ± 7.11 | 42.36 ± 7.38 | 32.58 ± 4.53 | 28.74 ± 9.19 |
| q_S2 | 163.10 ± 28.25 | 164.01 ± 11.17 | 65.08 ± 8.66 | 178.79 ± 8.70 | 152.42 ± 19.25 | 143.95 ± 11.23 | 44.34 ± 5.74 | 210.22 ± 50.08 |
| q_S3 | 17.33 ± 7.35 | 26.92 ± 5.32 | 15.65 ± 6.36 | 25.49 ± 3.22 | 13.55 ± 3.97 | 26.38 ± 8.32 | 19.90 ± 4.97 | 12.25 ± 6.02 |
| q_S | 235.97 ± 23.19 | 251.91 ± 10.84 | 126.17 ± 10.37 | 282.56 ± 9.20 | 203.17 ± 19.44 | 212.69 ± 16.60 | 96.82 ± 7.26 | 251.22 ± 53.24 |
| q_ym | 2.30 ± 0.15 | 2.89 ± 0.09 | 2.64 ± 0.21 | 4.15 ± 0.12 | 2.29 ± 0.18 | 1.95 ± 0.09 | 2.27 ± 0.18 | 2.75 ± 0.35 |
| c_P | 253.73 ± 15.06 | 246.19 ± 5.29 | 153.73 ± 8.11 | 235.90 ± 0.02 | 207.76 ± 11.16 | 274.77 ± 11.41 | 176.49 ± 8.16 | 232.49 ± 19.01 |
| c_SA | 489.51 ± 41.04 | 545.65 ± 20.10 | 272.73 ± 12.30 | 599.75 ± 0.02 | 430.03 ± 43.58 | 453.96 ± 28.00 | 261.87 ± 13.62 | 558.06 ± 96.85 |
| c_AA | 5.38 ± 1.18 | 21.82 ± 1.81 | 7.18 ± 0.76 | 96.73 ± 0.02 | 46.64 ± 10.77 | 57.14 ± 15.20 | 11.12 ± 1.09 | 78.24 ± 17.77 |
| c_CI | 0.11 ± 0.01 | 0.15 ± 0.01 | 0.21 ± 0.03 | 0.23 ± 0.01 | 0.15 ± 0.01 | 0.09 ± 0.01 | 0.20 ± 0.03 | 0.15 ± 0.01 |
| c_R | 0.07 ± 0.00 | 0.10 ± 0.00 | 0.16 ± 0.03 | 0.17 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.18 ± 0.03 | 0.09 ± 0.01 |
| c_So | 0.68 ± 0.03 | 0.71 ± 0.01 | 0.70 ± 0.03 | 0.75 ± 0.02 | 0.85 ± 0.02 | 0.63 ± 0.03 | 0.64 ± 0.03 | 0.79 ± 0.04 |
| c_Ru | 1.18 ± 0.03 | 1.26 ± 0.03 | 1.23 ± 0.03 | 1.25 ± 0.02 | 1.07 ± 0.01 | 1.26 ± 0.08 | 1.29 ± 0.05 | 1.21 ± 0.09 |
| Trait | ANOVA | Levene’s Test from Medians | Kruskal–Wallis Test for Equal Medians | ||
|---|---|---|---|---|---|
| F | p | p | Hc (Tie Corrected) | p | |
| SL | 63.07 | 1.797 × 10−45 | 4.961 × 10−9 | 141.3 | 2.66 × 10−27 |
| SFW | 9.482 | 5.01 × 10−10 | 0.0002302 | 57.16 | 5.554 × 10−10 |
| SSW | 27 | 3.081 × 10−25 | 0.0005318 | 103.7 | 1.896 × 10−19 |
| SSC | 5.649 | 0.000006414 | 0.00002497 | 39.41 | 1.63 × 10−6 |
| SDI | 55.53 | 5.242 × 10−42 | 0.00002992 | 138.8 | 9.156 × 10−27 |
| q_x1s | 1.346 | 0.2311 | 0.2123 | 9.921 | 0.1931 |
| q_x2s | 22.74 | 5.17 × 10−22 | 0.0001098 | 91.23 | 6.925 × 10−17 |
| q_x3s | 1.851 | 0.07999 | 0.05528 | 9.437 | 0.2228 |
| q_y1s | 3.028 | 0.00491 | 0.02729 | 24.66 | 0.000872 |
| q_y2s | 15.4 | 8.629 × 10−16 | 0.2018 | 79.47 | 1.769 × 10−14 |
| q_L | 19.35 | 3.015 × 10−19 | 0.3375 | 80.42 | 1.132 × 10−14 |
| q_S1 | 3.741 | 0.0008219 | 0.001609 | 23.95 | 0.001161 |
| q_S2 | 11.25 | 7.933 × 10−12 | 0.00008505 | 62.04 | 5.894 × 10−11 |
| q_S3 | 0.7227 | 0.6529 | 0.3255 | 8.768 | 0.2697 |
| q_S | 15.95 | 2.753 × 10−16 | 0.003657 | 72.27 | 5.138 × 10−13 |
| q_ym | 50.81 | 1.138 × 10−39 | 0.004768 | 131 | 3.922 × 10−25 |
| c_P | 13.39 | 6.557 × 10−14 | 0.8333 | 62.96 | 3.853 × 10−11 |
| c_SA | 16.74 | 5.364 × 10−17 | 0.004782 | 74.08 | 2.208 × 10−13 |
| c_AA | 18.07 | 3.666 × 10−18 | 3.871 × 10−16 | 116.1 | 4.92 × 10−22 |
| c_CI | 10.1 | 1.158 × 10−10 | 0.01301 | 84.56 | 1.613 × 10−15 |
| c_R | 10.09 | 1.192 × 10−10 | 0.000001117 | 87.43 | 4.161 × 10−16 |
| c_So | 6.757 | 3.934 × 10−7 | 0.1106 | 38.11 | 0.000002887 |
| c_Ru | 1.457 | 0.1854 | 0.3295 | 27.68 | 0.0002516 |
| Traits Set/Data Transformation | Percentage of Correctly Classified Spikes | |
|---|---|---|
| Species or Amphidiploid, 8 Classes | Spike Type, 3 Classes | |
| Manually estimated (5 traits) | 67.89 | 88.95 |
| Digitally estimated (26 traits) | 78.42 | 83.16 |
| Combined (31 traits) | 87.89 | 92.11 |
| Manually estimated/Box–Cox | 68.42 | 90.52 |
| Digitally estimated/Box–Cox | 82.11 | 85.26 |
| Combined/Box–Cox | 88.42 | 93.16 |
| WAG 8326, n = 8 | k1709, n = 9 | t-Test | F-Test | Mann–Whitney | |||
|---|---|---|---|---|---|---|---|
| Trait | Mean | Variance | Mean | Variance | p (Same Means) | p (Same Variances) | p (Equal) |
| SL | 43.25 | 13.36 | 60.44 | 13.78 | 8.5048 × 10−8 | 0.98 | 0.0006 |
| SWF | 14.00 | 5.14 | 12.11 | 1.11 | 0.04 | 0.05 | 0.02 |
| SSW | 8.25 | 4.21 | 8.89 | 0.86 | 0.41 | 0.04 | 0.35 |
| SSC | 19.88 | 7.84 | 20.44 | 1.53 | 0.59 | 0.03 | 0.92 |
| SDI | 43.59 | 25.59 | 32.25 | 5.81 | 2.3532 × 10−5 | 0.05 | 0.0006 |
| q_x1s | 15.44 | 84.86 | 36.65 | 433.88 | 0.02 | 0.04 | 0.06 |
| q_x2s | 26.60 | 79.64 | 41.15 | 201.29 | 0.02 | 0.24 | 0.03 |
| q_x3s | 4.65 | 16.85 | 6.63 | 9.47 | 0.28 | 0.44 | 0.16 |
| q_y1s | 7.46 | 3.16 | 3.24 | 4.23 | 0.0004 | 0.71 | 0.002 |
| q_y2s | 5.54 | 5.21 | 7.34 | 1.52 | 0.06 | 0.11 | 0.09 |
| q_L | 46.69 | 191.99 | 84.43 | 95.06 | 9.0908 × 10−6 | 0.35 | 0.0006 |
| q_S1 | 56.75 | 1350.90 | 79.90 | 6046.60 | 0.45 | 0.06 | 0.96 |
| q_S2 | 174.05 | 4175.40 | 216.74 | 4848.20 | 0.21 | 0.86 | 0.27 |
| q_S3 | 13.62 | 140.08 | 26.81 | 185.19 | 0.05 | 0.73 | 0.05 |
| q_S | 244.42 | 4942.00 | 323.44 | 1492.70 | 0.01 | 0.12 | 0.005 |
| q_ym | 5.28 | 0.65 | 3.88 | 0.47 | 0.001 | 0.65 | 0.003 |
| c_P | 252.60 | 1860.20 | 262.74 | 524.53 | 0.55 | 0.10 | 0.74 |
| c_SA | 527.99 | 16,810.00 | 691.58 | 4882.80 | 0.005 | 0.10 | 0.003 |
| c_AA | 220.86 | 2343.60 | 124.17 | 2159.90 | 0.0008 | 0.90 | 0.002 |
| c_Ci | 0.33 | 0.03 | 0.18 | 0.00 | 0.03 | 1.9949 × 10−5 | 0.04 |
| c_R | 0.34 | 0.02 | 0.12 | 0.00 | 0.0003 | 0.005 | 0.001 |
| Wheat Species or Amphidiploid | Abbreviation | Number of Accessions | Number of Plants |
|---|---|---|---|
| T. aestivum L. | TAE | 5 | 50 |
| T. spelta L. | TSP | 3 | 14 |
| T. antiquorum Heer ex Udach. | TAN | 2 | 20 |
| T. compactum Host | TCO | 5 | 63 |
| T. macha Dekapr. et Menabde | TMA | 2 | 9 |
| T. sphaerococcum Perc. | TSH | 2 | 18 |
| T. yunnanense King | TYU | 1 | 7 |
| Amphiploid speltiforme | ASP | 1 | 9 |
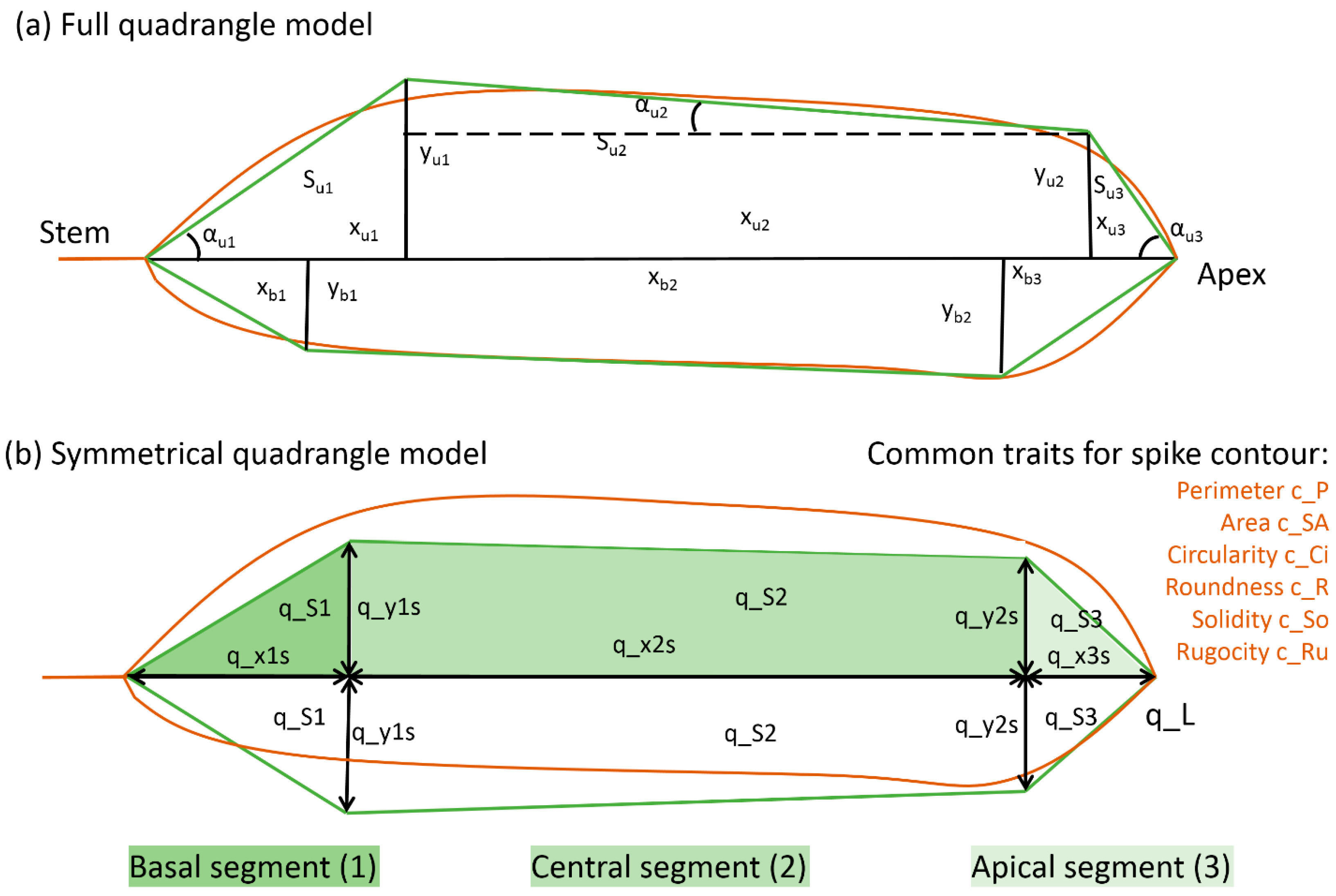
| Trait No. | Abbreviation | Name | Measurement Units |
|---|---|---|---|
| Manually estimated parameters | |||
| 1 | SL | Spike length | mm |
| 2 | SFW | Front width | mm |
| 3 | SSW | Side width | mm |
| 4 | SSC | Spikelet number | dimensionless |
| 5 | SDI | Density index | dm−1 |
| Independent parameters for the quadrangle model | |||
| 6 | q_x1s | Length of the basal spike segment | mm |
| 7 | q_x2s | Length of the central spike segment | mm |
| 8 | q_x3s | Length of the apical spike segment | mm |
| 9 | q_y1s | Width of the basal segment | mm |
| 10 | q_y2s | Width of the apical segment | mm |
| Derived parameters for the quadrangle model | |||
| 11 | q_L | Spike length (q_x1s + q_x2s + q_x3s) | mm |
| 12 | q_S1 | Area of the basal spike segment | mm2 |
| 13 | q_S2 | Area of the central spike segment | mm2 |
| 14 | q_S3 | Area of the apical spike segment | mm2 |
| 15 | q_S | Area of the quadrangle for half a spike | mm2 |
| 16 | q_ym | Width index (q_S/q_L) | mm |
| 17 | q_x1ns | Normalized length of the basal spike segment (q_x1s/q_L) | dimensionless |
| 18 | q_x2ns | Normalized length of the central spike segment (q_x2s/q_L) | dimensionless |
| 19 | q_x3ns | Normalized length of the apical spike segment (q_x3s/q_L) | dimensionless |
| 20 | q_y1ns | Normalized width of the basal spike segment (q_y1s/q_L) | dimensionless |
| 21 | q_y2ns | Normalized width of the apical spike segment (q_y2s/q_L) | dimensionless |
| 22 | q_S1S | Normalized area of the basal spike segment (q_S1/q_S) | dimensionless |
| 23 | q_S2S | Normalized area of the central spike segment (q_S2/q_S) | dimensionless |
| 24 | q_S3S | Normalized area of the apical spike segment (q_S3/q_S) | dimensionless |
| General size and shape parameters for the spike contour | |||
| 25 | c_P | Perimeter | mm |
| 26 | c_SA | Spike body projection area | mm2 |
| 27 | c_AA | Awn area | mm2 |
| 28 | c_Ci | Circularity | dimensionless |
| 29 | c_R | Roundness | dimensionless |
| 30 | c_So | Solidity | dimensionless |
| 31 | c_Ru | Rugosity | dimensionless |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komyshev, E.G.; Genaev, M.A.; Kruchinina, Y.V.; Koval, V.S.; Goncharov, N.P.; Afonnikov, D.A. Evaluation of the Spike Diversity of Seven Hexaploid Wheat Species and an Artificial Amphidiploid Using a Quadrangle Model Obtained from 2D Images. Plants 2024, 13, 2736. https://doi.org/10.3390/plants13192736
Komyshev EG, Genaev MA, Kruchinina YV, Koval VS, Goncharov NP, Afonnikov DA. Evaluation of the Spike Diversity of Seven Hexaploid Wheat Species and an Artificial Amphidiploid Using a Quadrangle Model Obtained from 2D Images. Plants. 2024; 13(19):2736. https://doi.org/10.3390/plants13192736
Chicago/Turabian StyleKomyshev, Evgenii G., Mikhail A. Genaev, Yuliya V. Kruchinina, Vasily S. Koval, Nikolay P. Goncharov, and Dmitry A. Afonnikov. 2024. "Evaluation of the Spike Diversity of Seven Hexaploid Wheat Species and an Artificial Amphidiploid Using a Quadrangle Model Obtained from 2D Images" Plants 13, no. 19: 2736. https://doi.org/10.3390/plants13192736







