Organogenesis in a Broad Spectrum of Grape Genotypes and Agrobacterium-Mediated Transformation of the Podarok Magaracha Grapevine Cultivar
Abstract
1. Introduction
2. Results
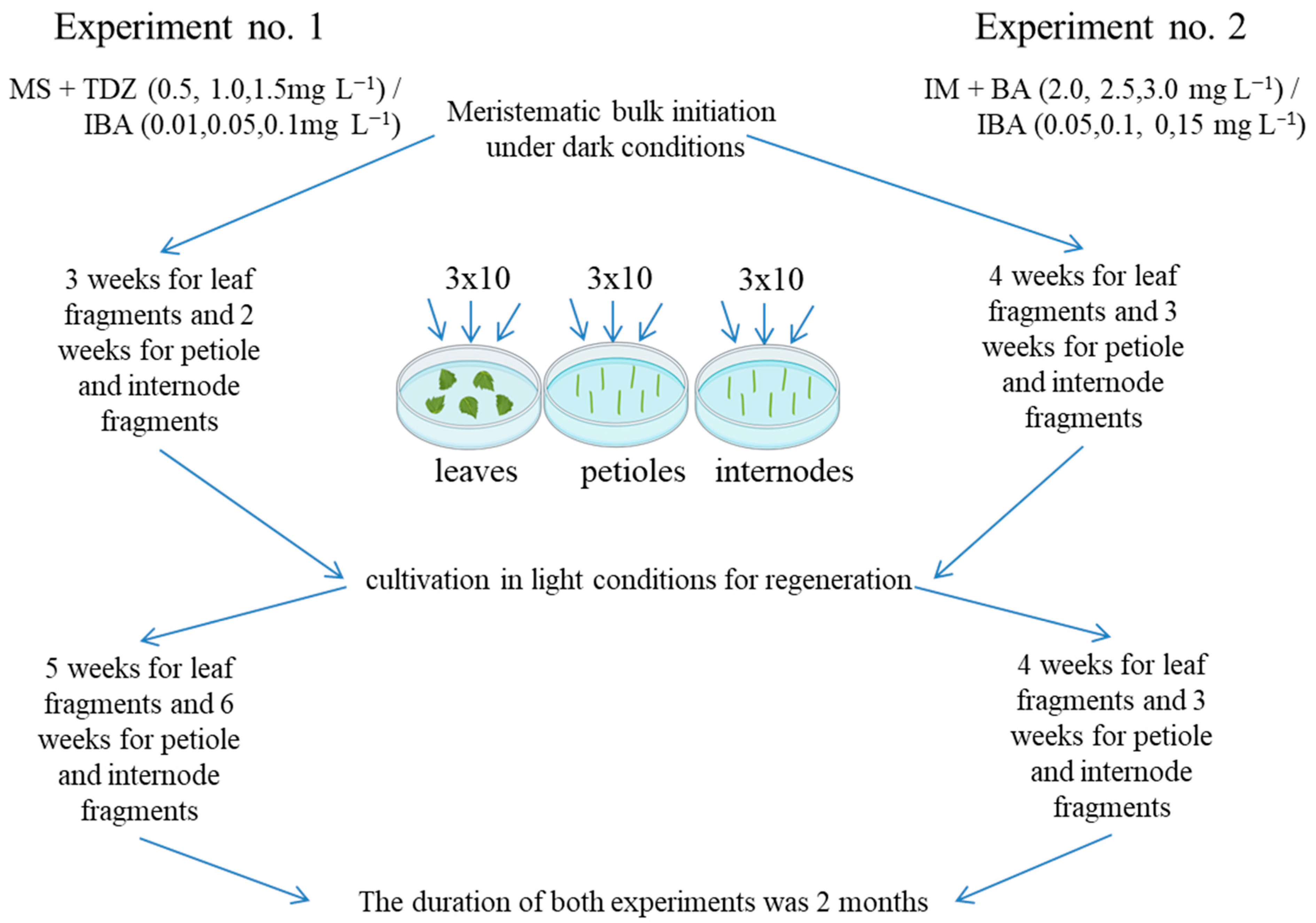
2.1. Development of a Regeneration Protocol
2.2. Enhancing Efficiencies for the Most Responsible Genotypes
2.3. Development of an Elongation Protocol
2.4. Tissue-Culture Cycle (Direct Organogenesis)
2.5. Agrobacterium-Mediated Transformation of ‘Podarok Magaracha’
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Development of a Regeneration Protocol
4.3. Development of an Elongation Protocol
4.4. Agrobacterium-Mediated Transformation
4.4.1. Transient Transformation
4.4.2. Stable Transformation
4.5. PCR Analysis
4.6. Southern Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabbadini, S.; Capriotti, L.; Molesini, B.; Pandolfini, T.; Navacchi, O.; Limera, C.; Ricci, A.; Mezzetti, B. Comparison of regeneration capacity and Agrobacterium mediated-cell transformation efficiency of different cultivars and rootstocks of Vitis spp. via organogenesis. Sci. Rep. 2019, 9, 582. [Google Scholar] [CrossRef]
- Gray, D.J.; Li, Z.T.; Dhekney, S.A. Precision breeding of grapevine (Vitis vinifera L.) for improved traits. Plant Sci. 2014, 228, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bregitzer, P. Plant regeneration and callus type in barley: Effects of genotype and culture media. Crop Sci. 1992, 32, 1108–1112. [Google Scholar] [CrossRef]
- Lemaux, P.G.; Cho, M.-J.; Zhang, S.; Bregitzer, P. Transgenic cereals: Hordeum vulgare L. (barley). In Molecular Improvement of Cereal Crops; Vasiled, I.K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 255–316. [Google Scholar]
- Barro, F.; Martin, A.; Lazzeri, P.A.; Barcelo, P. Medium optimization for efficient somatic embryogenesis and plant regeneration from immature inflorescences and immature scutella of elite cultivars of wheat, barley and tritordeum. Euphytica 1999, 108, 161–167. [Google Scholar] [CrossRef]
- Jacobsen, J.; Matthews, P.; Abbott, D.; Wang, M.-B.; Waterhouse, P.; Buza, L.; Thornton, S.; Gubler, F. Barley transformation breeding: Further progress and remaining problems. In Proceedings of the 9th Australian Barley Technical Symposium, Melbourne, Australia, 12–16 September 1999; pp. 18–19. [Google Scholar]
- Filippov, M.; Miroshnichenko, D.; Vernikovskaya, D.; Dolgov, S. The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell Tissue Organ Cult. 2006, 84, 192–201. [Google Scholar] [CrossRef]
- Costa, D.L.; Malnoy, M.; Lecourieux, D.; Deluc, L.; Ouaked-Lecourieux, F.; Thomas, M.; Torregrosa, L.J.M. The state-of-the-art of grapevine biotechnology and new breeding technologies (NBTS). OENO One 2019, 53, 189–212. [Google Scholar] [CrossRef]
- Butiuc-Keul, A.; Coste, A. Biotechnologies and Strategies for Grapevine Improvement. Horticulturae 2023, 9, 62. [Google Scholar] [CrossRef]
- Çabuk, B.; Özgen, M. The Effect of Different 2, 4-D Doses on Callus Induction and Chromosomal Structure in Maize (Zea mays L.). IJOEAR 2016, 2, 188–194. [Google Scholar]
- Gerdakaneh, M.; Mohamadi, M.; Badakhshan, H.; Arji, I. The effect of growth regulators on micropropagatin of GF677 rootstock under liquid mediumconditions. J. Plant Prod. 2020, 27, 43–57. [Google Scholar]
- Sundararajan, S.; Sivaraman, B.; Rajendran, V.; Ramalingam, S. Tissue culture and agrobacterium-mediated genetic transformation studies in four commercially important indica rice cultivars. J. Crop Sci. Biotechnol. 2017, 20, 175–183. [Google Scholar] [CrossRef]
- Chimdessa, E. Composition and preparation of plant tissue culture medium. Tissue Cult. Bioeng. 2020, 3, 120. [Google Scholar]
- Dhar, U.; Joshi, M. Efficient plant regeneration protocol through callus for Saussurea Obvallata (DC.) Edgew. (Asteraceae): Effect of explant type, age and plant growth regulators. Plant Cell Rep. 2005, 24, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, M.; Chiaiese, P.; Di Matteo, A.; Errico, A.; Corrado, G. Accumulation of ascorbic acid in tomato cell culture: Influence of the genotype, source explant and time of in vitro cultivation. Antioxidants 2020, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, L.; Ricci, A.; Molesini, B.; Mezzetti, B.; Pandolfini, T.; Piunti, I.; Sabbadini, S. Efficient protocol of de novo shoot organogenesis from somatic embryos for grapevine genetic transformation. Front. Plant Sci. 2023, 14, 1172758. [Google Scholar] [CrossRef]
- Mullins, M.G.; Srinivasan, C. Somatic embryos and plantlets from an ancient clone of the grapevine (cv. Cabernet-Sauvignon) by apomixes in vitro. J. Exp. Bot. 1976, 27, 1022–1030. [Google Scholar]
- Gray, D.J. Somatic embryogenesis in grape. In Somatic Embryogenesis in Woody Plants; Jain, S., Gupta, P., Newton, R., Eds.; Springer: New York, NY, USA, 1995; pp. 191–217. [Google Scholar]
- Nakano, M.; Sakakibara, T.; Watanabe, Y.; Mii, M. Establishment of embryogenic culture in several cultivars of Vitis vinifera x Vitis labruscana. VITIS—J. Grapevine Res. 1997, 36, 141–145. [Google Scholar]
- Franks, T.; He, D.G.; Thomas, M. Regeneration of transgenic Vitis vinifera L. Sultana plants: genotypic and phenotypic analysis. Mol. Breed. 1998, 4, 321–333. [Google Scholar]
- Iocco, P.; Franks, T.; Thomas, M. Genetic transformation of major wine grape cultivars of Vitis vinifera L. Transgenic Res. 2001, 10, 105–112. [Google Scholar] [CrossRef]
- Lopez-Perez, A.J.; Carreno, J.; Martinez, C.A.; Dabauza, M. High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. VITIS—J. Grapevine Res. 2005, 44, 79–85. [Google Scholar]
- Cutanda, M.C.; Bouquet, A.; Chatelet, P.; Lopez, G.; Botella, O.; Montero, F.J.; Torregrosa, L. Somatic embryogenesis and plant regeneration of Vitis vinifera cultivars ‘Macabeo’ and ‘Tempranillo’. VITIS—J. Grapevine Res. 2008, 47, 159–162. [Google Scholar]
- Zlenko, V.A.; Likhovskoy, V.V.; Volynkin, V.A.; Khvatkov, P.A.; Vasilyk, I.A.; Dolgov, S.V. Induction of in vitro somatic embryogenesis grapes (Vitis vinifera L.) of domestic and foreign breeding. Biotechnologiya 2017, 33, 35–44. (In Russian) [Google Scholar] [CrossRef]
- Xie, X.; Aguero, C.B.; Wang, Y.; Walker, A.M. Genetic transformation of grape varieties and rootstocks via organogenesis. Plant Cell Tissue Organ Cult. 2016, 126, 541–552. [Google Scholar] [CrossRef]
- Thomas, M.R.; Locco, P.; Franks, T. Transgenic grapevines status and future. Acta Hortic. 2000, 528, 279–287. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.; Kretzchmar, A.; Souza, J.A. In vitro Propagation of Grapevine Cultivars with Potential for South of Brazil. Am. J. Plant Sci. 2015, 6, 1806–1815. [Google Scholar] [CrossRef][Green Version]
- Dutt, M.; Li, Z.T.; Dhekney, S.A.; Gray, D.J. Transgenic plants from shoot apical meristems of Vitis vinifera L. “Thompson Seedless” via Agrobacterium-mediated transformation. Plant Cell Rep. 2007, 26, 2101–2110. [Google Scholar] [CrossRef]
- Favre, J. Premiers resultats concernant l’obtention in vitro de neoformations caulinaires chez la vigne. Ann. L’amelioration Plantes 1977, 27, 151–169. (In French) [Google Scholar]
- Rajasekaran, K.; Mullins, M.G. Organogenesis in internode explants of grapevines. VITIS—J. Grapevine Res. 1981, 20, 218–227. [Google Scholar]
- Kurmi, U.S.; Sharma, D.K.; Tripathi, M.K.; Tiwari, R.; Baghel, B.S.; Tiwari, S. Plant regeneration of Vitis vinifera (L) via direct and indirect organogenesis from cultured nodal segments. J. Agric. Technol. 2011, 7, 721–737. [Google Scholar]
- Zhang, P.; Yu, Z.-Y.; Cheng, Z.-M.; Zhang, Z.; Tao, J.-M. In vitro explants regeneration of the grape ‘Wink’ (Vitis vinifera L. ‘Wink’). J. Plant Breed. Crop Sci. 2011, 3, 276–282. [Google Scholar]
- Clog, E.; Bass, P.; Walter, B. Plant regeneration by organogenesis in Vitis rootstock species. Plant Cell Rep. 1990, 8, 726–728. [Google Scholar] [CrossRef]
- Stamp, J.A.; Colby, S.M.; Meredith, C.P. Direct shoot organogenesis and plant regeneration from leaves of grape (Vitis spp.). Plant Cell Tissue Organ Cult. 1990, 22, 127–133. [Google Scholar] [CrossRef]
- Stamp, J.A.; Colby, S.M.; Meredith, C.P. Improved shoot organogenesis from leaves of grape. J. Am. Soc. Hortic. Sci. 1990, 115, 1038–1042. [Google Scholar] [CrossRef]
- Torregrosa, L.; Bouquet, A. Adventitious bud formation and shoot development from in vitro leaves of Vitis x Muscadinia hybrids. Plant Cell Tissue Organ Cult. 1996, 45, 245–252. [Google Scholar] [CrossRef]
- Nicholson, K.L.; Tarlyn, N.; Armour, T.; Swanson, M.E.; Dhingra, A. Effect of phyllotactic position and cultural treatments toward successful direct shoot organogenesis in dwarf ‘Pixie’grapevine (Vitis vinifera L.). Plant Cell Tissue Organ Cult. 2012, 111, 123–129. [Google Scholar] [CrossRef]
- Ehab, M.R.M.; Hemaid, I.A.S.; Omar, A.A.; Naif, M.K. Producing Transgenic Thompson Seedless Grape (Vitis vinifera L.) plants using Agrobacterium tumefaciens. Int. J. Agric. Biol. 2016, 18, 661–670. [Google Scholar]
- Kumsa, F. Effect of growth regulators on indirect organogenesis of two grapevines (Vitis vinifera L.) cultivars. Afr. J. Biotechnol. 2017, 16, 852–859. [Google Scholar]
- Reisch, B.I.; Martens, M.H.; Cheng, Z.M. High frequency regeneration from grapevine petioles: Extension to new genotypes. VITIS—J. Grapevine Res. 1990, 29, 419–422. [Google Scholar]
- Li, J.F.; Zhang, Z.; Zhuang, Z.M.; Tong, Z.G.; Tao, J.M. Plant regeneration of grape rootstock ‘5BB’ (Vitis berlandieri × V. riparia) in vitro. Acta Bot. Boreal.-Occident. Sin. 2007, 27, 1323–1328. (In Chinese) [Google Scholar]
- Mezzetti, B.; Pandolfini, T.; Navacchi, O.; Landi, L. Genetic transformation of Vitis vinifera via organogenesis. BMC Biotechnol. 2002, 2, 18. [Google Scholar] [CrossRef]
- Kumsa, F.; Feyissa, T. In vitro regeneration of two grapevine (Vitis vinifera L.) varieties from leaf explants. Afr. J. Biotechnol. 2019, 18, 92–100. [Google Scholar]
- Singh, S.K.; Khawale, R.N.; Singh, S.P. Techniques for rapid in vitro multiplication of Vitis vinifera L. cultivars. J. Hortic. Sci. Biotechnol. 2004, 19, 267–272. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of plant growth regulators on in vitro propagation of Vitis ficifolia var. ganebu and its interspecific hybrid grape. Asian J. Plant Sci. 2005, 4, 466–471. [Google Scholar] [CrossRef]
- Quan, D.L.; Chang, Y.Y. Study on direct regenerating adventitious buds from different organs of grape. J. Gansu Agric. Univ. 2005, 40, 173–177. [Google Scholar]
- Korban, S.S.; Oconnor, P.A.; Elobeidy, A. Effects of thidiazuron naphthaleneacetic acid, dark incubation and genotype on shoot organogenesis from Malus leaves. Hortic. Sci. 1992, 67, 341–349. [Google Scholar] [CrossRef]
- Toreegrosa, L.; Bouquet, A.; Goussard, P.G. In vitro Culture and Propagation of Grapevine. In Molecular Biology & Biotechnology of the Grapevine; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 281–326. [Google Scholar]
- Deng, J.; Liu, X.; Xue, R.G. Induction of multiple shoots from stem explants of Chardonny grape. J. Fruit Sci. 2009, 26, 222–225. [Google Scholar]
- Scorza, R.; Cordts, J.M.; Gray, D.J.; Gonsalves, D.; Emershad, R.L.; Ramming, D.W. Producing transgenic ‘Thompson Seedless’ grape (Vitis vinifera L.) plants. J. Am. Soc. Hortic. Sci. 1996, 121, 616–619. [Google Scholar] [CrossRef]
- Scorza, R.; Cordts, J.M.; Ramming, D.W.; Emershad, R.L. Transformation of grape (Vitis vinifera L.) zygotic-derived somatic embryos and regeneration of transgenic plants. Plant Cell Rep. 1995, 14, 589–592. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Shvedova, A.N.; Khvatkov, P.A.; Dolgov, S.V. Optimization of factors affecting the efficiency of Agrobacterium-mediated transformation of Wolffia arrhiza. Appl. Biochem. Microbiol. 2023, 59, 1177–1182. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant Species. HortScience 1981, 16, 453. [Google Scholar]
- Driver, J.; Kuniyuki, A. In vitro Propagation of Paradox walnut rootstock. Hortic. Sci. 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Hood, E.E.; Gelvin, S.B.; Melchers, L.S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plants. Transgen. Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Travin, D.Y.; Watson, Z.L.; Metelev, M.; Ward, F.R.; Osterman, I.A.; Khven, I.M.; Khabibullina, N.F.; Serebryakova, M.; Mergaert, P.; Polikanov, Y.S.; et al. Structure of ribosome-bound azole-modified peptide phazolicin rationalizes its species-specific mode of bacterial translation inhibition. Nat. Commun. 2019, 10, 4563. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
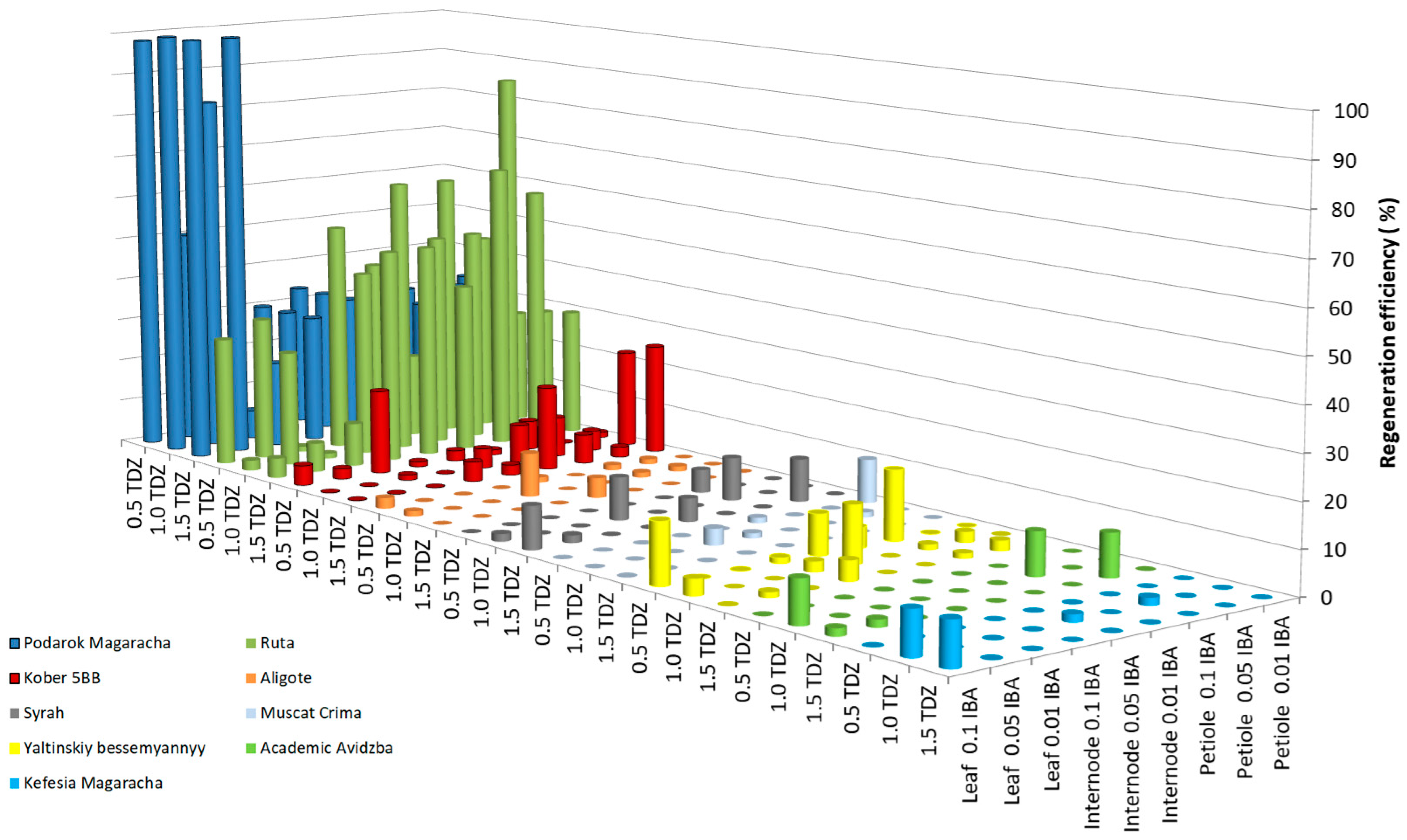
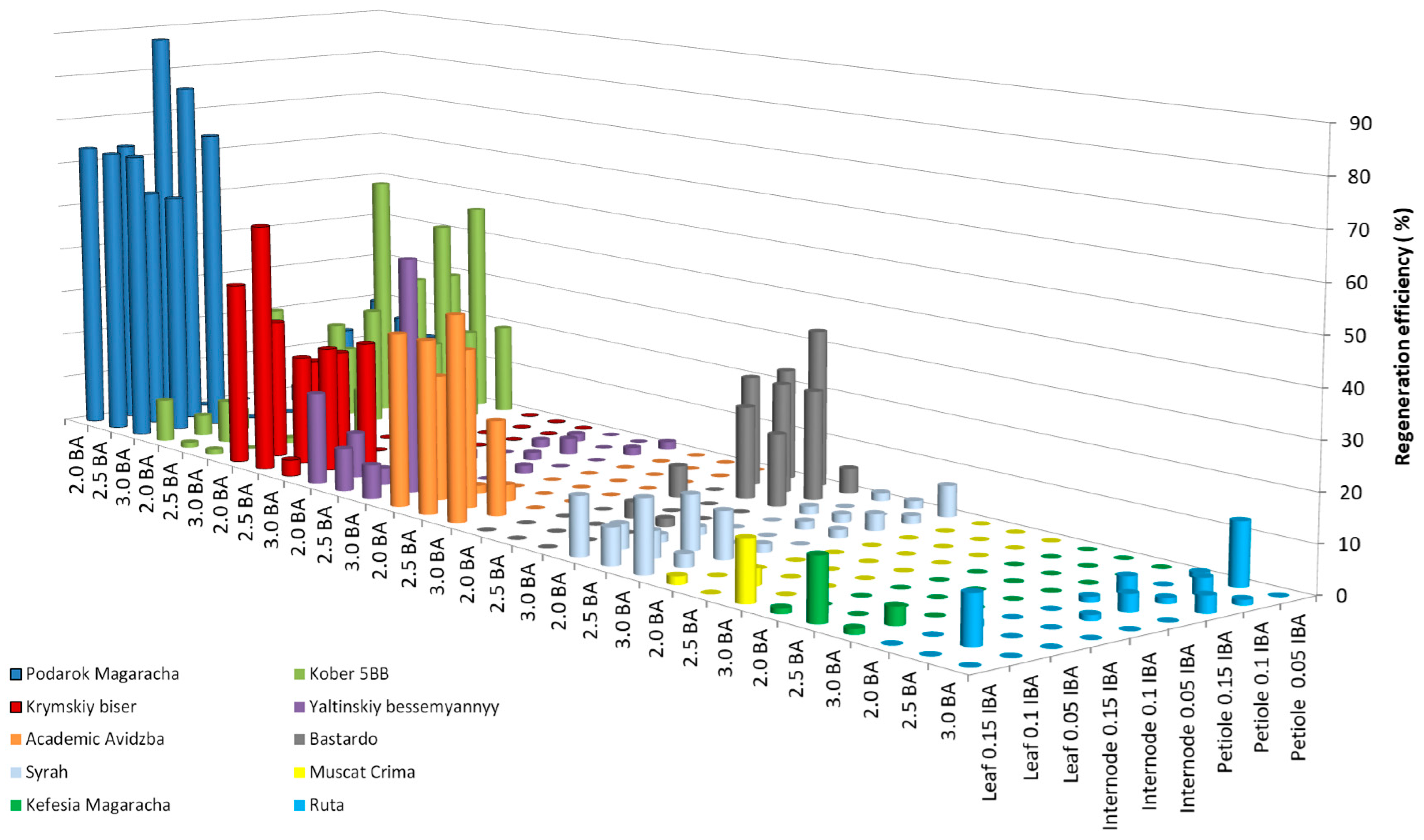
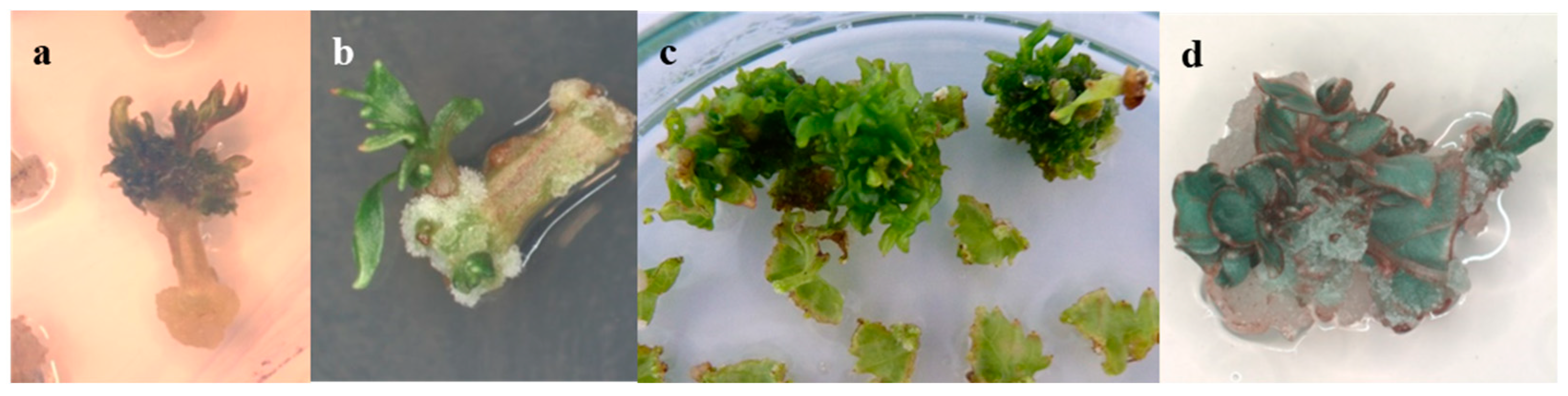
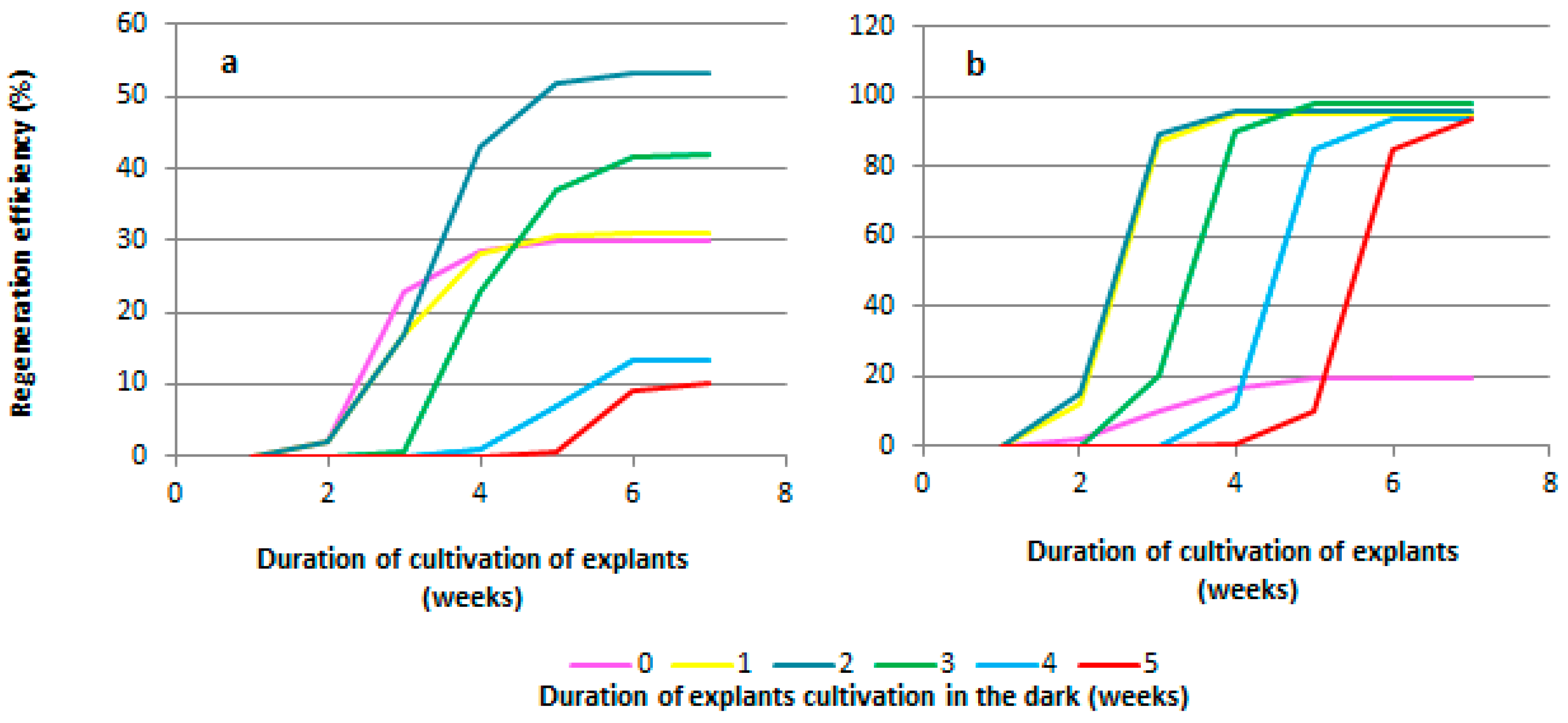


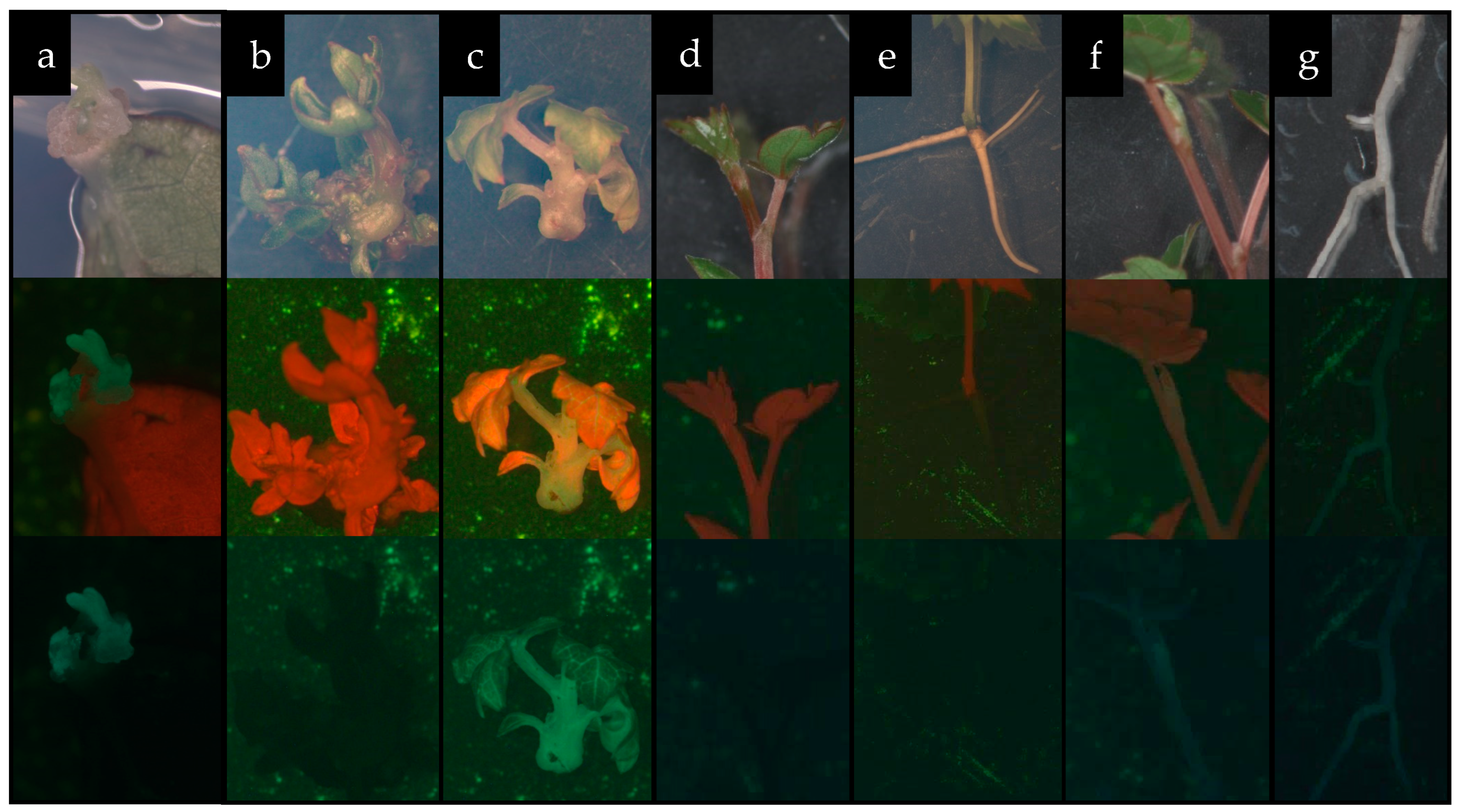
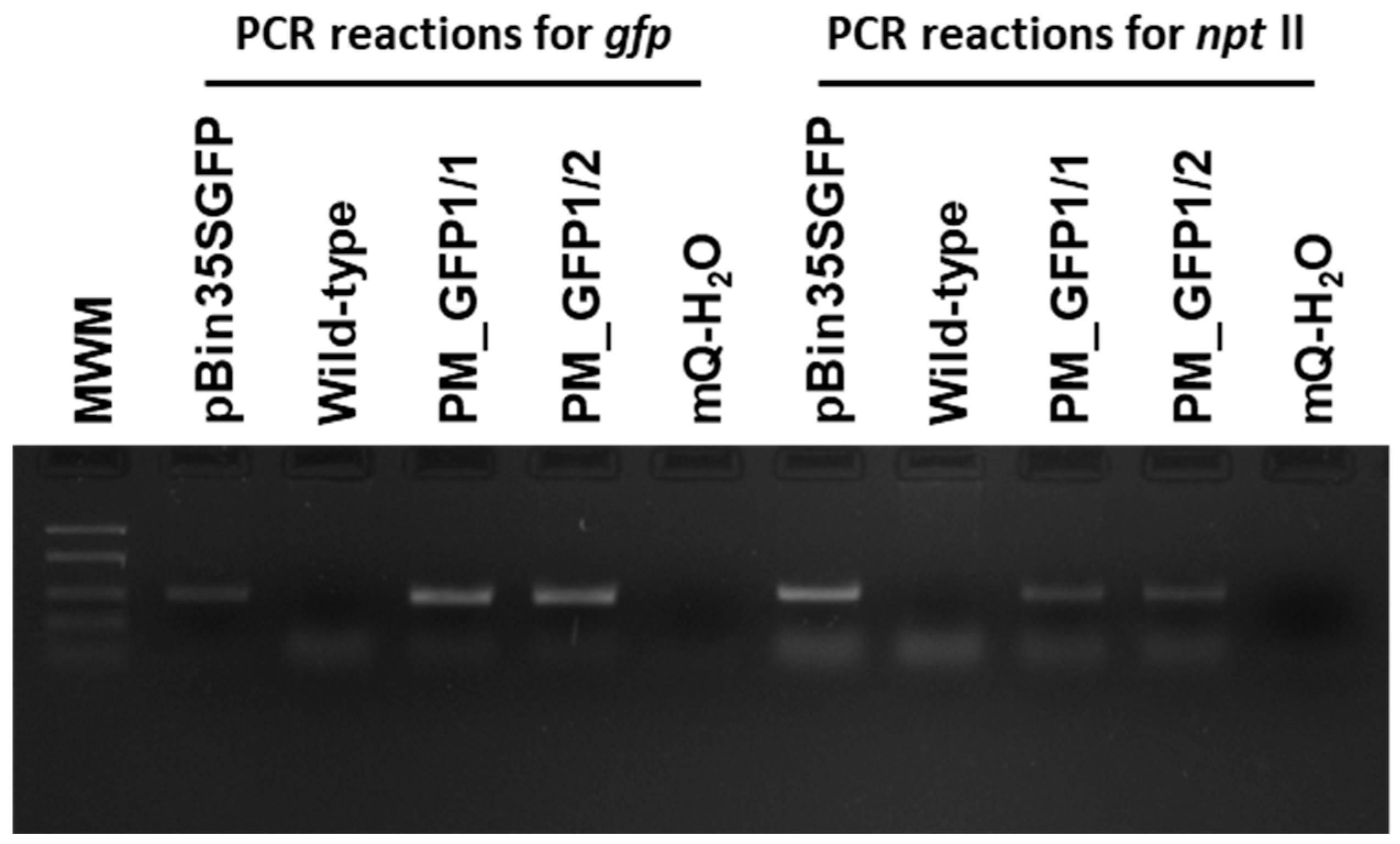


| Genotype | MS | PIV | NN | DKW | WPM | IM |
|---|---|---|---|---|---|---|
| Kober 5BB | 0 a | 0 a | 9.7 bc | 18.0 c | 1.7 ab | 52.1 d |
| Podarok Magaracha | 50.0 a | 43.3 a | 32.7 a | 60.2 a | 64.1 a | 97.6 b |
| Duration of Cocultivation of Explants with Agrobacterium (h) | Inoculum Concentrations (OD600) | Duration of Cultivation of Explants on a Selective Medium (Days) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 14 | 21 | 28 | 42 | 56 | 70 | |||
| 24 | 0.2 | 0.0 | 15.5 | 17.2 | 24.1 | 24.1 | 22.4 | 17.2 | 10.3 | 8.6 | 8.6 | 6.9 | 6.9 | bc |
| 0.4 | 0.0 | 19.0 | 27.6 | 41.4 | 44.8 | 32.8 | 25.9 | 20.7 | 7.0 | 3.5 | 3.5 | 3.5 | a | |
| 0.6 | 0.0 | 15.5 | 31.0 | 57.6 | 59.2 | 57.9 | 50.0 | 36.2 | 15.5 | 12.1 | 12.1 | 12.1 | fghij | |
| 0.8 | 0.0 | 39.7 | 46.6 | 60.3 | 65.5 | 44.8 | 27.6 | 19.0 | 10.3 | 8.6 | 6.9 | 6.9 | bc | |
| 1.0 | 0.0 | 25.9 | 43.1 | 55.2 | 53.5 | 39.7 | 27.6 | 15.5 | 10.3 | 10.3 | 6.9 | 6.9 | bc | |
| 48 | 0.2 | - | 1.7 | 36.2 | 75.9 | 67.2 | 58.6 | 41.4 | 39.7 | 31.0 | 24.1 | 8.5 | 8.5 | cdef |
| 0.4 | - | 10.3 | 58.6 | 82.8 | 79.3 | 74.1 | 48.3 | 34.5 | 19.0 | 10.5 | 8.5 | 8.5 | cdef | |
| 0.6 | - | 17.2 | 69.0 | 86.2 | 82.8 | 74.1 | 48.3 | 36.2 | 19.0 | 12.1 | 8.6 | 5.1 | ab | |
| 0.8 | - | 36.2 | 87.9 | 93.1 | 91.4 | 82.8 | 70.7 | 56.9 | 31.0 | 17.2 | 10.3 | 8.5 | cdef | |
| 1.0 | - | 19.0 | 46.6 | 67.2 | 60.3 | 48.3 | 37.9 | 32.8 | 22.4 | 13.5 | 10.3 | 10.3 | efghi | |
| 72 | 0.2 | - | - | 32.8 | 74.1 | 67.2 | 50.0 | 36.6 | 49.1 | 22.4 | 19.0 | 17.2 | 17.2 | klmn |
| 0.4 | - | - | 46.6 | 82.6 | 79.3 | 62.1 | 41.4 | 41.0 | 22.4 | 17.2 | 13.8 | 13.8 | hijkl | |
| 0.6 | - | - | 51.7 | 94.8 | 86.2 | 72.4 | 65.5 | 50.1 | 34.5 | 24.1 | 20.7 | 19.0 | mn | |
| 0.8 | - | - | 81.0 | 96.6 | 94.8 | 87.9 | 82.8 | 56.7 | 37.9 | 27.6 | 25.7 | 24.3 | pq | |
| 1.0 | - | - | 70.7 | 86.2 | 84.5 | 75.9 | 67.2 | 56.7 | 43.1 | 36.2 | 29.3 | 27.6 | qr | |
| 96 | 0.2 | - | - | - | 68.4 | 79.3 | 77.6 | 56.7 | 34.5 | 25.9 | 22.4 | 19.0 | 17.2 | mn |
| 0.4 | - | - | - | 82.8 | 82.8 | 77.6 | 65.5 | 46.6 | 37.9 | 19.0 | 17.2 | 13.8 | ghijkl | |
| 0.6 | - | - | - | 87.9 | 89.3 | 80.7 | 68.4 | 39.2 | 35.7 | 20.7 | 17.2 | 15.5 | jklmn | |
| 0.8 | - | - | - | 89.7 | 94.8 | 94.8 | 74.1 | 37.9 | 37.9 | 31.0 | 25.5 | 24.1 | opq | |
| 1.0 | - | - | - | 64.0 | 67.2 | 60.3 | 51.7 | 46.6 | 41.4 | 37.9 | 29.3 | 29.3 | r | |
| 120 | 0.2 | - | - | - | - | 69.0 | 91.4 | 62.1 | 44.8 | 24.1 | 19.0 | 12.1 | 10.3 | defghi |
| 0.4 | - | - | - | - | 84.1 | 91.4 | 65.5 | 41.4 | 19.0 | 13.8 | 8.5 | 8.5 | cdef | |
| 0.6 | - | - | - | - | 91.4 | 93.1 | 79.3 | 58.6 | 33.1 | 25.9 | 19.0 | 19.0 | n | |
| 0.8 | - | - | - | - | 94.8 | 93.1 | 75.9 | 50.0 | 39.1 | 31.0 | 10.3 | 10.3 | fghi | |
| 1.0 | - | - | - | - | 50.0 | 51.7 | 43.7 | 41.4 | 34.5 | 31.0 | 17.2 | 13.8 | ijkl | |
| BA Concentration, mg L−1 | Number of GFP-Expressing Regenerated Plants (pcs per 100 Explants) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IAA Concentration (mg L−1) | IBA Concentration (mg L−1) | |||||||||
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 1.5 | 0 a | 0 a | 0 a | 1 b | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| 2.0 | 0 a | 0 a | 0 a | 1 b | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| 2.5 | 0 a | 0 a | 1 b | 4 c | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| 3.0 | 0 a | 0 a | 0 a | 2 b | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maletich, G.; Pushin, A.; Rybalkin, E.; Plugatar, Y.; Dolgov, S.; Khvatkov, P. Organogenesis in a Broad Spectrum of Grape Genotypes and Agrobacterium-Mediated Transformation of the Podarok Magaracha Grapevine Cultivar. Plants 2024, 13, 2779. https://doi.org/10.3390/plants13192779
Maletich G, Pushin A, Rybalkin E, Plugatar Y, Dolgov S, Khvatkov P. Organogenesis in a Broad Spectrum of Grape Genotypes and Agrobacterium-Mediated Transformation of the Podarok Magaracha Grapevine Cultivar. Plants. 2024; 13(19):2779. https://doi.org/10.3390/plants13192779
Chicago/Turabian StyleMaletich, Galina, Alexander Pushin, Evgeniy Rybalkin, Yuri Plugatar, Sergey Dolgov, and Pavel Khvatkov. 2024. "Organogenesis in a Broad Spectrum of Grape Genotypes and Agrobacterium-Mediated Transformation of the Podarok Magaracha Grapevine Cultivar" Plants 13, no. 19: 2779. https://doi.org/10.3390/plants13192779
APA StyleMaletich, G., Pushin, A., Rybalkin, E., Plugatar, Y., Dolgov, S., & Khvatkov, P. (2024). Organogenesis in a Broad Spectrum of Grape Genotypes and Agrobacterium-Mediated Transformation of the Podarok Magaracha Grapevine Cultivar. Plants, 13(19), 2779. https://doi.org/10.3390/plants13192779





