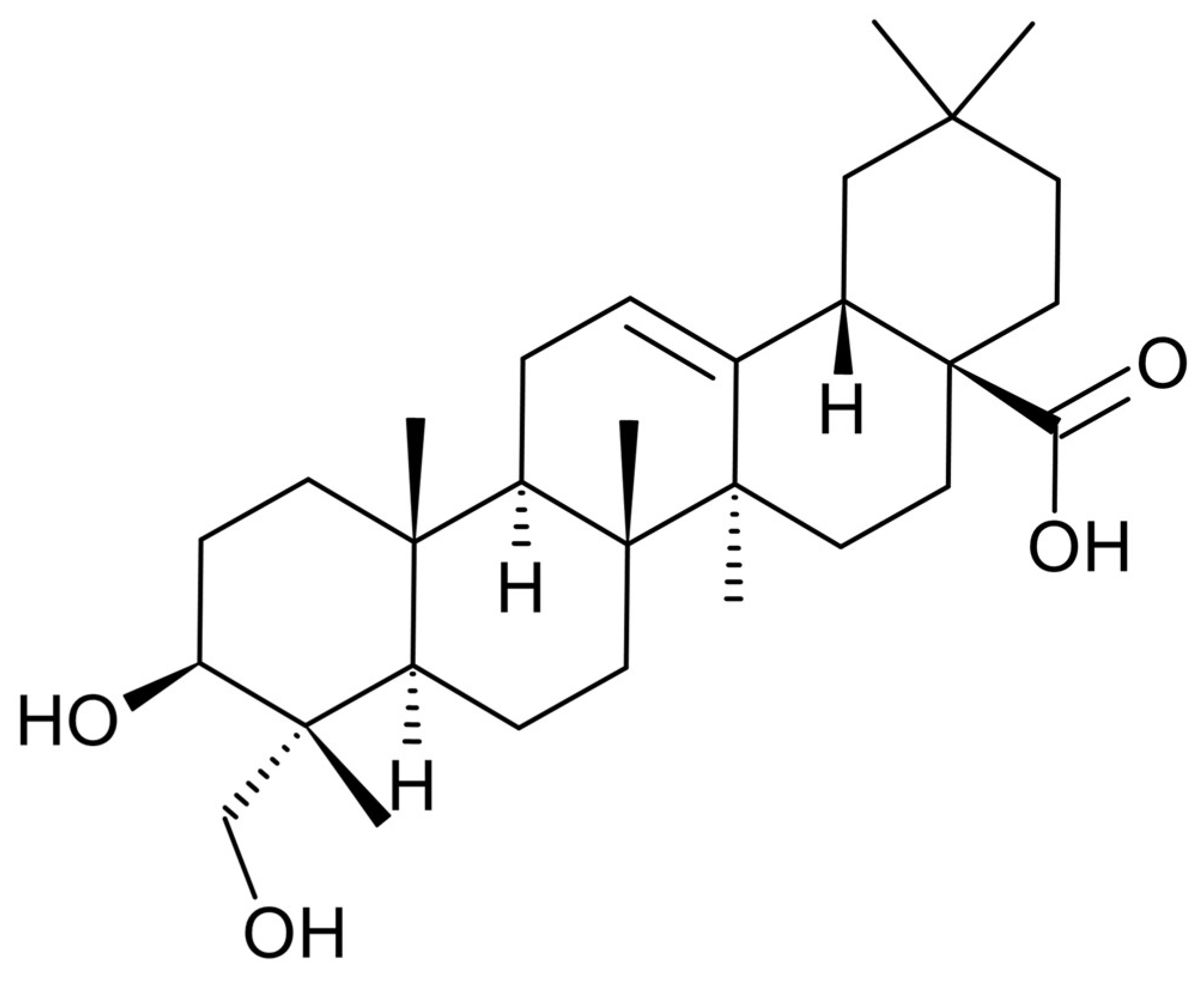
Hederagenin from Hedera helix Promotes Fat Browning in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results
2.1. Effect of HDG on Cell Viability
2.2. Effect of HDG on Lipid Accumulation
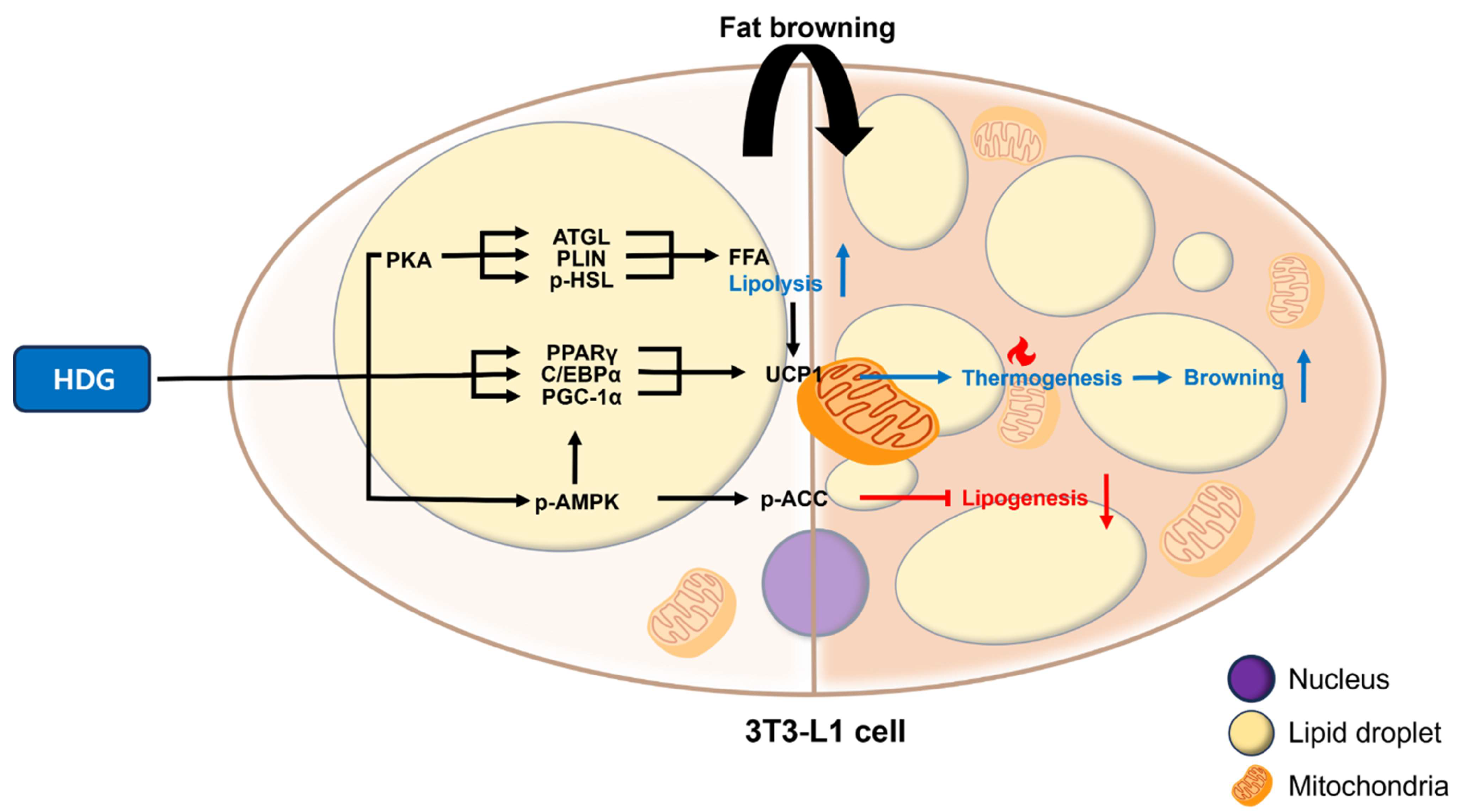
2.3. Effect of HDG on Mitochondrial Biogenesis and Adipocyte Browning
2.4. Effect of HDG on Lipogenesis and Lipolysis
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Cell Culture and Differentiation
4.3. Cell Viability Assay
4.4. Oil Red O Staining
4.5. Mitochondrial Analysis and Immunofluorescence
4.6. Western Blot Analysis
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Endocrine Society Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Basen-Engquist, K.; Chang, M. Obesity and Cancer Risk: Recent Review and Evidence. Curr. Oncol. Rep. 2011, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Munoz, C.M.; López, M.; Albericio, F.; Makowski, K. Targeting Energy Expenditure—Drugs for Obesity Treatment. Pharmaceuticals 2021, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Phelan, S. Long-Term Weight Loss Maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular Bioenergetics as a Target for Obesity Therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef]
- Aherne, W.; Hull, D. Brown Adipose Tissue and Heat Production in the Newborn Infant. J. Pathol. Bacteriol. 1966, 91, 223–234. [Google Scholar] [CrossRef]
- Heaton, J.M. The Distribution of Brown Adipose Tissue in the Human. J. Anat. 1972, 112, 35–39. [Google Scholar]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Singh, A.K.; Aryal, B.; Chaube, B.; Rotllan, N.; Varela, L.; Horvath, T.L.; Suárez, Y.; Fernández-Hernando, C. Brown Adipose Tissue Derived ANGPTL4 Controls Glucose and Lipid Metabolism and Regulates Thermogenesis. Mol. Metab. 2018, 11, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Nuutila, P.; Noponen, T.; Parkkola, R.; Viljanen, T.; Enerbäck, S.; Rissanen, A.; Pietiläinen, K.H.; Virtanen, K.A. Blunted Metabolic Responses to Cold and Insulin Stimulation in Brown Adipose Tissue of Obese Humans. Obesity 2013, 21, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Kubo, N.; Kawahara, M.; Okamatsu-Ogura, Y.; Miyazaki, Y.; Otsuka, R.; Fukuchi, K. Evaluation of Glucose Uptake and Uncoupling Protein 1 Activity in Adipose Tissue of Diabetic Mice upon β-Adrenergic Stimulation. Mol. Imaging Biol. 2019, 21, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Seale, P. Control of Brown and Beige Fat Development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yun, J.W. β-Carotene Stimulates Browning of 3T3-L1 White Adipocytes by Enhancing Thermogenesis via the Β3-AR/P38 MAPK/SIRT Signaling Pathway. Phytomedicine 2022, 96, 153857. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Zhang, J. Peanut Shell Extract and Luteolin Regulate Lipid Metabolism and Induce Browning in 3T3-L1 Adipocytes. Foods 2022, 11, 2696. [Google Scholar] [CrossRef]
- Lee, H.S.; Heo, C.U.; Song, Y.-H.; Lee, K.; Choi, C.-I. Naringin Promotes Fat Browning Mediated by UCP1 Activation via the AMPK Signaling Pathway in 3T3-L1 Adipocytes. Arch. Pharm. Res. 2023, 46, 192–205. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, L.; He, C.; Wang, C.; Lv, Y.; Li, H.; An, Y.; Duan, Y.; Dai, H.; Zhang, H.; et al. Mulberry Leaf Flavonoids Activate BAT and Induce Browning of WAT to Improve Type 2 Diabetes via Regulating the AMPK/SIRT1/PGC-1α Signaling Pathway. Chin. J. Nat. Med. 2023, 21, 812–829. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, S.M.; Lim, S.H.; Choi, C.-I. Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals 2023, 16, 1727. [Google Scholar] [CrossRef]
- Bedir, E.; Kırmızıpekmez, H.; Sticher, O.; Çalış, İ. Triterpene Saponins from the Fruits of Hedera Helix. Phytochemistry 2000, 53, 905–909. [Google Scholar] [CrossRef]
- Christensen, L.P.; Lam, J.; Thomasen, T. Polyacetylenes from the Fruits of Hedera Helix. Phytochemistry 1991, 30, 4151–4152. [Google Scholar] [CrossRef]
- Stauss-Grabo, M.; Atiye, S.; Warnke, A.; Wedemeyer, R.S.; Donath, F.; Blume, H.H. Observational Study on the Tolerability and Safety of Film-Coated Tablets Containing Ivy Extract (Prospan® Cough Tablets) in the Treatment of Colds Accompanied by Coughing. Phytomedicine 2011, 18, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Shin, Y.J.; Kim, N.; Yoo, G.; Park, S.; Kim, S.H. Determination of Saponins and Flavonoids in Ivy Leaf Extracts Using HPLC-DAD. J. Chromatogr. Sci. 2015, 53, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Gosens, R.; Meurs, H.; Häberlein, H. Pre-Treatment with α-Hederin Increases β-Adrenoceptor Mediated Relaxation of Airway Smooth Muscle. Phytomedicine 2011, 18, 214–218. [Google Scholar] [CrossRef]
- Rai, A. The Antiinflammatory and Antiarthritic Properties of Ethanol Extract of Hedera Helix. Indian J. Pharm. Sci. 2013, 75, 99–102. [Google Scholar] [CrossRef]
- Bun, S.-S.; Elias, R.; Baghdikian, B.; Ciccolini, J.; Ollivier, E.; Balansard, G. Alpha-Hederin Potentiates 5-FU Antitumor Activity in Human Colon Adenocarcinoma Cells. Phytother. Res. 2008, 22, 1299–1302. [Google Scholar] [CrossRef]
- Saiah, H.; Allem, R.; Kebir, F.Z. Antioxidant and Antibacterial Activities of Six Algerian Medicinal Plants. Int. J. Pharm. Pharm. Sci. 2016, 8, 367–374. [Google Scholar]
- Qabaha, K.; Abbadi, J.; Yaghmour, R.; Hijawi, T.; Naser, S.A.; Al-Rimawi, F. Unveiling the Antibacterial and Antioxidant Potential of Hedera Helix Leaf Extracts: Recent Findings. Can. J. Physiol. Pharmacol. 2023, 102, 26–32. [Google Scholar] [CrossRef]
- 2Saeed Khan, S.; Adil, A.; Naeem, S.; Jaffar, N.; Khatoon, H.; Ansar, H.; Shafiq, Y. Evaluation of Acute and Chronic Antidiabetic Activity of Ivy (Hedera helix L.) Aqueous Leaf Extract in Rat Model. Pak. J. Biol. Sci. 2020, 23, 1357–1368. [Google Scholar] [CrossRef]
- 3Huang, X.; Shen, Q.-K.; Guo, H.-Y.; Li, X.; Quan, Z.-S. Pharmacological Overview of Hederagenin and Its Derivatives. RSC Med. Chem. 2023, 14, 1858–1884. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of White Adipose Tissue: Lessons from Experimental Models. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20160051. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef] [PubMed]
- Tiraby, C.; Tavernier, G.; Lefort, C.; Larrouy, D.; Bouillaud, F.; Ricquier, D.; Langin, D. Acquirement of Brown Fat Cell Features by Human White Adipocytes *. J. Biol. Chem. 2003, 278, 33370–33376. [Google Scholar] [CrossRef]
- Harms, M.J.; Ishibashi, J.; Wang, W.; Lim, H.-W.; Goyama, S.; Sato, T.; Kurokawa, M.; Won, K.-J.; Seale, P. Prdm16 Is Required for the Maintenance of Brown Adipocyte Identity and Function in Adult Mice. Cell Metab. 2014, 19, 593–604. [Google Scholar] [CrossRef]
- Emont, M.P.; Kim, D.-I.; Wu, J. Development, Activation, and Therapeutic Potential of Thermogenic Adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 13–19. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.H.; Yi, S.A.; Park, J.W.; Han, J.-W.; Lee, J. Ginsenoside Rg3 Induces Browning of 3T3-L1 Adipocytes by Activating AMPK Signaling. Nutrients 2020, 12, 427. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The New Role of AMP-Activated Protein Kinase in Regulating Fat Metabolism and Energy Expenditure in Adipose Tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Son, E.; Im, G.; Kim, D.-S. Herbal Combination of Phyllostachys Pubescens and Scutellaria Baicalensis Inhibits Adipogenesis and Promotes Browning via AMPK Activation in 3T3-L1 Adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Yan, M.; Audet-Walsh, É.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic AMPK Activation via Loss of FLCN Induces Functional Beige Adipose Tissue through PGC-1α/ERRα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Torró-Montell, L.; Cortés-Castell, E.; Sirvent-Segura, E.; Veciana-Galindo, C.; Gil-Guillén, V.; Rizo-Baeza, M. Influence of Olive Extracts on the Expression of Genes Involved in Lipid Metabolism in Medaka Fish. Molecules 2019, 24, 3068. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-F.; Jiao, J.; Zhang, W.; Xu, J.-Y.; Zhang, W.; Fu, C.-L.; Qin, L.-Q. Lipolysis and Thermogenesis in Adipose Tissues as New Potential Mechanisms for Metabolic Benefits of Dietary Fiber. Nutrition 2017, 33, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Schreiber, R.; Haemmerle, G.; Lass, A.; Fledelius, C.; Jacobsen, P.; Tornqvist, H.; Zechner, R.; Zimmermann, R. Adipose Triglyceride Lipase and Hormone-Sensitive Lipase Are the Major Enzymes in Adipose Tissue Triacylglycerol Catabolism *. J. Biol. Chem. 2006, 281, 40236–40241. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.A.; Bickel, P.E. Minireview: Lipid Droplets in Lipogenesis and Lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-Adrenergic Control of Lipolysis and Thermogenesis in Adipose Tissues. J. Exp. Biol. 2018, 221, jeb165381. [Google Scholar] [CrossRef]
- Li, Y.; Fromme, T.; Schweizer, S.; Schöttl, T.; Klingenspor, M. Taking Control over Intracellular Fatty Acid Levels Is Essential for the Analysis of Thermogenic Function in Cultured Primary Brown and Brite/Beige Adipocytes. EMBO Rep. 2014, 15, 1069–1076. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, M.; Liu, H.; Xie, J.; Yan, J.; Hou, X.; Liu, J. Zeaxanthin Promotes Browning by Enhancing Mitochondrial Biogenesis through the PKA Pathway in 3T3-L1 Adipocytes. Food Funct. 2021, 12, 6283–6293. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, M.; Sharma, A.; Kim, K.; Lee, H.-J. Black Ginseng and Ginsenoside Rb1 Promote Browning by Inducing UCP1 Expression in 3T3-L1 and Primary White Adipocytes. Nutrients 2019, 11, 2747. [Google Scholar] [CrossRef]





| Gene | Forward | Reverse |
|---|---|---|
| Aco1 | ATCCAGACTTCCAACATFAG | AACCACATGATTTCTTCAGG |
| Atgl | TTCACCATCCGCTTGTTGGAG | AGATGGTCACCCAATTTCCTC |
| Cd137 | GGTCTGTGCTTAAGACCGGG | TCTTAATAGCTGGTCCTCCCTC |
| Cebpa | AGGTGCTGGAGTTGACCAGT | CAGCCTAGAGATCCAGCGAC |
| Cebpb | ACGAGTACAAGATGCGGCG | TGAACAAGTTCCGCAGGGTG |
| Cidea | CGGGAATAGCCAGAGTCACC | TGTGCATCGGATGTCGTAGG |
| Cited1 | AACCTTGGAGTGAAGGATCGC | GTAGGAGAGCCTATTGGAGATGT |
| Cox4 | TGACGGCCTTGGACGG | CGATCAGCGTAAGTGGGGA |
| Cpt1 | GTGTTGGAGGTGACAGACTT | CACTTTCTCTTTCCACAAGG |
| Fgf21 | CGTCTGCCTCAGAAGGACTC | TCTACCATGCTCAGGGGGTC |
| Hsl | GCACTGTGACCTGCTTGGT | CTGGCACCCTCACTCCATA |
| Nrf1 | GCTAATGGCCTGGTCCAGAT | CTGCGCTGTCCGATATCCTG |
| Pgc1a | ATGTGCAGCCAAGACTCTGTA | CGCTACACCACTTCAATCCAC |
| Plin1 | GCAAGAAGAGCTGAGCAGAC | AATCTGCCCACGAGAAAGGA |
| Ppara | GAGAGGGCACACGCTAGGAA | GAACACCAATGTTCGGAGCC |
| Pparg | CAAGAATACCAAAGTGCGATCAA | GAGCTGGGTCTTTTCAGAATAATAAG |
| Prdm16 | GATGGGAGATGCTGACGGAT | TGATCTGACACATGGCGAGG |
| Tbx1 | AGCGAGGCGGAAGGGA | CCTGGTGACTGTGCTGAAGT |
| Tfam | ATGTGGAGCGTGCTAAAAGC | GGATAGCTACCCATGCTGGAA |
| Tmem26 | CCATGGAAACCAGTATTGCAGC | ATTGGTGGCTCTGTGGGATG |
| Ucp1 | CCTGCCTCTCTCGGAAACAA | GTAGCGGGGTTTGATCCCAT |
| GAPDH | TTGTTGCCATCAACGACCCC | GCCGTTGAATTTGCCGTGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Lee, H.S.; Lim, S.H.; Choi, G.; Choi, C.-I. Hederagenin from Hedera helix Promotes Fat Browning in 3T3-L1 Adipocytes. Plants 2024, 13, 2789. https://doi.org/10.3390/plants13192789
Choi SM, Lee HS, Lim SH, Choi G, Choi C-I. Hederagenin from Hedera helix Promotes Fat Browning in 3T3-L1 Adipocytes. Plants. 2024; 13(19):2789. https://doi.org/10.3390/plants13192789
Chicago/Turabian StyleChoi, Seung Min, Ho Seon Lee, Sung Ho Lim, Gayoung Choi, and Chang-Ik Choi. 2024. "Hederagenin from Hedera helix Promotes Fat Browning in 3T3-L1 Adipocytes" Plants 13, no. 19: 2789. https://doi.org/10.3390/plants13192789





