Abstract
The constantly growing need to increase the production of agricultural products in changing climatic conditions makes it necessary to accelerate the development of new cultivars that meet the modern demands of agronomists. Currently, the breeding process includes the stages of genotyping and phenotyping to optimize the selection of promising genotypes. One of the most popular phenotypic methods is the pulse-amplitude modulated (PAM) fluorometry, due to its non-invasiveness and high information content. In this review, we focused on the opportunities of using chlorophyll fluorescence (ChlF) parameters recorded using PAM fluorometry to assess the state of plants in drought and heat stress conditions and predict the economically significant traits of wheat, as one of the most important agricultural crops, and also analyzed the relationship between the ChlF parameters and genetic markers.
Keywords:
breeding; chlorophyll fluorescence; PAM fluorometry; crop yield; stress tolerance; drought; heat; wheat 1. Introduction
Wheat is one of the most widely cultivated crops. It is critical to human nutrition, accounting for about 18% of all calories and up to 19% of protein consumed [1]. Simultaneously, with the growing population and the increasing need to step up the pace of production, there is a reduction in arable land, which further increases the requirements for crop productivity. Not all regions in which wheat is grown have favorable climatic conditions, and therefore grain yield (GY) is largely limited by abiotic stressors such as drought and elevated temperatures [2]. In response to demands from agricultural producers, breeders are working to develop new wheat cultivars that can provide high yields, including when grown in unfavorable conditions [3,4].
Currently, traditional approaches to the selection of genotypes for the development of new crop cultivars are enhanced by the use of genomic and phenomic technologies [5,6,7]. The latter are currently regarded as an essential element of breeding programs. At the same time, protocols for introducing phenotypic methods into the system for selecting promising genotypes are still being developed and improved. In particular, optical phenotyping methods are actively being introduced into this process [8,9]. Optical methods include spectral methods, in particular, multi- and hyperspectral techniques, thermal imaging, the assessment of photosynthetic activity based on chlorophyll fluorescence (ChlF) parameters, and others. Methods based on the recording and interpretation of data on the intensity of ChlF have long been firmly established in the practice of plant research [10,11,12,13]. These methods include the pulse-amplitude modulated (PAM) fluorometry, the fast polyphasic rise of the induction curve (OJIP), fast repetition rate (FRR) fluorometry, etc. [14,15,16]. Their use in the breeding process is a promising approach to assess the health of plants at early stages of development and select genotypes that have the greatest potential to achieve target traits.
One of the most informative methods for recording ChlF is the PAM fluorometry. The PAM parameters, calculated based on the ChlF intensity, reflect the activity of numerous processes occurring in the photosynthetic apparatus [10,11,12]. It is known that the PAM parameters are closely related to CO₂ assimilation [15,17]. An analysis of the changes in ChlF parameters makes it possible to detect changes in the physiological state of plants in response to the influence of various factors, including the availability of water and minerals, temperature, lighting, biotic factors, etc. [18,19,20,21,22,23,24].
This review discusses the potential and examples of the use of PAM fluorometry in the breeding process, both for predicting significant wheat traits, including in combination with genotypic predictors, and for assessing the current plant state in various conditions, including field studies and stress modeling in a controlled environment. Due to the larger amount of data available, we analyze the results obtained on Triticum aestivum (unless otherwise noted).
2. Briefly about PAM Fluorometry
PAM fluorometry is based on recording the intensity of ChlF under different light conditions [25]. In this case, such parameters as the minimum level of fluorescence under weak measuring light and the maximum level of fluorescence during a saturation pulse of high intensity light in a dark-adapted state (F0 and Fm) and under the actinic light (F0′ and Fm′), as well as the current fluorescence level under actinic light (Ft), are recorded. In the simplest case, F0 is recorded after a period of dark adaptation; then, a pulse of saturation light is applied, causing the closure of all working photosystems (electron acceptors QA), and Fm is recorded [26]. Fv/Fm (the maximum quantum efficiency of photosystem II (PSII)), calculated using Fm and F0 values, is the most commonly used PAM parameter for assessing the condition of plants [22,27,28,29,30]. The PAM method is also used to study the processes accompanying changes in fluorescence intensity after the actinic light is turned on (Kautsky effect), which reflects transient processes, including the rate of activation of the Calvin–Benson cycle, as well as photosynthetic activity in the light-adapted state [31,32,33,34]. Using the PAM method, it is possible to evaluate the contribution of the photochemical and non-photochemical quenching of fluorescence to the realization of the energy of absorbed light [32,34]. The most commonly analyzed PAM parameters are shown in Table 1.

Table 1.
Commonly used ChlF parameters. Prepared by the authors based on data from Maxwell and Johnson [15], Baker [17]; Kalaji et al. [11,12]; and Klughammer and Schreiber [35].
Among the advantages of PAM fluorometry, it is important to note the high information content and sensitivity of PAM parameters, which allows for a comprehensive assessment of the state of the photosynthetic apparatus and makes the PAM method one of the most used for studying the activity of photosynthesis. On the other hand, it has some limitations. Systems using PAM technology have a relatively high cost due to the technical complexity of the integrated equipment, but manufacturers have devoted much effort to making PAM fluorometers more affordable and easier to operate [36]. A major limitation is the relative low speed and, in some cases, the methodological complexity of obtaining data due to the fairly long period needed (tens of minutes) for dark adaptation [37]. This factor does not allow for the use of PAM fluorometry in studies comparable in throughput to other optical methods, such as the registration of solar-induced fluorescence or reflectance parameters. In addition, researchers encounter difficulties in interpreting PAM parameters. The complex structure of the crown and individual leaves in vascular plants, as well as a number of other features, make it difficult to interpret the results obtained [13]. At the same time, such features themselves are of great interest for study. Attempts to reduce the impact of these limitations on the use of this method are currently being made. PAM-imaging systems, making it possible to record ChlF parameters simultaneously on a lot of plants or on a wide area of a large plant, have become widespread. In addition, attempts to record initial photosynthetic parameters to estimate Fv/Fm without prior dark adaptation using machine-learning methods are being made [38]. The development of technologies and approaches to recording and interpreting ChlF data enhances the prospects for using PAM fluorometry for large-scale plant studies. Combined with this fact, extended information about the state of plants and their response to external factors [11,12,39] makes methods based on recording ChlF, among which PAM fluorometry is the most widely used, promising for use in the breeding process. PAM parameters can be used to assess the sensitivity of various genotypes to stressors, acting both as independent predictors of stress tolerance and in combination with other phenotypic and genotypic markers.
3. Detection of Drought and Heat Stress Using ChlF Parameters
PAM fluorometry, which makes it possible to assess the current level of photosynthetic activity, is widely used to assess the effects of stress. This is due to the fact that photosynthesis is a complex process that is highly sensitive to environmental conditions [40]. Moreover, the rates of photosynthesis directly affect plant productivity, so the maintenance of its activity serves as an indicator of potential survival and yield under stressful conditions [41]. Changes in photosynthetic activity can be caused both by the direct influence of environmental factors on the functioning of the photosynthetic apparatus, and by changes in other physiological processes that are closely related to photosynthesis, including transpiration, the transport of assimilates, etc. [42]. Thus, the use of methods based on recording ChlF allows researchers to obtain fairly complete and accurate information about the state of the photosynthetic apparatus under the influence of stressors of various natures [12,23].
3.1. Drought Stress
Drought is one of the main causes of crop losses in agricultural plants, including wheat [43,44,45]. At the same time, researchers face the challenge of studying the state of plants under conditions of water deficiency before the appearance of irreversible changes caused by drought [46]. Due to the sensitivity of the light-dependent reactions of photosynthesis to changes in water content, ChlF parameters are widely used to assess plant responses to drought. At the same time, various ChlF parameters can act as criteria for assessing the susceptibility of plants to drought-induced changes [12].
Fv/Fm is one of the traditional ChlF parameters used to diagnose the state of plants. As a rule, under optimal conditions, the value of Fv/Fm, which reflects the maximum quantum yield of PSII, ranges from 0.77 to 0.84 in different plants [47,48,49,50]. The convenience of measuring this parameter due to its independence from the current light intensity and gas composition of the air determines its frequent use to assess the effects of various stressors. Fv/Fm decreases under water deficiency, but sensitivity to the intensity of water stress varies among environments. Thus, a 15-day soil moisture deficit in 6-week-old wheat plants grown in greenhouse conditions caused a decrease in the Fv/Fm level from 0.73 to 0.49 [51].
At the same time, the sensitivity of the parameter Fv/Fm to drought depends on the strength and duration of water deficit. In particular, no differences were found between Fv/Fm in rainfed and irrigated conditions in the field [52]. Fv/Fm also did not change significantly in response to soil drought stress in pot experiments [53,54]. In addition, Fv/Fm responds poorly to moderate drought or early stages of severe drought stress [53,54,55]. Moderate physiological drought caused by polyethylene glycol did not cause changes in Fv/Fm at medium N supply, while more severe drought significantly reduced the value of this parameter [56]. Due to the high stability of Fv/Fm, its use as a criterion of genotype tolerance at early stages or under moderate water stress seems unpromising. A decrease in Fv/Fm in many cases is associated with irreversible damage to the photosynthetic apparatus, including the inactivation of photosynthetic reaction centers [57], while the activity of photosynthetic processes changes long before the onset of structural rearrangements in photosystems [58]. At the same time, the simplicity and high reproducibility of measuring Fv/Fm both in laboratory and field conditions make it possible to propose it as a quantitative criterion for the depth of stress that a specific genotype can withstand during selection screening. Thus, this parameter is convenient for determining critical threshold values of drought tolerance of a genotype.
ChlF parameters, which reflect not the integrity, but the efficiency of the photosynthetic apparatus, are more sensitive to the effects of various stressors, including drought. These parameters primarily include ΦPSII, qP, ETR, as well as qN and NPQ [51,56,59]. Significant changes in these parameters occur even with a relatively weak change in water status.
ΦPSII is one of the main ChlF parameters characterizing the efficiency of using the energy of absorbed light into photochemical processes; the parameter qP, which is close to ΦPSII, reflects the proportion of open photosystems II capable of participating in electron transport along the photosynthetic electron transport chain (PETC) [15]. Water deficiency causes the suppression of the linear electron flow, which manifests as a decrease in the values of the ΦPSII and qP parameters [51,60,61,62,63,64]. In particular, a 10-day drought caused a significant decrease in ΦPSII in winter wheat plants at different stages of development; Moreover, the depth of stress changes increased with increasing drought intensity [60]. The dependence of qP on drought intensity has also been shown. In particular, a significant suppression of qP was observed starting from day 6 of drought stress and statistically significantly increased with increasing treatment duration [62]. Along with pot experiments, a decrease in ΦPSII and qP in wheat has also been observed under rainfed conditions in the field [65], as well as when physiological drought is induced by PEG [56,66]. It is worth noting that these parameters show similar dynamics and comparable percentage changes during drought. However, their level depends on the intensity of light supplied to the plant [17]. To take into account the intensity of actinic light that acts when recording ChlF parameters, the parameter ETR, which characterizes the rate of electron transport along the PETC, is used [49]. In general, the direction of drought-induced changes in ETR coincides with those for ΦPSII and qP. However, the sensitivity of these parameters may vary due to genotypic differences and recording conditions [51,52,56].
A group of ChlF parameters that reflect the thermal dissipation of energy from absorbed sunlight, such as NPQ and qN, also show significant changes during drought stress development. These parameters have fairly high variability and sensitivity to changes in external conditions both in controlled and field conditions in T. aestivum [51,53,56,60,62,65,67]. The parameters NPQ and qN show an increase even with a moderate water deficit. In particular, qN in plants subjected to water deficiency was higher compared to the control after 4 days of treatment in a pot experiment [53]. Moreover, the level of qN and NPQ increases significantly with increasing drought duration [60,62]. It should be noted that along with the typical increase in NPQ during drought, a decrease in this parameter can be observed at the terminal stage of drought [68,69].
Drought-induced changes in the described parameters in T. aestivum begin at different stages of water stress, which leads to their different sensitivity and applicability. In most cases, ChlF parameters reflecting the efficiency of linear electron flow in PETC respond to water deficiency earlier than others (for example, Fv/Fm) [69]. This is due to the high sensitivity of electron transport to fluctuations in the activity of physiological processes and the rapid response to changes in signaling cascades. An increase in the intensity and/or duration of drought stress causes rearrangements and damage to the structure of photosystems, which manifests itself as a decrease in Fv/Fm. The further development of a drought of extreme intensity can cause damage to thylakoid membranes, which leads to a drop in the pH gradient and a decrease in NPQ, along with a reduction in photosynthetic activity.
The observed changes in ChlF parameters under drought stress in higher plants, including wheat, may be a result of changes in both the activity of the photosynthetic apparatus and other physiological processes. The complex of mechanisms that generate a signal of the water deficiency and cause changes in stomatal aperture includes the involvement of a large number of signaling molecules, the key of which are abscisic acid, calcium ions and reactive oxygen species (ROS) [70]. As a result of drought-induced stomatal closure, the availability of CO2 is reduced, which, together with a decrease in mesophyll conductivity for CO2 and the activity of enzymes such as RuBisCO, has a negative effect on the activity of the Calvin–Benson cycle and suppresses the entire process of photosynthesis, including the light-dependent stages [71]. The inhibition of PETC leads to the excessive formation of ROS, which, on the one hand, perform a signaling function, triggering numerous cascades of reactions; on the other hand, ROS cause damage to thylakoid membranes and proteins of the photosynthetic apparatus, leading to a decrease in the parameters of photosynthetic activity [72]. Such changes may be irreversible and require de novo synthesis of a number of photosynthetic proteins.
Considering the high sensitivity of ChlF parameters to the action of drought stress, it is necessary to take into account that drought is a slowly developing stress factor under natural conditions. The strategy for developing drought tolerance includes, among other things, rearrangements in the photosynthetic apparatus [73,74]. This is also reflected in the ChlF parameters recorded during drought. On the other hand, the complexity of the parameters helps to identify features of drought tolerance mechanisms in specific genotypes [73], which can be useful in breeding.
3.2. Heat Stress
One of the most important impacts of climate change is the earlier onset of persistently high temperatures during the growing season [75]. Also, over the past century, the average annual temperature in regions where wheat and other major crops are grown has increased by 1 °C [76]. Wheat, like many C3 plants, has a temperature optimum for active photosynthesis, growth and development in the range from 17 to 25 ℃ [77]. At the same time, a significant part of wheat cultivation areas is located in regions where wheat is exposed to heat stress.
One of the most popular ChlF parameters used to assess heat stress in wheat is Fv/Fm. It makes it possible to assess the depth of stress in plants and the degree of damage to the photosynthetic apparatus. In particular, moderate heating for 5 days caused a decrease in Fv/Fm in wheat plants; Moreover, the value of this parameter reached the control level after the cessation of the stressor [78]. The promise of Fv/Fm for detecting stress changes during short-term exposure to a stressor was also shown in other works. In particular, Fv/Fm acted as a sensitive indicator of the development of heat stress of varying intensity, as well as the recovery of photosynthetic capacity after its end [79]. In the work [80], a temperature increase by 2–3 °C for 4–8 days caused a decrease in Fv/Fm, and the magnitude of such a decrease varied in different years of the experiment. A long-term moderate increase in growing temperature can also negatively affect to the Fv/Fm level [81]. Taking into account the fairly high sensitivity and convenience of recording Fv/Fm both in laboratory and field studies, it should be noted that the Fv/Fm level characterizes the functional integrity of the photosynthetic apparatus, which changes quite quickly when exposed to elevated temperatures. At the same time, its change is preceded by a change in ChlF parameters, which reflect the current activity of photosynthesis, including electron transport through the PETC. In particular, exposure to heating to 40 °C for 30 min did not cause changes in Fv/Fm, while a more sensitive parameter, NPQ, was significantly reduced in T. aestivum [82]. The physiological basis of Fv/Fm tolerance to mild or early-stage heat stress may be due to changes in the functioning of PETC, which are aimed at protecting PSII from damage. It was shown that short-term heating in the dark (30–60 min) caused a decrease in the Fv/Fm value, which was due to the degradation of the D1 protein [83]. At the same time, damage to PSII did not occur during similar heating in light, which may be due to the intensification of non-photochemical quenching and the activation of cyclic electron flow. Such changes lead to a decrease in the linear electron flow, which manifests as a drop in the parameters ΦPSII and qP [84,85,86,87]. The activation of the mechanisms protecting the photosynthetic apparatus from damage during heating causes a later response of Fv/Fm to the development of stress and, as a consequence, lower sensitivity to changes compared to parameters characterizing the activity of electron transport in PETC.
The parameters reflecting the intensity of the linear electron flow are very sensitive to changes in ambient temperature. In particular, a short-term gradual heating of wheat leaves caused the response of ΦPSII in the physiological temperature range (when moving from 25 to 30 °C) during the first minute [88]. In this case, a moderate short-term rise in temperature caused an increase in ΦPSII, followed by a decrease with a further temperature growth (on average, when the temperature reached 40–45 °C) [88,89]. Heating wheat leaves to 40 °C for several hours caused a drop in ΦPSII and ETR by about half [90]. Similarly, increasing the temperature in the phytotron to 40 °C for 4 h induced a decrease in ΦPSII and qP [91].
The decrease in linear electron transport rate caused by moderate thermal exposure is usually accompanied by an increase in non-photochemical quenching [90,91,92]. At the same time, more intense exposure may form a different picture of the photosynthetic response. In particular, the short-term (30 min) heating of wheat grown hydroponically to 44 °C caused a slight increase in the energy-dependent component of NPQ (NPQF), accompanied by a statistically significant decrease in ΦPSII; at the same time, exposure to a temperature of 46.5 °C caused a simultaneous decrease in both ΦPSII and NPQF [86]. Similar results (a simultaneous decrease in both ΦPSII and NPQ) were also shown in a pot experiment after heating wheat seedlings to 45 °C for 30 min [93]. Along with intense short-term heating, long-term exposure to lower (but still very elevated) temperatures (38–42 °C) can also induce such changes in the activity of the light-dependent stage of photosynthesis [94,95].
It is worth noting that variations in the tolerance of different genotypes of T. aestivum to elevated temperatures are also manifested in different stability of the photosynthetic apparatus. Such differences appear both in the short-term [29,87,88,93,96] and long-term [29,81,84,85] heat stress.
The main targets for the effect of heat stress on photosynthesis are the Calvin–Benson cycle, the oxygen-evolving complex, PETC and photophosphorylation [97]. In particular, when the temperature changes, the activity of RuBisCO, RuBisCO activase, and enzymes involved in the regeneration of ribulose-1,5-bisphosphate significantly changes [98,99]. This effect is caused by going beyond the temperature optimum, a decrease in ATP content, and oxidative stress. In addition to the reactions directly involved in CO2 assimilation, an increase in temperature can affect the functioning of the PETC; at the same time, PSII is more susceptible to heat damage compared to PSI. The main targets here are the D1 protein, the plastoquinone pool, cytochrome b559, the oxygen-evolving complex, etc. [100]. When exposed to critical temperatures, a decrease in chlorophyll content and the destruction of the lipid bilayer of membranes and protein structure can occur [101]. All of these processes lead to a decrease in photosynthetic activity, which is detected with a high sensitivity using ChlF parameters.
It is worth noting that plants have powerful adaptation mechanisms for survival in conditions of elevated temperatures, including the maintenance and recovery of photosynthetic capacity. The disclosure and regulation of such mechanisms will help optimize the heat tolerance of crop plants, particularly wheat, to improve agricultural efficiency [101].
In general, considering the efficiency of PAM fluorometry for assessing the depth of drought and heat stress on wheat plants, the following can be summarized: ChlF parameters are sensitive indicators for these stress factors. Their changes were observed at different stages of stressors of different intensity. The earliest changes are typical for parameters reflecting PETC efficiency (ΦPSII, qP, ETR) and non-photochemical quenching (NPQ, qN). It should be taken into account that ΦPSII, qP and ETR are characterized by a decrease with increasing stress depth, whereas NPQ and qN are characterized by a rise at the initial stages of stress development with a subsequent drop with increasing stress intensity and depth. Fv/Fm, reflecting the integrity of PSII, responds to the stressor later in comparison with the above parameters, but at the same time has a high reproducibility of measurements, which underpins its wide use. These features should be taken into account when developing specific protocols for assessing the state of plants under stress conditions.
4. Predicting Crop Traits Using ChlF Parameters
In addition to the detection of stress states in agricultural plants, the use of phenotypic traits recorded by non-invasive optical methods for the selection of potentially productive and stress-tolerant genotypes in the breeding process is in active development [102,103,104]. In particular, the work [93] showed that the quantum yield of PSII in the light-adapted state (ΦPSIIef) in young wheat seedlings was positively correlated with the accumulation of plant fresh weight at a later age. In addition, a parameter demonstrating the rate at which the quantum yield reached a steady-state value after turning on the light (half-time) was negatively correlated with the fresh and dry weight of older plants. The steady-state and transient parameters of photosynthetic activity reflect the rate of the biosynthesis process, which influence the final characteristics of mature plants. It is known that the accumulation of biomass in plants that have not reached maturity can be associated with the biomass of mature plants [105], and that, in turn, with the yield [4,106,107]. ChlF parameters also act as predictors of significant traits for other plant species [108,109,110]. Thus, the use of a combination of several ChlF parameters in conjunction with machine learning made it possible to predict the accumulation of fresh weight in lettuce plants [111]. In addition to the value of aboveground biomass, ChlF parameters show potential for predicting yield-related traits. In particular, F0 and Fv/Fm were significantly correlated with wheat GY under well-watered conditions in some types of environments [52]. It is important to take into account that ChlF parameters recorded at different stages of plant development have different potential for prediction. In particular, such an effect has been clearly demonstrated in barley plants [112].
When assessing the potential tolerance of wheat to adverse environmental conditions, such as drought and elevated temperatures, researchers focus on two types of predictors based on ChlF. In the first case, phenotypic parameters recorded in plants under optimal conditions act as predictors [52,93]. In this case, the predictive potential of the parameters used is determined by the initial activity of the photosynthetic apparatus. In laboratory conditions, this effect was shown when assessing the potential tolerance of wheat seedlings to elevated temperatures, which acted as a short-term stressor, and to soil drought (longer-term stress) [93]. Tolerance to soil moisture deficit showed a strong negative correlation with the NPQ value in the light-adapted state and the maximum NPQ level after turning on the actinic light in younger non-stressed plants; the heat tolerance was positively correlated with Fv/Fm and a dark level of ΦPSII (5 min after turning the actinic light off). It is worth noting that the predictive potential of ChlF parameters recorded in non-stressed plants requires in-depth research. In particular, it may be limited by the fact that the entire spectrum of genotypic variations affecting the physiological state and final agronomic traits of plants under stress conditions may not be manifested.
The second type of tolerance predictors are ChlF parameters recorded in plants that have already been exposed to stress factors, for example, in uncontrolled field conditions. In this case, the relationship between the phenotypic parameters of young plants under adverse conditions and their final economically significant traits may be due primarily to the stability of the photosynthetic apparatus and plant defense systems, including the work of heat shock proteins, the antioxidant system, etc. In particular, under rainfed (water-limited) conditions, F0 and Fv/Fm recorded at anthesis can show a statistically significant correlation with GY, but such a relationship can be ambiguous and unstable when growing plants in the different environments [52]. Also, the relationship of potential fluorescent predictors with yield and its components can be assessed by a set of phenotypic and agronomic characteristics recorded under conditions of different water availability. For example, in the work [113], the ChlF parameters F0′, Fm′, Y (NO), NPQ, ETRmax and Y (II), recorded at the stage of grain filling, significantly correlated with GY, harvest index, the number of kernels per spike, and 1000 kernel weight in two water conditions (well-watered and water-limited conditions). At the same time, the strongest positive relationship with GY in wheat was shown for Fm′ and Y (NO), negative—for NPQ.
In general, the use of phenotyping for the selection of promising genotypes at stages of plant development prior to full maturity in the breeding process opens up broad prospects for accelerating and optimizing the wheat breeding process. ChlF parameters are highly informative indicators of plant physiological state. In addition, the photosynthetic process and the accompanying changes in ChlF parameters are quite well studied, which facilitates the elucidation of the mechanisms of the relationship between identified predictors and target traits. At the same time, the use of several types of predictors is promising. Such predictors may include steady-state ChlF parameters, as well as parameters characterizing the light-induced dynamics of the efficiency of PETC operation, both under optimal and stressful conditions. In addition to a wide range of traits that can be studied at a fairly high speed using high-throughput phenotyping systems [114], the approach of integrating two interrelated components (phenotyping and genotyping) into the early selection process is being actively introduced [115,116,117]. This approach increases the reliability and accuracy of prediction, and therefore it is important to consider the available information on the relationship between ChlF parameters and genetic markers.
5. The Relationship between ChlF Parameters and Genotypic Characteristics
Bread wheat (Triticum aestivum L.) is an allohexaploid, which includes three genomes, A, B and D, with a size of 16–17 Gb [118]. The genome size of wheat is many times larger than that of other plant species [119]. Wheat, like other important agricultural crops (barley and rye), belongs to the Triticeae tribe. These crops have undergone many changes during the process of domestication [118,120]. The complete genome sequence of T. aestivum was formed as a result of hybridization of three diploid ancestors. The first hybridization event occurred between T. urartu (AA) and a yet unidentified species with a B genome, a close relative or ancestor of Aegilops Speltoides, with the formation of the tetraploid T. dicoccoides. The D genome was added to the domesticated tetraploid T. dicoccoides from Ae. Tauschii, forming the complete hexaploid genome of T. aestivum [118]. The D genome is characterized by fewer polymorphic markers, which indicates less genetic diversity due to a low recombination frequency [119,121]. The wheat D genome plays a key role in tolerance to biotic and abiotic stress, and also ensures the high baking qualities of wheat grain [122,123]. The three wheat genomes have approximately the same percentage of transposable elements (86%, 85% and 83% of the sequence for A, B, and D, respectively) [120]. However, the sizes of the genomes differ: B genome (5.18 Gb), A genome (4.93 Gb), and D genome (3.95 Gb). The largest number of markers in genome-wide association studies (GWAS) is determined in the B genome, and the smallest in the D genome [119,124], which is primarily explained by genetic diversity and the size of genomes.
ChlF parameters reflect the activity of photosynthetic processes for which certain genes are responsible. The peculiarity of photosynthetic genes is their movement from the chloroplast genome to the nuclear genome during evolution, while maintaining functional activity directly in the chloroplasts. To date, it has been determined that about 10–15% of plant genes are involved in photosynthesis [105]. However, GWAS analyses of traits associated with photosynthetic activity often identify single nucleotide polymorphisms (SNPs) associated with more than just photosynthesis [125]. An analysis, performed on the basis of the NCBI Genes database (https://www.ncbi.nlm.nih.gov/gene (accessed on 5 June 2024)), revealed trends in the distribution of T. aestivum photosynthesis-related genes along chromosomes (Figure 1). The distribution of genes between genomes is even, but the largest number of genes is in the D genome and linkage groups 1, 2, 5 and 6.
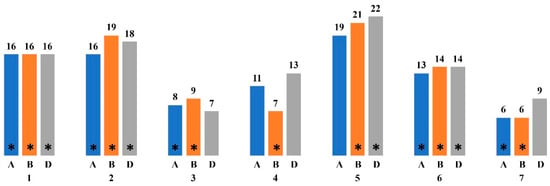
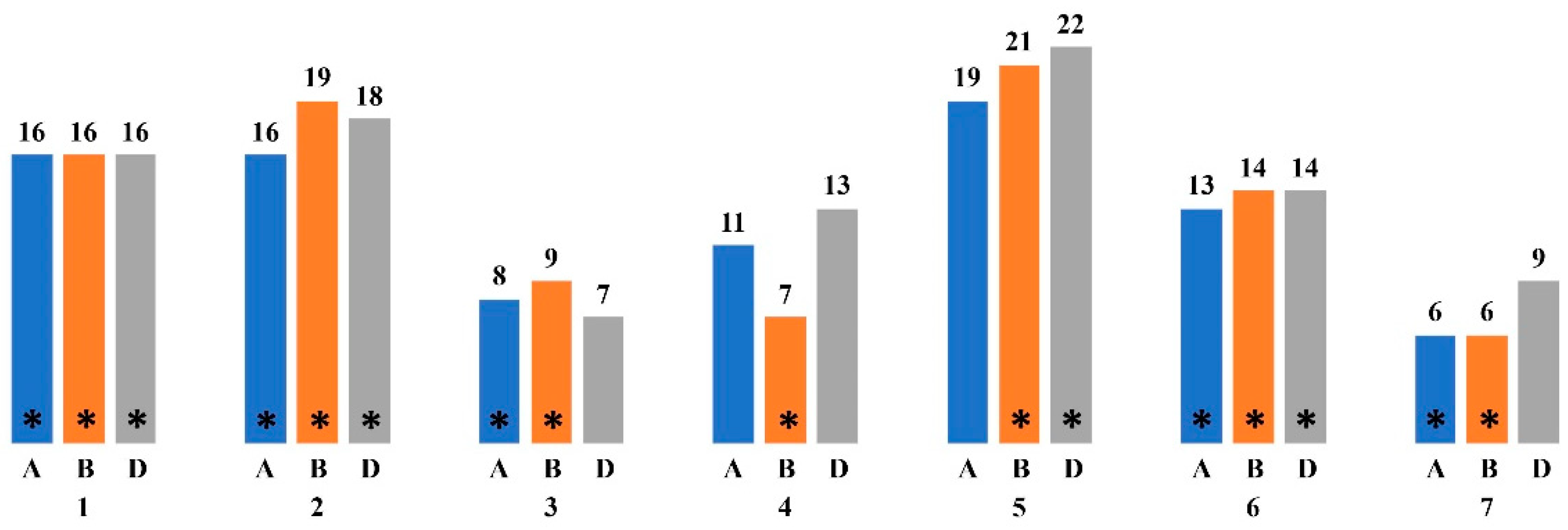
Figure 1.
The distribution of the genes characterized as involved in photosynthesis along the chromosomes of T. aestivum based on the NCBI Genes database (https://www.ncbi.nlm.nih.gov/gene (accessed on 5 June 2024)). * indicates chromosomes on which loci associated with ChlF parameters and chlorophyll content have been identified; the letters A, B and D indicate genomes; 1–7 indicate chromosomes; the number of genes described is indicated by the numbers above the corresponding columns.
Loci associated with fluorescence parameters and chlorophyll content were identified on chromosomes 1A, 1B, 1D, 2A, 2B, 2D, 3A, 3B, 4B, 5B, 5D, 6A, 6B, 6D, 7A, 7B. Particularly, chromosomes from the B genome are often described as including loci associated with photosynthetic parameters and chlorophyll in bread wheat and triticale [126,127,128,129,130,131]. The loci associated with chlorophyll content under stress conditions such as drought are found on chromosomes 1B, 2A, 2B, 3A, 3B, 4A, 5A, 6B, 6D and 7B [106,107]. Under the influence of heat stress, such loci are identified on chromosomes 1A 2B, 2D, 4B, 4D, 5D, 6A and 6B [128,131].
ChlF parameters, reflecting the activity of photosynthetic processes, are polygenic traits, like many important classical breeding traits such as yield and tolerance to stress. The quantitative trait loci (QTLs) identified in studies are associated not only with ChlF parameters, but also with other significant traits. Seven QTL clusters associated with both photosynthesis and GY, including those limited by the marker pairs Xgwm335.2–Xgwm186 and Xgwm296.3–Xbarc168, have been identified [132]. QTLs for Fv/Fm and grain weight per ear were identified on chromosome 6B [127]. The region of the stable SNP peak Excalibur_rep_c68899_1400 on chromosome 2B is described as being responsible for ΦPSII and wheat yield [133]. The locus in the Xbarc99–Xbarc169 region on chromosome 1D was responsible for the net rate of photosynthesis and GY, and the locus in the Xbcd1095–Xfbb113 region on chromosome 2B was additionally responsible for chlorophyll content [129]. Under drought conditions, shared loci have also been identified for ChlF parameters and GY on chromosome 7D [133], as well as ChlF parameters and drought tolerance in wheat on chromosome 1B [134].
The heritability of ChlF parameters should be discussed in detail. The results available to date show both the strong and weak heritability of ChlF parameters. In particular, a strong heritability of ChlF parameters has been shown in field studies on Hordeum vulgare plants [112]. At the same time, a low heritability of ChlF parameters was found on T. aestivum plants [52]. However, in this case, a limited number of measurements during each growing season should be taken into account. Considering the variability in weather conditions (in different cultivation years), the high sensitivity of ChlF parameters to environmental conditions may be a possible reason for the conclusion of the low heritability of traits. The above-mentioned high heritability of ChlF parameters under controlled conditions [112] supports this assumption.
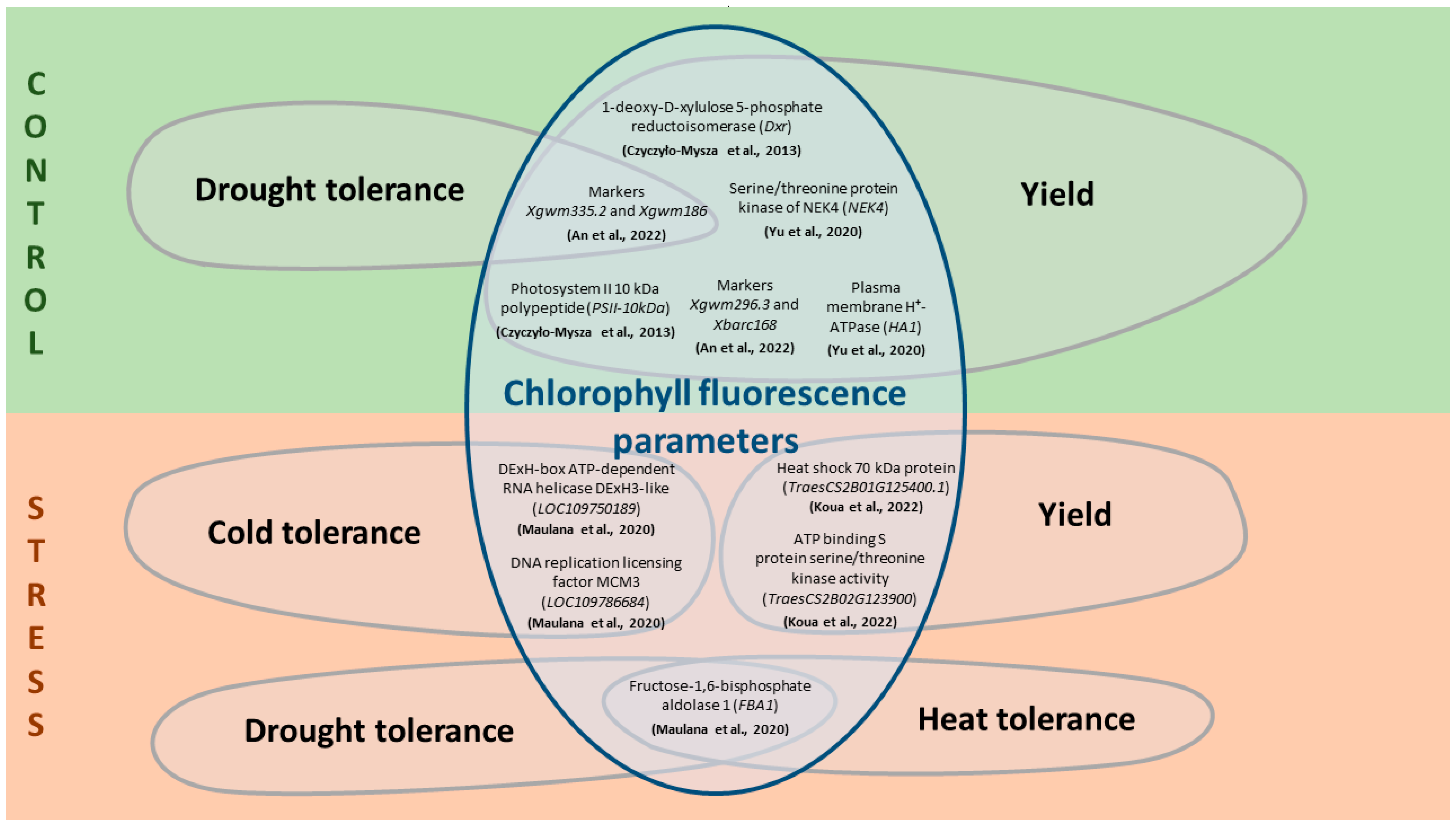
To analyze the interaction of traits associated with ChlF and significant breeding traits, it is worth noting the candidate genes localized in regions of interest (Figure 2 and Table S1). The following gene ontology terms are described for most of the identified genes: ATP binding, protein phosphorylation, photosynthesis, electron transfer, oxidoreductase activity, and chaperone proteins [124,133]. The genes localized in the regions associated with the considered traits have been described both under optimal plant growth conditions and under stress conditions [127,128,133].
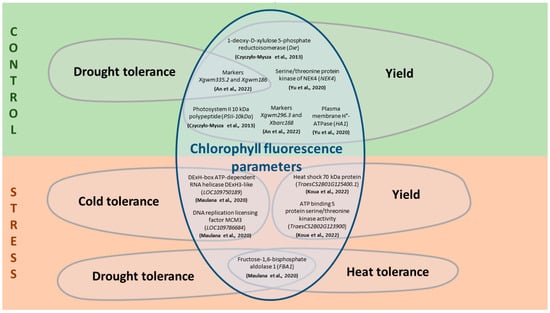
Figure 2.
Relationship between ChlF parameters and significant breeding traits through some candidate genes under control and stress conditions for T. aestivum. The scheme is based on data from [127,129,132,133,135], a detailed description of the measured ChlF parameters and associated genes is described in Table S1.
Under non-stress conditions, the SNP (IWB14950) for chlorophyll content is located in a region of chromosome 1B that has sequence similarity to the heat shock protein (LOC109759017), involved in heat stress tolerance [128]. On chromosome 1D, the SNP locus IWB17397 for GY and net photosynthetic rate contained the serine/threonine protein kinase NEK4 gene [129]. On chromosome 2B, the SNP locus IWA1040 for GY, net photosynthetic rate and, additionally, chlorophyll content, is associated with the plasma membrane H+-ATPase gene [129]. QTLs for Fv/Fm were found on chromosomes 2A, 3A, 6A, 7A, 2B, 5B, 6B, 1D and 2D [127]. Moreover, three of the five regions on chromosome 6B contained QTL for ChlF parameters that also coincided with QTL for plant yield parameters [127]. The identified candidate genes are involved in photosynthesis and energy metabolism [127].
Under drought conditions, multiple significant SNPs associated with the DNA replication licensing factor MCM3 gene, which is involved in the formation of tolerance to salt and cold stress in other crop species, were identified for chlorophyll content on chromosome 1B [135,136]. Also, under the influence of drought, two significant SNPs (IWB60417 and IWB11846), associated with candidate genes (DExH-box ATP-dependent RNA helicase DExH3-like (LOC109750189) and the fructose-1,6-bisphosphate aldolase 1 (FBA1), respectively) were identified for Fv/Fm on chromosome 3A [135]. The first gene is involved in plant development and tolerance to abiotic stresses, such as low temperature and freezing [137], while the latter is involved in plant stress reactions, such as drought and heat stress responses [138]. A locus for the combination of ChlF and yield traits has been identified on chromosome 2B. The locus, which has a pleiotropic effect for the quantum yield of PSII and GY under drought conditions, contained 31 genes responsible for the activity of peroxidase, nonspecific serine/threonine protein kinase and heat shock proteins [133]. In tetraploid wheat T. timopheevii, changes in photosynthesis parameters and yield under drought conditions caused by the activity of several genes localized in the distal region of the short arm of chromosome 2A [139]. TraesCS2A01G044900.1, encoding cytochrome P450, may be one of the candidates for plant stress response and yield effects [140]. TraesCS2A01G035100.1 encodes the chloroplast protein CP12, which has been shown to be involved in the regulation of the activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoribulokinase (PRK) [141,142]. TraesCS2A01G355400.1 encodes S-adenosylmethionine decarboxylase, an enzyme in the biosynthesis of polyamines involved in the regulation of tolerance to oxidative stress caused by drought [143]. Under heat stress, chlorophyll content markers are found on several chromosomes, including 2D, where candidate genes involved in stress responses and growth regulation are localized [128]. For example, plastid lipid-associated protein is involved in increasing plant yield under stress [144]. The N-terminal domain of heat shock protein is involved in plant responses to various environmental stresses, including heat [128]. IAA-amino acid hydrolase ILR1-like hydrolyzes amino acid conjugates of IAA (indole-3-acetic acid), which acts as a plant growth regulator [145]. Also, under heat stress, a gene encoding the K⁺ antiporter, which is involved in the response of plants to salt stress [146], was found on chromosome 4B [128].
It is worth noting a recently discovered pattern confirming the genetic relationship between ChlF parameters and significant breeding traits. An increase in the efficiency of photosynthesis among created cultivars occurs simultaneously with an increase in breeding progress. This is possible due to the selection of wheat cultivars that have allele variations that contribute not only to improved yield, but also to increased photosynthetic activity. For example, modern cultivars released after 2010 that have shown higher yields also have a higher chlorophyll content and quantum yield of PSII than cultivars released before 1980. Interestingly, for the quantum yield of PSII, the difference between these two groups was greater under drought than in control conditions, suggesting that selection has increased wheat yield under drought conditions through the accumulation of genetic variants that have a positive effect on photosynthetic activity under suboptimal conditions [133].
6. Conclusions
The method for recording ChlF parameters, based on PAM fluorometry, has high potential for use in breeding. One of the fields of its application is the assessment of the tolerance of genotypes to the effects of unfavorable factors during the growing season or when modeling stressors under controlled conditions. The prediction of economically significant traits at early stages of plant development is another potential niche for including ChlF parameters in the breeding process. In these cases, ChlF parameters can be used both as independent indicators of the potential of the genotypes under study and in combination with other phenotypic and genotypic characteristics. It is more appropriate to measure these parameters as predictors under controlled conditions due to the high influence of environmental factors on their values. Integrating PAM fluorometry into the process of selecting promising genotypes requires a deep understanding of the physiological basis of the relationship of ChlF parameters with the productivity potential of agricultural plants and with the nature of their response to changes in environmental conditions during the growth period, which directly affects the yield. In particular, a detailed study of the contribution of changes in the activity of a certain physiological process to the modulation of ChlF parameters and their impact on the final yield is necessary. It is also necessary to identify a set of genetic markers associated with parameters recorded using PAM fluorometry. This will provide a more meaningful and reliable selection of genome editing points or genotypes for traditional crossbreeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13192778/s1, Table S1: Relationship between ChlF parameters and significant breeding traits through some candidate genes under control and stress conditions for T. aestivum.
Author Contributions
Conceptualization, V.V. and O.S.; writing—original draft preparation, F.A., P.P. and O.S.; writing—review and editing, V.V.; visualization, P.P.; funding acquisition, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation (project no. 23-26-00212).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. ISBN 978-3-030-90672-6. [Google Scholar]
- Iqbal, N.; Hayat, M.T.; Tahir, A.A.; Akhtar, S.; Bangash, N.; Nazeer, A.; Saleem, A.R.; Riyazuddin, R. Global Prospects of Climate-Resilient Agriculture. In Climate-Resilient Agriculture, Vol 1; Hasanuzzaman, M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–25. ISBN 978-3-031-37423-4. [Google Scholar]
- Mondal, S.; Sallam, A.; Sehgal, D.; Sukumaran, S.; Farhad, M.; Navaneetha Krishnan, J.; Kumar, U.; Biswal, A. Advances in Breeding for Abiotic Stress Tolerance in Wheat. In Genomic Designing for Abiotic Stress Resistant Cereal Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 71–103. ISBN 978-3-030-75874-5. [Google Scholar]
- Langridge, P.; Reynolds, M. Breeding for Drought and Heat Tolerance in Wheat. Theor. Appl. Genet. 2021, 134, 1753–1769. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.; Alharbi, S.; Alotaibi, M.; Al Mosallam, M.; Motawei, M.; Alrajhi, A. Wheat Omics: Classical Breeding to New Breeding Technologies. Saudi J. Biol. Sci. 2021, 28, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Robbins, K.; Morales, N.; Shu, Q.; Cen, H. Advances in Optical Phenotyping of Cereal Crops. Trends Plant Sci. 2022, 27, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.P.A.; Veeramuthu, D.; Maharajan, T.; Soosaimanickam, M. The Era of Plant Breeding: Conventional Breeding to Genomics-assistedBreeding for Crop Improvement. Curr. Genom. 2023, 24, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Chawade, A.; Van Ham, J.; Blomquist, H.; Bagge, O.; Alexandersson, E.; Ortiz, R. High-Throughput Field-Phenotyping Tools for Plant Breeding and Precision Agriculture. Agronomy 2019, 9, 258. [Google Scholar] [CrossRef]
- Watt, M.; Fiorani, F.; Usadel, B.; Rascher, U.; Muller, O.; Schurr, U. Phenotyping: New Windows into the Plant for Breeders. Annu. Rev. Plant Biol. 2020, 71, 689–712. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Lysenko, V.; Rajput, V.D.; Kumar Singh, R.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Chalenko, E.; Yadronova, O.; Dmitriev, P.; et al. Chlorophyll Fluorometry in Evaluating Photosynthetic Performance: Key Limitations, Possibilities, Perspectives and Alternatives. Physiol. Mol. Biol. Plants 2022, 28, 2041–2056. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee; Strasser, B.J.; Strasser, R.J. ChlorophyllaFluorescence Induction in Higher Plants: Modelling and Numerical Simulation. J. Theor. Biol. 1998, 193, 131–151. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kolber, Z.S.; Prášil, O.; Falkowski, P.G. Measurements of Variable Chlorophyll Fluorescence Using Fast Repetition Rate Techniques: Defining Methodology and Experimental Protocols. Biochim. Biophys. Acta (BBA)—Bioenerg. 1998, 1367, 88–106. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W.; Demmig-Adams, B. Chlorophyll Fluorescence as a Tool to Monitor Plant Response to the Environment. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 583–604. ISBN 978-1-4020-3217-2. [Google Scholar]
- Guidi, L.; DeglInnocenti, E. Imaging of Chlorophyll a Fluorescence: A Tool to Study Abiotic Stress in Plants. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A., Ed.; InTech: Houston, TX, USA, 2011; ISBN 978-953-307-394-1. [Google Scholar]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Ohnishi, M.; Furutani, R.; Sohtome, T.; Suzuki, T.; Wada, S.; Tanaka, S.; Ifuku, K.; Ueno, D.; Miyake, C. Photosynthetic Parameters Show Specific Responses to Essential Mineral Deficiencies. Antioxidants 2021, 10, 996. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the Photosynthetic Apparatus Functions by Chlorophyll Fluorescence and P700 Absorbance in C3 and C4 Plants under Physiological Conditions and under Salt Stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Eduardo Aucique-Perez, C.; Elizabeth Román Ramos, A. Chlorophyll a Fluorescence: A Method of Biotic Stress Detection. In Challenges in Plant Disease Detection and Recent Advancements; Bahadur, A., Ed.; IntechOpen: London, UK, 2024; ISBN 978-0-85466-143-5. [Google Scholar]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous Recording of Photochemical and Non-Photochemical Chlorophyll Fluorescence Quenching with a New Type of Modulation Fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of Chlorophyll Fluorescence and Primary Photochemistry in Chloroplasts by Dibromothymoquinone. Biochim. Biophys. Acta (BBA)—Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll Fluorescence Technique as a Rapid Tool for in Vitro Screening of Olive Cultivars (Olea europaea L.) Tolerant to Drought Stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Phenotyping of Wheat Cultivars for Heat Tolerance Using Chlorophyll a Fluorescence. Funct. Plant Biol. 2012, 39, 936. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; He, L.; Zhang, H.-Y.; Guo, B.-B.; Zhu, Y.-J.; Wang, C.-Y.; Guo, T.-C. Assessment of Plant Nitrogen Status Using Chlorophyll Fluorescence Parameters of the Upper Leaves in Winter Wheat. Eur. J. Agron. 2015, 64, 78–87. [Google Scholar] [CrossRef]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Krause, G.H.; Jahns, P. Pulse Amplitude Modulated Chlorophyll Fluorometry and Its Application in Plant Science. In Light-Harvesting Antennas in Photosynthesis; Green, B.R., Parson, W.W., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2003; Volume 13, pp. 373–399. ISBN 978-90-481-5468-5. [Google Scholar]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B. Govindjee Modeling Chlorophyll a Fluorescence Transient: Relation to Photosynthesis. Biochem. Mosc. 2014, 79, 291–323. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of Photosynthetic Energy Partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A Tool for Large-Scale Plant Phenotyping Connected to the Open PhotosynQ Network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to Correctly Determine the Different Chlorophyll Fluorescence Parameters and the Chlorophyll Fluorescence Decrease Ratio RFd of Leaves with the PAM Fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Xia, Q.; Tang, H.; Fu, L.; Tan, J.; Govindjee, G.; Guo, Y. Determination of Fv/Fm from Chlorophyll a Fluorescence without Dark Adaptation by an LSSVM Model. Plant Phenomics 2023, 5, 0034. [Google Scholar] [CrossRef]
- Moustakas, M.; Eleftheriou, E.P.; Ouzounidou, G. Short-Term Effects of Aluminium at Alkaline pH on the Structure and Function of the Photosynthetic Apparatus. Photosynthetica 1997, 34, 169–177. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the World: Improving Photosynthetic Efficiency for Sustainable Crop Production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Foyer, C.H. Sink Regulation of Photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of Drought on Agronomic Traits of Rice and Wheat: A Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Zörb, C.; Geilfus, C.-M. Drought and Crop Yield. Plant Biol. J. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Nyaupane, S.; Poudel, M.R.; Panthi, B.; Dhakal, A.; Paudel, H.; Bhandari, R. Drought Stress Effect, Tolerance, and Management in Wheat—A Review. Cogent Food Agric. 2024, 10, 2296094. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon Yield of O2 Evolution and Chlorophyll Fluorescence Characteristics at 77 K among Vascular Plants of Diverse Origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Pfündel, E. Estimating the Contribution of Photosystem I to Total Leaf Chlorophyll Fluorescence. Photosynth. Res. 1998, 56, 185–195. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ullah, Z.; Sher, H.; Abbas, Z.; Rasheed, A. Water Stress Effects on Stay Green and Chlorophyll Fluorescence with Focus on Yield Characteristics of Diverse Bread Wheats. Planta 2023, 257, 104. [Google Scholar] [CrossRef]
- Del Pozo, A.; Méndez-Espinoza, A.M.; Garriga, M.; Estrada, F.; Castillo, D.; Matus, I.; Lobos, G.A. Phenotypic Variation in Leaf Photosynthetic Traits, Leaf Area Index, and Carbon Discrimination of Field-Grown Wheat Genotypes and Their Relationship with Yield Performance in Mediterranean Environments. Planta 2023, 258, 22. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Subash, N.; Haris, A.; Sikka, A.K. Influence of Water Stress on Leaf Photosynthetic Characteristics in Wheat Cultivars Differing in Their Susceptibility to Drought. Photosynthetica 2006, 44, 125–129. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.-B.; Olsovska, K.; Brestic, M. Photosynthetic Proton and Electron Transport in Wheat Leaves under Prolonged Moderate Drought Stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef]
- Hnilicka, F.; Lysytskyi, S.; Rygl, T.; Hnilickova, H.; Pecka, J. Effect of Short-Term Water Deficit on Some Physiological Properties of Wheat (Triticum aestivum L.) with Different Spike Morphotypes. Agronomy 2023, 13, 2892. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shangguan, Z. Leaf Gas Exchange and Fluorescence of Two Winter Wheat Varieties in Response to Drought Stress and Nitrogen Supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Photoinhibition and Zeaxanthin Formation in Intact Leaves: A Possible Role of the Xanthophyll Cycle in the Dissipation of Excess Light Energy. Plant Physiol. 1987, 84, 218–224. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, Z. Drought-Induced Changes in Chlorophyll Fluorescence of Young Wheat Plants. Biotechnol. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Wang, F.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Adaptation to and Recovery from Drought Stress at Vegetative Stages in Wheat (Triticum aestivum) Cultivars. Funct. Plant Biol. 2016, 43, 1159. [Google Scholar] [CrossRef]
- Man, J.; Yu, Z.; Shi, Y. Radiation Interception, Chlorophyll Fluorescence and Senescence of Flag Leaves in Winter Wheat under Supplemental Irrigation. Sci. Rep. 2017, 7, 7767. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of Physiological Indicators Associated with Drought Tolerance in Wheat under Drought and Re-Watering Conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef]
- Radzikowska-Kujawska, D.; John, P.; Piechota, T.; Nowicki, M.; Kowalczewski, P.Ł. Response of Winter Wheat (Triticum aestivum L.) to Selected Biostimulants under Drought Conditions. Agriculture 2022, 13, 121. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Zhang, W. Physiological Characteristics, Crop Growth and Grain Yield of Twelve Wheat Varieties Cultivated in the North China Plain. Agronomy 2023, 13, 3041. [Google Scholar] [CrossRef]
- Kang, J.; Chu, Y.; Ma, G.; Zhang, Y.; Zhang, X.; Wang, M.; Lu, H.; Wang, L.; Kang, G.; Ma, D.; et al. Physiological Mechanisms Underlying Reduced Photosynthesis in Wheat Leaves Grown in the Field under Conditions of Nitrogen and Water Deficiency. Crop J. 2023, 11, 638–650. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Cui, J.-M.; Su, Y.-Q.; Zhang, C.-M.; Ma, J.; Zhang, Z.-W.; Yuan, M.; Liu, W.-J.; Zhang, H.-Y.; Yuan, S. Comparison of Phosphorylation and Assembly of Photosystem Complexes and Redox Homeostasis in Two Wheat Cultivars with Different Drought Resistance. Sci. Rep. 2017, 7, 12718. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental Drought-Priming Enhances Tolerance to Post-Anthesis Drought in Offspring of Wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef]
- Ma, S.-C.; Li, F.-M.; Yang, S.-J.; Li, C.-X.; Jiang, L.-N.; Yunusa, I. Characteristics of Flag Leaf Photosynthesis and Root Respiration of Four Historical Winter Wheat Varieties Released over Recent Decades in Semi-Arid Northwest China. Aust. J. Crop Sci. 2013, 7, 1100–1105. [Google Scholar]
- Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.K. Modulation of Photosynthesis and Other Proteins during Water–Stress. Mol. Biol. Rep. 2021, 48, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G.; et al. Adjustment of Photosynthetic Activity to Drought and Fluctuating Light in Wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef]
- Jonwal, S.; Verma, N.; Sinha, A.K. Regulation of Photosynthetic Light Reaction Proteins via Reversible Phosphorylation. Plant Sci. 2022, 321, 111312. [Google Scholar] [CrossRef]
- Shah, H.; Hellegers, P.; Siderius, C. Climate Risk to Agriculture: A Synthesis to Define Different Types of Critical Moments. Clim. Risk Manag. 2021, 34, 100378. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, D.K.Y.; Shaghaleh, H.; Chang, T.; Alhaj Hamoud, Y. Response of Photosynthesis in Wheat (Triticum aestivum L.) Cultivars to Moderate Heat Stress at Meiosis and Anthesis Stages. Agronomy 2023, 13, 2251. [Google Scholar] [CrossRef]
- Xu, J.; Lowe, C.; Hernandez-Leon, S.G.; Dreisigacker, S.; Reynolds, M.P.; Valenzuela-Soto, E.M.; Paul, M.J.; Heuer, S. The Effects of Brief Heat During Early Booting on Reproductive, Developmental, and Chlorophyll Physiological Performance in Common Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 886541. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yao, X.; Liu, H.; Liu, B.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Comparison of the Abilities of Vegetation Indices and Photosynthetic Parameters to Detect Heat Stress in Wheat. Agric. For. Meteorol. 2019, 265, 121–136. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Maqsood, H.; Ullah, N.; Khan, A.; Waqas, M.; Sattar, N.; Tasneem, M.; Xu, X.; Zhangli, H.; et al. Strengthening Leaf Physiological Functioning and Grain Yield Formation in Heat-Stressed Wheat through Potassium Application. Front. Plant Sci. 2022, 13, 1005773. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.; Rosenqvist, E. Wheat Cultivars Selected for High Fv/Fm under Heat Stress Maintain High Photosynthesis, Total Chlorophyll, Stomatal Conductance, Transpiration and Dry Matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Marutani, Y.; Yamauchi, Y.; Miyoshi, A.; Inoue, K.; Ikeda, K.; Mizutani, M.; Sugimoto, Y. Regulation of Photochemical Energy Transfer Accompanied by Structural Changes in Thylakoid Membranes of Heat-Stressed Wheat. Int. J. Mol. Sci. 2014, 15, 23042–23058. [Google Scholar] [CrossRef]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to Photosystem II Due to Heat Stress without Light-Driven Electron Flow: Involvement of Enhanced Introduction of Reducing Power into Thylakoid Membranes. Planta 2012, 236, 753–761. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Sharma, D.K.; Ottosen, C.-O. Heat Stress and Recovery of Photosystem II Efficiency in Wheat (Triticum aestivum L.) Cultivars Acclimated to Different Growth Temperatures. Environ. Exp. Bot. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Hairat, S.; Khurana, P. Photosynthetic Efficiency, Temperature Induction Response, Carbon Isotope Discrimination Correlate with Expression Profiling in Indian Wheat Cultivars. Plant Signal. Behav. 2016, 11, e1179416. [Google Scholar] [CrossRef][Green Version]
- Yudina, L.; Sukhova, E.; Gromova, E.; Nerush, V.; Vodeneev, V.; Sukhov, V. A Light-Induced Decrease in the Photochemical Reflectance Index (PRI) Can Be Used to Estimate the Energy-Dependent Component of Non-Photochemical Quenching under Heat Stress and Soil Drought in Pea, Wheat, and Pumpkin. Photosynth. Res. 2020, 146, 175–187. [Google Scholar] [CrossRef]
- Li, C.; Ma, M.; Zhang, T.; Feng, P.; Chen, X.; Liu, Y.; Brestic, M.; Galal, T.M.; Al-Yasi, H.M.; Yang, X. Comparison of Photosynthetic Activity and Heat Tolerance between near Isogenic Lines of Wheat with Different Photosynthetic Rates. PLoS ONE 2021, 16, e0255896. [Google Scholar] [CrossRef]
- Sherstneva, O.; Abdullaev, F.; Kior, D.; Yudina, L.; Gromova, E.; Vodeneev, V. Prediction of Biomass Accumulation and Tolerance of Wheat Seedlings to Drought and Elevated Temperatures Using Hyperspectral Imaging. Front. Plant Sci. 2024, 15, 1344826. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Tajti, J.; Hamow, K.Á.; Marček, T.; Ivanovska, B.; Szalai, G.; Pál, M.; Zalewska, E.D.; Darkó, É. Acclimation of Photosynthetic Processes and Metabolic Responses to Elevated Temperatures in Cereals. Physiol. Plant. 2021, 171, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Marutani, Y.; Yamauchi, Y.; Higashiyama, M.; Miyoshi, A.; Akimoto, S.; Inoue, K.; Ikeda, K.; Mizutani, M.; Sugimoto, Y. Essential Role of the PSI–LHCII Supercomplex in Photosystem Acclimation to Light and/or Heat Conditions by State Transitions. Photosynth. Res. 2017, 131, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological Characteristics and Metabolomics of Transgenic Wheat Containing the Maize C4 Phosphoenolpyruvate Carboxylase (PEPC) Gene under High Temperature Stress. Protoplasma 2017, 254, 1017–1030. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought Priming at Vegetative Growth Stages Improves Tolerance to Drought and Heat Stresses Occurring during Grain Filling in Spring Wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Sherstneva, O.; Khlopkov, A.; Gromova, E.; Yudina, L.; Vetrova, Y.; Pecherina, A.; Kuznetsova, D.; Krutova, E.; Sukhov, V.; Vodeneev, V. Analysis of Chlorophyll Fluorescence Parameters as Predictors of Biomass Accumulation and Tolerance to Heat and Drought Stress of Wheat. Funct. Plant Biol. 2021, 49, 155–169. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High Temperature Specifically Affects the Photoprotective Responses of Chlorophyll B-Deficient Wheat Mutant Lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef]
- Kreslavski, V.; Tatarinzev, N.; Shabnova, N.; Semenova, G.; Kosobryukhov, A. Characterization of the Nature of Photosynthetic Recovery of Wheat Seedlings from Short-Term Dark Heat Exposures and Analysis of the Mode of Acclimation to Different Light Intensities. J. Plant Physiol. 2008, 165, 1592–1600. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat Stress: An Overview of Molecular Responses in Photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and Molecular Insights on Wheat Responses to Heat Stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Tang, M.; Jin, X.-Q.; Li, H.; Chen, L.-S.; Wang, Q.-L.; Sun, A.-Z.; Yi, Y.; Guo, F.-Q. Regulation of Calvin–Benson Cycle Enzymes under High Temperature Stress. Abiotech 2022, 3, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring High Temperature Responses of Photosynthesis and Respiration to Improve Heat Tolerance in Wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N. Imaging Technologies for Plant High-Throughput Phenotyping: A Review. Front. Agr. Sci. Eng. 2018, 5, 406–419. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Anshori, M.F.; Dirpan, A.; Sitaresmi, T.; Rossi, R.; Farid, M.; Hairmansis, A.; Purwoko, B.; Suwarno, W.B.; Nugraha, Y. An Overview of Image-Based Phenotyping as an Adaptive 4.0 Technology for Studying Plant Abiotic Stress: A Bibliometric and Literature Review. Heliyon 2023, 9, e21650. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Singh, U.; Godwin, D.C.; Bowen, W.T. Cereal Growth, Development and Yield. In Understanding Options for Agricultural Production; Tsuji, G.Y., Hoogenboom, G., Thornton, P.K., Eds.; Systems Approaches for Sustainable Agricultural Development; Springer: Dordrecht, The Netherlands, 1998; Volume 7, pp. 79–98. ISBN 978-90-481-4940-7. [Google Scholar]
- Okuyama, L.A.; Federizzi, L.C.; Barbosa Neto, J.F. Correlation and Path Analysis of Yield and Its Components and Plant Traits in Wheat. Cienc. Rural. 2004, 34, 1701–1708. [Google Scholar] [CrossRef]
- White, E.M.; Wilson, F.E.A. Responses of Grain Yield, Biomass and Harvest Index and Their Rates of Genetic Progress to Nitrogen Availability in Ten Winter Wheat Varieties. Ir. J. Agric. Food Res. 2006, 45, 85–101. [Google Scholar]
- Li, R.; Guo, P.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of Chlorophyll Content and Fluorescence Parameters as Indicators of Drought Tolerance in Barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Juzoń, K.; Czyczyło-Mysza, I.; Ostrowska, A.; Marcińska, I.; Skrzypek, E. Chlorophyll Fluorescence for Prediction of Yellow Lupin (Lupinus luteus L.) and Pea (Pisum sativum L.) Susceptibility to Drought. Photosynthetica 2019, 57, 950–959. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, N.; Kaiser, E.; Li, G.; An, D.; Sun, Q.; Chen, W.; Liu, W.; Luo, W. Integrating Chlorophyll Fluorescence Parameters into a Crop Model Improves Growth Prediction under Severe Drought. Agric. For. Meteorol. 2021, 303, 108367. [Google Scholar] [CrossRef]
- Moriyuki, S.; Fukuda, H. High-Throughput Growth Prediction for Lactuca Sativa L. Seedlings Using Chlorophyll Fluorescence in a Plant Factory with Artificial Lighting. Front. Plant Sci. 2016, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Stein, M.; Oshana, L.; Zhao, W.; Matsubara, S.; Stich, B. Exploring Natural Genetic Variation in Photosynthesis-Related Traits of Barley in the Field. J. Exp. Bot. 2024, 75, 4904–4925. [Google Scholar] [CrossRef]
- Méndez-Espinoza, A.M.; Romero-Bravo, S.; Estrada, F.; Garriga, M.; Lobos, G.A.; Castillo, D.; Matus, I.; Aranjuelo, I.; Del Pozo, A. Exploring Agronomic and Physiological Traits Associated With the Differences in Productivity Between Triticale and Bread Wheat in Mediterranean Environments. Front. Plant Sci. 2019, 10, 404. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-Throughput Phenotyping: Breaking through the Bottleneck in Future Crop Breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Furbank, R.T.; Jimenez-Berni, J.A.; George-Jaeggli, B.; Potgieter, A.B.; Deery, D.M. Field Crop Phenomics: Enabling Breeding for Radiation Use Efficiency and Biomass in Cereal Crops. New Phytol. 2019, 223, 1714–1727. [Google Scholar] [CrossRef]
- Bhat, J.A.; Deshmukh, R.; Zhao, T.; Patil, G.; Deokar, A.; Shinde, S.; Chaudhary, J. Harnessing High-Throughput Phenotyping and Genotyping for Enhanced Drought Tolerance in Crop Plants. J. Biotechnol. 2020, 324, 248–260. [Google Scholar] [CrossRef]
- Shen, Y.; Adnan, M.; Ma, F.; Kong, L.; Wang, M.; Jiang, F.; Hu, Q.; Yao, W.; Zhou, Y.; Zhang, M.; et al. A High-Throughput Method for Precise Phenotyping Sugarcane Stalk Mechanical Strength Using near-Infrared Spectroscopy. Plant Methods 2022, 19, 101. [Google Scholar] [CrossRef]
- Middleton, C.P.; Senerchia, N.; Stein, N.; Akhunov, E.D.; Keller, B.; Wicker, T.; Kilian, B. Sequencing of Chloroplast Genomes from Wheat, Barley, Rye and Their Relatives Provides a Detailed Insight into the Evolution of the Triticeae Tribe. PLoS ONE 2014, 9, e85761. [Google Scholar] [CrossRef]
- Eltaher, S.; Sallam, A.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Poland, J.; Baenziger, P.S. Genetic Diversity and Population Structure of F3:6 Nebraska Winter Wheat Genotypes Using Genotyping-By-Sequencing. Front. Genet. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Wicker, T.; Gundlach, H.; Spannagl, M.; Uauy, C.; Borrill, P.; Ramírez-González, R.H.; De Oliveira, R.; Mayer, K.F.X.; Paux, E.; Choulet, F. Impact of Transposable Elements on Genome Structure and Evolution in Bread Wheat. Genome Biol. 2018, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Zhang, W.; Akhunov, E.; Sherman, J.; Ma, Y.; Luo, M.-C.; Dubcovsky, J. Analysis of Gene-Derived SNP Marker Polymorphism in US Wheat (Triticum aestivum L.) Cultivars. Mol Breed. 2009, 23, 23–33. [Google Scholar] [CrossRef]
- Martynov, S.P.; Dobrotvorskaya, T.V.; Mitrofanova, O.P. Genealogical Analysis of the Use of Aegilops (Aegilops L.) Genetic Material in Wheat (Triticum aestivum L.). Russ. J. Genet. 2015, 51, 855–862. [Google Scholar] [CrossRef]
- Yang, B.; Chen, N.; Dang, Y.; Wang, Y.; Wen, H.; Zheng, J.; Zheng, X.; Zhao, J.; Lu, J.; Qiao, L. Identification and Validation of Quantitative Trait Loci for Chlorophyll Content of Flag Leaf in Wheat under Different Phosphorus Treatments. Front. Plant Sci. 2022, 13, 1019012. [Google Scholar] [CrossRef]
- Gudi, S.; Saini, D.K.; Halladakeri, P.; Singh, G.; Singh, S.; Kaur, S.; Goyal, P.; Srivastava, P.; Mavi, G.S.; Sharma, A. Genome-Wide Association Study Unravels Genomic Regions Associated with Chlorophyll Fluorescence Parameters in Wheat (Triticum aestivum L.) under Different Sowing Conditions. Plant Cell Rep. 2023, 42, 1453–1472. [Google Scholar] [CrossRef]
- Van Bezouw, R.F.H.M.; Keurentjes, J.J.B.; Harbinson, J.; Aarts, M.G.M. Converging Phenomics and Genomics to Study Natural Variation in Plant Photosynthetic Efficiency. Plant J. 2019, 97, 112–133. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Xu, P.; Jia, J.-Z.; Zhou, R.-H. Quantitative Trait Loci for Leaf Chlorophyll Fluorescence Traits in Wheat. Aust. J. Crop Sci. 2010, 4, 571–579. [Google Scholar]
- Czyczyło-Mysza, I.; Tyrka, M.; Marcińska, I.; Skrzypek, E.; Karbarz, M.; Dziurka, M.; Hura, T.; Dziurka, K.; Quarrie, S.A. Quantitative Trait Loci for Leaf Chlorophyll Fluorescence Parameters, Chlorophyll and Carotenoid Contents in Relation to Biomass and Yield in Bread Wheat and Their Chromosome Deletion Bin Assignments. Mol Breed. 2013, 32, 189–210. [Google Scholar] [CrossRef]
- Maulana, F.; Ayalew, H.; Anderson, J.D.; Kumssa, T.T.; Huang, W.; Ma, X.-F. Genome-Wide Association Mapping of Seedling Heat Tolerance in Winter Wheat. Front. Plant Sci. 2018, 9, 1272. [Google Scholar] [CrossRef]
- Yu, M.; Chen, H.; Mao, S.-L.; Dong, K.-M.; Hou, D.-B.; Chen, G.-Y. Contribution of Photosynthetic- and Yield-Related Traits towards Grain Yield in Wheat at the Individual Quantitative Trait Locus Level. Biotechnol. Biotechnol. Equip. 2020, 34, 1188–1197. [Google Scholar] [CrossRef]
- Gołębiowska, G.; Dyda, M.; Wajdzik, K. Quantitative Trait Loci and Candidate Genes Associated with Cold-Acclimation and Microdochium Nivale Tolerance/Susceptibility in Winter Triticale (x Triticosecale). Plants 2021, 10, 2678. [Google Scholar] [CrossRef]
- Devate, N.B.; Krishna, H.; Parmeshwarappa, S.K.V.; Manjunath, K.K.; Chauhan, D.; Singh, S.; Singh, J.B.; Kumar, M.; Patil, R.; Khan, H.; et al. Genome-Wide Association Mapping for Component Traits of Drought and Heat Tolerance in Wheat. Front. Plant Sci. 2022, 13, 943033. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, C.; Li, H.; Zheng, Q.; Li, B.; Li, Z. An Analysis of the Genetic Relation between Photosynthesis and Yield-Related Traits in Wheat. Agriculture 2022, 12, 560. [Google Scholar] [CrossRef]
- Koua, A.P.; Oyiga, B.C.; Dadshani, S.; Benaouda, S.; Sadeqi, M.B.; Rascher, U.; Léon, J.; Ballvora, A. Chromosome 3A Harbors Several Pleiotropic and Stable Drought-responsive Alleles for Photosynthetic Efficiency Selected through Wheat Breeding. Plant Direct 2022, 6, e438. [Google Scholar] [CrossRef]
- Yang, D.; Jing, R.; Chang, X.; Li, W. Quantitative Trait Loci Mapping for Chlorophyll Fluorescence and Associated Traits in Wheat (Triticum aestivum). J. Integr. Plant Biol. 2007, 49, 646–654. [Google Scholar] [CrossRef]
- Maulana, F.; Huang, W.; Anderson, J.D.; Ma, X.-F. Genome-Wide Association Mapping of Seedling Drought Tolerance in Winter Wheat. Front. Plant Sci. 2020, 11, 573786. [Google Scholar] [CrossRef]
- Tuteja, N.; Tran, N.Q.; Dang, H.Q.; Tuteja, R. Plant MCM Proteins: Role in DNA Replication and Beyond. Plant Mol. Biol. 2011, 77, 537–545. [Google Scholar] [CrossRef]
- Liu, Y.; Imai, R. Function of Plant DExD/H-Box RNA Helicases Associated with Ribosomal RNA Biogenesis. Front. Plant Sci. 2018, 9, 125. [Google Scholar] [CrossRef]
- Lu, W.; Tang, X.; Huo, Y.; Xu, R.; Qi, S.; Huang, J.; Zheng, C.; Wu, C. Identification and Characterization of Fructose 1,6-Bisphosphate Aldolase Genes in Arabidopsis Reveal a Gene Family with Diverse Responses to Abiotic Stresses. Gene 2012, 503, 65–74. [Google Scholar] [CrossRef]
- Osipova, S.; Permyakov, A.; Konstantinov, D.; Shchukina, L.; Rudikovskaya, E.; Permyakova, M.; Pshenichnikova, T. Variability of Photosynthesis Parameters and Yield in Recombinant Lines of Bread Wheat with Introgressions from Triticum timopheevii into 2A Chromosome under Different Water Supply Conditions. Cereal Res. Commun. 2023, 52, 101–113. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Arunachalam, C.; Malla, K.B.; Kahla, A.; Perochon, A.; Jia, J.; Thapa, G.; Doohan, F.M. A Wheat Cytochrome P450 Enhances Both Resistance to Deoxynivalenol and Grain Yield. PLoS ONE 2018, 13, e0204992. [Google Scholar] [CrossRef] [PubMed]
- Graciet, E.; Gans, P.; Wedel, N.; Lebreton, S.; Camadro, J.-M.; Gontero, B. The Small Protein CP12: A Protein Linker for Supramolecular Complex Assembly. Biochemistry 2003, 42, 8163–8170. [Google Scholar] [CrossRef]
- Gontero, B.; Maberly, S.C. An Intrinsically Disordered Protein, CP12: Jack of All Trades and Master of the Calvin Cycle. Biochem. Soc. Trans. 2012, 40, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of Polyamines on the Grain Filling of Wheat under Drought Stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Rottet, S.; Besagni, C.; Kessler, F. The Role of Plastoglobules in Thylakoid Lipid Remodeling during Plant Development. Biochim. Biophys. Acta (BBA)—Bioenerg. 2015, 1847, 889–899. [Google Scholar] [CrossRef]
- LeClere, S.; Tellez, R.; Rampey, R.A.; Matsuda, S.P.T.; Bartel, B. Characterization of a Family of IAA-Amino Acid Conjugate Hydrolases from Arabidopsis. J. Biol. Chem. 2002, 277, 20446–20452. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).