Dynamics of Hydrogen Peroxide Accumulation During Tip Growth of Infection Thread in Nodules and Cell Differentiation in Pea (Pisum sativum L.) Symbiotic Nodules
Abstract
1. Introduction
2. Results
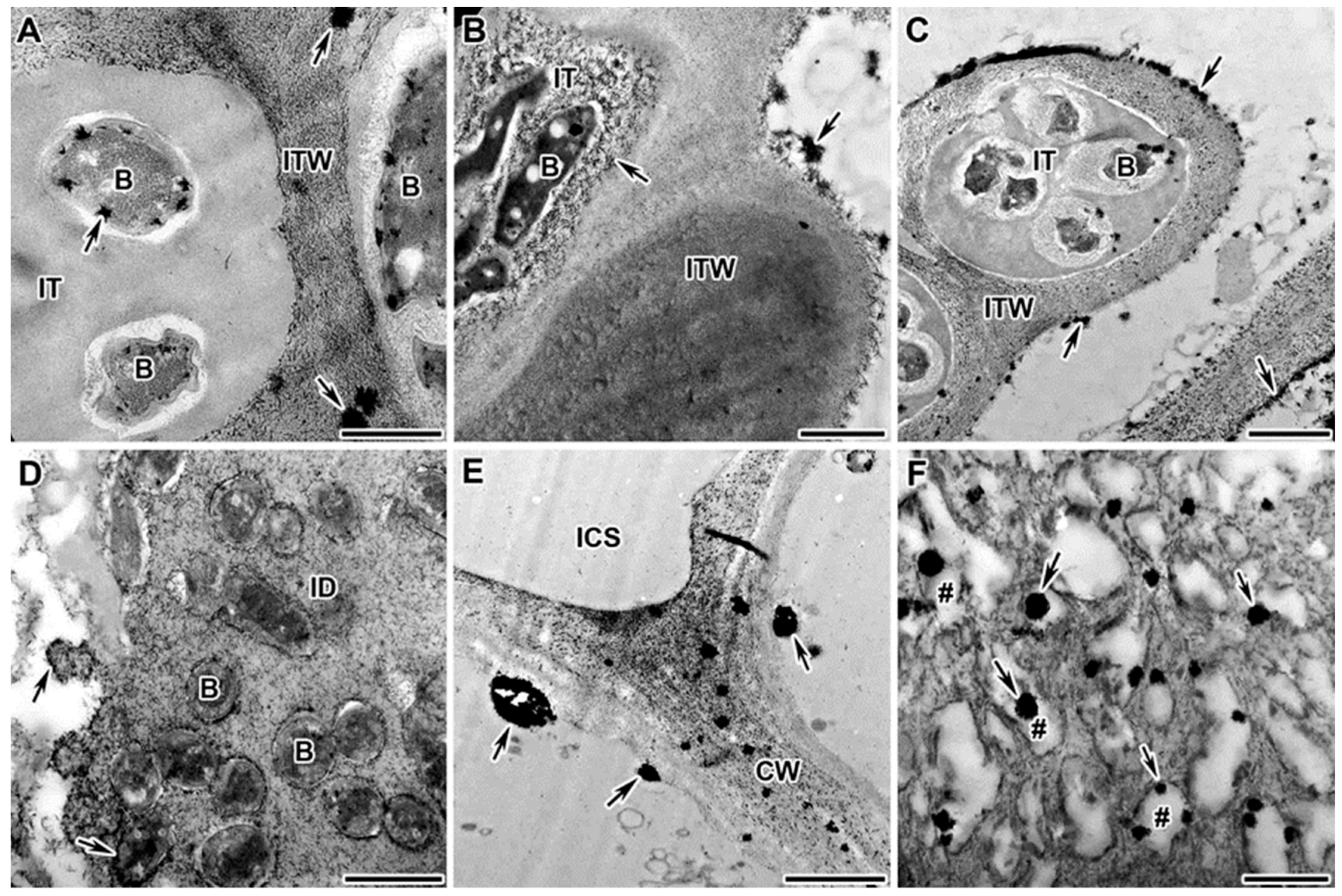
2.1. Dynamics of Distribution of Hydrogen Peroxide in Infection Threads
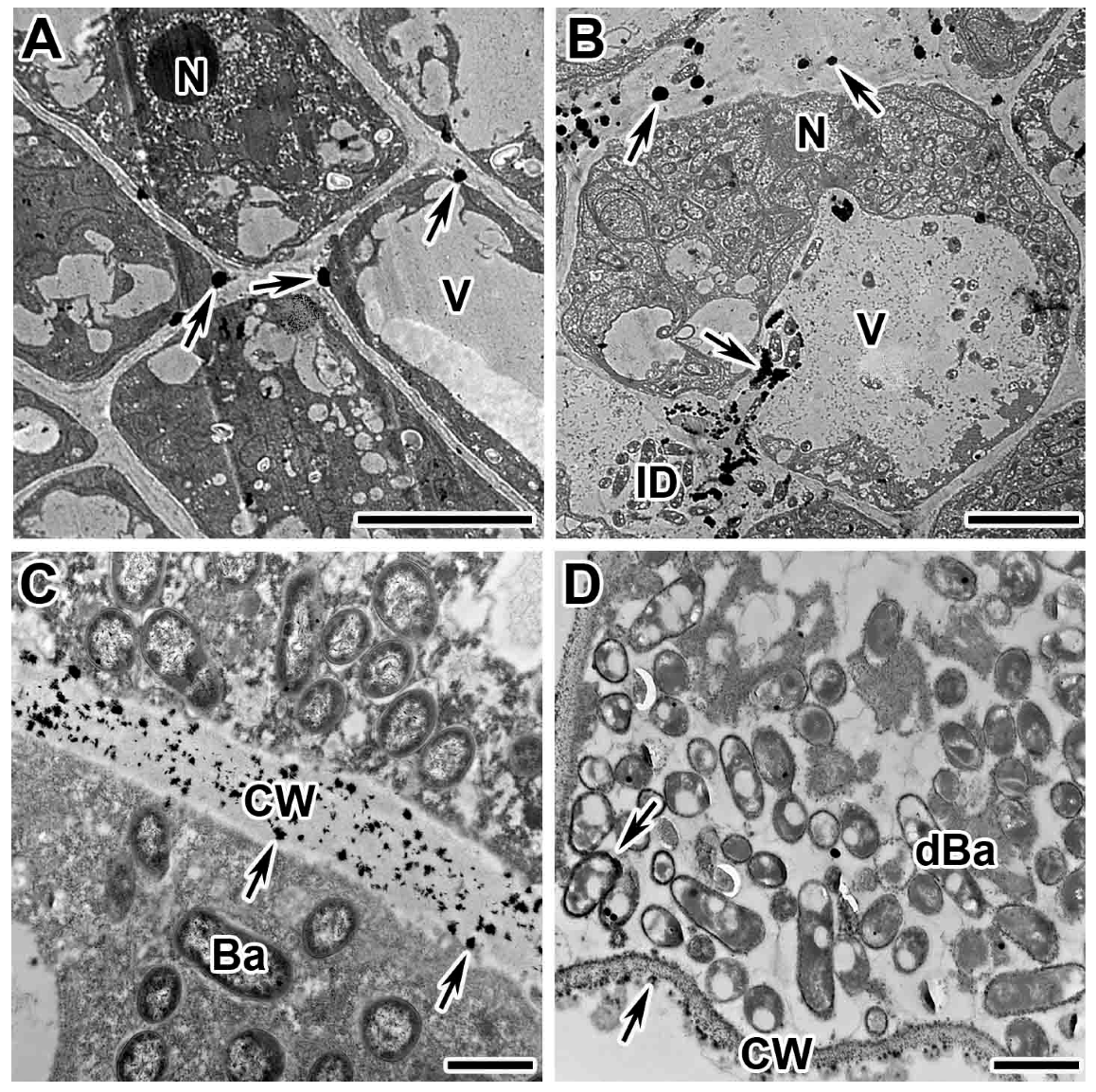
2.2. Dynamics of Distribution of Hydrogen Peroxide in Cells of Wild-Type Nodules
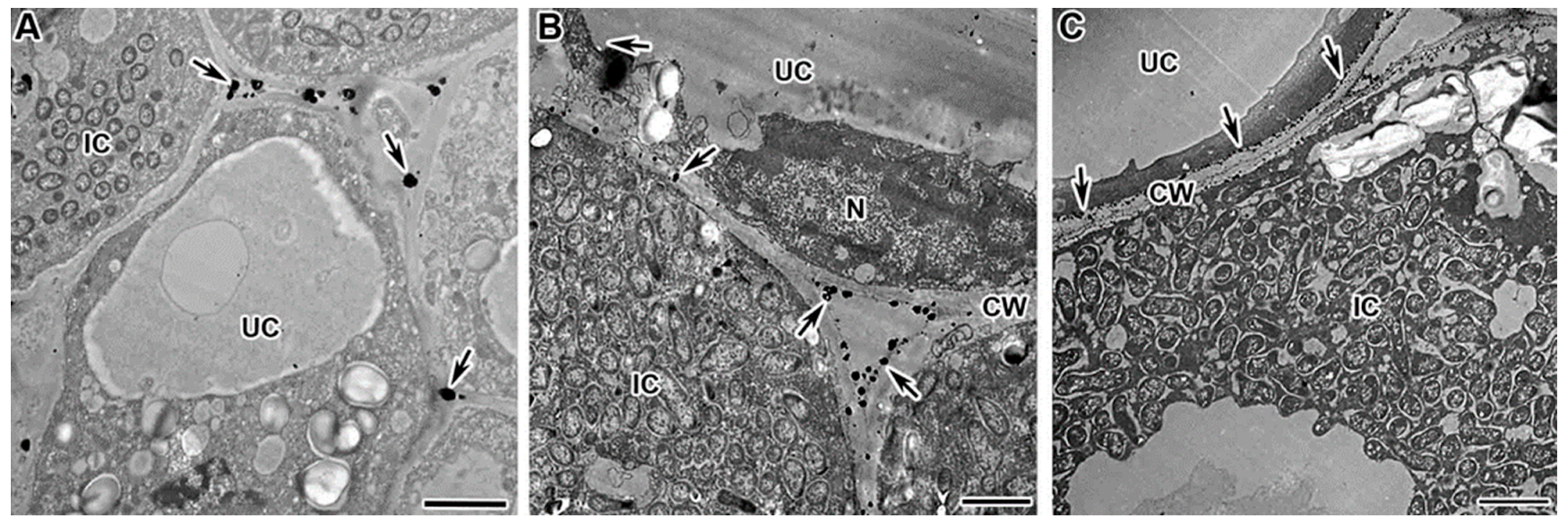
2.3. Dynamics of Distribution of Hydrogen Peroxide in Infected Cells of Mutant Nodules
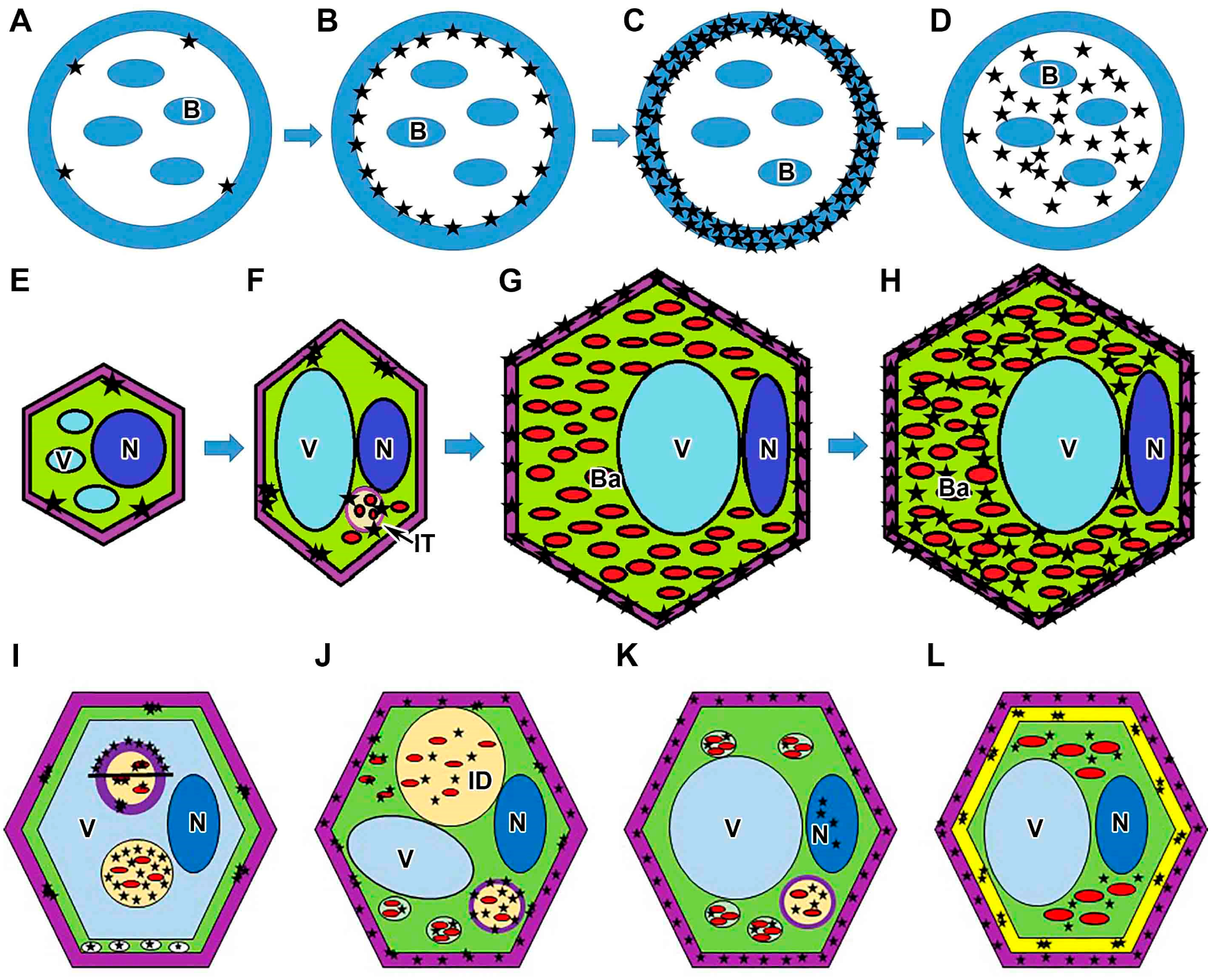
3. Discussion
4. Materials and Methods
4.1. Plant Material and Bacterial Strain
4.2. Plant Growth Conditions
4.3. Histochemical Localization of H2O2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minguillón, S.; Matamoros, M.A.; Duanmu, D.; Becana, M. Signaling by reactive molecules and antioxidants in legume nodules. New Phytol. 2022, 236, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Damiani, I.; Drain, A.; Guichard, M.; Balzergue, S.; Boscari, A.; Boyer, J.-C.; Brunaud, V.; Cottaz, S.; Rancurel, C.; Da Rocha, M.; et al. Nod factor effects on root hair-specific transcriptome of Medicago truncatula: Focus on plasma membrane transport systems and reactive oxygen species networks. Front. Plant Sci. 2016, 7, 794. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.; Pucciariello, C.; Mandon, K.; Innocenti, G.; Jamet, A.; Baudouin, E.; Hérouart, D.; Frendo, P.; Puppo, A. Reactive oxygen and nitrogen species and glutathione: Key players in the legume–Rhizobium symbiosis. J. Exp. Bot. 2006, 57, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; De Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005, 165, 683–701. [Google Scholar] [CrossRef]
- Puppo, A.; Pauly, N.; Boscari, A.; Mandon, K.; Brouquisse, R. Hydrogen peroxide and nitric oxide: Key regulators of the legume–Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013, 18, 2202–2219. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Becana, M. Redox control of the legume-Rhizobium symbiosis. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 67–96. [Google Scholar]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. Signals in plant disease resistance. Bull. L’institut Pasteur 1995, 93, 167–186. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. CMLS 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Fernández-García, N.; Wienkoop, S.; Loscos, J.; Saiz, A.; Becana, M. Mitochondria are an early target of oxidative modifications in senescing legume nodules. New Phytol. 2013, 197, 873–885. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Alloing, G.; Mandon, K.; Frendo, P. Redox regulation of differentiation in symbiotic nitrogen fixation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1469–1478. [Google Scholar] [CrossRef]
- Montiel, J.; Arthikala, M.-K.; Cárdenas, L.; Quinto, C. Legume NADPH oxidases have crucial roles at different stages of nodulation. Int. J. Mol. Sci. 2016, 17, 680. [Google Scholar] [CrossRef]
- Chang, C.; Damiani, I.; Puppo, A.; Frendo, P. Redox changes during the legume-Rhizobium symbiosis. Mol. Plant 2009, 2, 370–377. [Google Scholar] [CrossRef]
- Frendo, P.; Matamoros, M.; Alloing, G.; Becana, M. Thiol-based redox signaling in the nitrogen-fixing symbiosis. Front. Plant Sci. 2013, 4, 376. [Google Scholar] [CrossRef]
- Becana, M.; Matamoros, M.A.; Udvardi, M.; Dalton, D.A. Recent insights into antioxidant defenses of legume root nodules. New Phytol. 2010, 188, 960–976. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Sci. 2000, 159, 75–85. [Google Scholar] [CrossRef]
- Alloing, G.; Mandon, K.; Boncompagni, E.; Montrichard, F.; Frendo, P. Involvement of glutaredoxin and thioredoxin systems in the nitrogen-fixing symbiosis between legumes and rhizobia. Antioxidants 2018, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yin, J.; Peng, Y.; Xie, J.; Wu, H.; He, D.; Li, X.; Cheng, G. Glutathione is involved in detoxification of peroxide and root nodule symbiosis of Mesorhizobium huakuii. Curr. Microbiol. 2020, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.A.; Chernova, E.N.; Kulaeva, O.A.; Tsyganova, A.V.; Kusakin, P.G.; Russkikh, I.V.; Tikhonovich, I.A.; Tsyganov, V.E. The regulation of pea (Pisum sativum L.) symbiotic nodule infection and defense responses by glutathione, homoglutathione, and their ratio. Front. Plant Sci. 2022, 13, 843565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Karunakaran, R.; East, A.K.; Munoz-Azcarate, O.; Poole, P.S. Glutathione affects the transport activity of Rhizobium leguminosarum 3841 and is essential for efficient nodulation. FEMS Microbiol. Lett. 2017, 364, fnx045. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Moran, J.F.; Iturbe-Ormaetxe, I.; Rubio, M.C.; Becana, M. Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol. 1999, 121, 879–888. [Google Scholar] [CrossRef]
- El Msehli, S.; Lambert, A.; Baldacci-Cresp, F.; Hopkins, J.; Boncompagni, E.; Smiti, S.A.; Hérouart, D.; Frendo, P. Crucial role of (homo)glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol. 2011, 192, 496–506. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Andrio, E.; Marino, D.; Marmeys, A.; de Segonzac, M.D.; Damiani, I.; Genre, A.; Huguet, S.; Frendo, P.; Puppo, A.; Pauly, N. Hydrogen peroxide-regulated genes in the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 2013, 198, 179–189. [Google Scholar] [CrossRef]
- Desikan, R.; Hancock, J.T.; Neill, S.J. Oxidative stress signalling. In Plant Responses to Abiotic Stress; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 121–149. [Google Scholar] [CrossRef]
- Peleg-Grossman, S.; Golani, Y.; Kaye, Y.; Melamed-Book, N.; Levine, A. NPR1 protein regulates pathogenic and symbiotic interactions between Rhizobium and legumes and non-Legumes. PLoS ONE 2009, 4, e8399. [Google Scholar] [CrossRef]
- Peleg-Grossman, S.; Melamed-Book, N.; Levine, A. ROS production during symbiotic infection suppresses pathogenesis-related gene expression. Plant Signal. Behav. 2012, 7, 409–415. [Google Scholar] [CrossRef]
- Lohar, D.P.; Haridas, S.; Gantt, J.S.; VandenBosch, K.A. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume–rhizobia symbiosis. New Phytol. 2007, 173, 39–49. [Google Scholar] [CrossRef]
- Montiel, J.; Nava, N.; Cárdenas, L.; Sánchez-López, R.; Arthikala, M.-K.; Santana, O.; Sánchez, F.; Quinto, C. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by rhizobia. Plant Cell Physiol. 2012, 53, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Arthikala, M.-K.; Montiel, J.; Sánchez-López, R.; Nava, N.; Cárdenas, L.; Quinto, C. Respiratory burst oxidase homolog gene A is crucial for Rhizobium infection and nodule maturation and function in common bean. Front. Plant Sci. 2017, 8, 2003. [Google Scholar] [CrossRef]
- Marino, D.; Andrio, E.; Danchin, E.G.J.; Oger, E.; Gucciardo, S.; Lambert, A.; Puppo, A.; Pauly, N. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol. 2011, 189, 580–592. [Google Scholar] [CrossRef]
- Montiel, J.; Reid, D.; Grønbæk, T.H.; Benfeldt, C.M.; James, E.K.; Ott, T.; Ditengou, F.A.; Nadzieja, M.; Kelly, S.; Stougaard, J. Distinct signaling routes mediate intercellular and intracellular rhizobial infection in Lotus japonicus. Plant Physiol. 2021, 185, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.; et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef] [PubMed]
- Ramu, S.K.; Peng, H.-M.; Cook, D.R. Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol. Plant Microbe Interact. 2002, 15, 522–528. [Google Scholar] [CrossRef]
- Cook, D.; Dreyer, D.; Bonnet, D.; Howell, M.; Nony, E.; VandenBosch, K. Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 1995, 7, 43–55. [Google Scholar] [CrossRef]
- Wisniewski, J.-P.; Rathbun, E.A.; Knox, J.P.; Brewin, N.J. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: Implications for pea nodule Initiation by Rhizobium leguminosarum. Mol. Plant Microbe Interact. 2000, 13, 413–420. [Google Scholar] [CrossRef]
- Jamet, A.; Sigaud, S.; Van de Sype, G.; Puppo, A.; Hérouart, D. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant Microbe Interact. 2003, 16, 217–225. [Google Scholar] [CrossRef]
- Jamet, A.; Mandon, K.; Puppo, A.; Hérouart, D. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J. Bacteriol. 2007, 189, 8741–8745. [Google Scholar] [CrossRef]
- Harrison, J.; Jamet, A.; Muglia, C.I.; Van de Sype, G.; Aguilar, O.M.; Puppo, A.; Frendo, P. Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 168–174. [Google Scholar] [CrossRef]
- Muglia, C.; Comai, G.; Spegazzini, E.; Riccillo, P.M.; Aguilar, O.M. Glutathione produced by Rhizobium tropici is important to prevent early senescence in common bean nodules. FEMS Microbiol. Lett. 2008, 286, 191–198. [Google Scholar] [CrossRef]
- Yang, L.; El Msehli, S.; Benyamina, S.; Lambert, A.; Hopkins, J.; Cazareth, J.; Pierre, O.; Hérouart, D.; Achi-Smiti, S.; Boncompagni, E.; et al. Glutathione deficiency in Sinorhizobium meliloti does not impair bacteroid differentiation but induces early senescence in the interaction with Medicago truncatula. Front. Plant Sci. 2020, 11, 137. [Google Scholar] [CrossRef]
- Benyamina, S.M.; Baldacci-Cresp, F.; Couturier, J.; Chibani, K.; Hopkins, J.; Bekki, A.; de Lajudie, P.; Rouhier, N.; Jacquot, J.-P.; Alloing, G.; et al. Two Sinorhizobium meliloti glutaredoxins regulate iron metabolism and symbiotic bacteroid differentiation. Environ. Microbiol. 2013, 15, 795–810. [Google Scholar] [CrossRef]
- Alesandrini, F.; Mathis, R.; Van de Sype, G.; Hérouart, D.; Puppo, A. Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol. 2003, 158, 131–138. [Google Scholar] [CrossRef]
- Wang, L.; Rubio, M.C.; Xin, X.; Zhang, B.; Fan, Q.; Wang, Q.; Ning, G.; Becana, M.; Duanmu, D. CRISPR/Cas9 knockout of leghemoglobin genes in Lotus japonicus uncovers their synergistic roles in symbiotic nitrogen fixation. New Phytol. 2019, 224, 818–832. [Google Scholar] [CrossRef]
- Santos, R.; Hérouart, D.; Sigaud, S.; Touati, D.; Puppo, A. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant Microbe Interact. 2001, 14, 86–89. [Google Scholar] [CrossRef]
- Rubio, M.C.; James, E.K.; Clemente, M.R.; Bucciarelli, B.; Fedorova, M.; Vance, C.P.; Becana, M. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol. Plant Microbe Interact. 2004, 17, 1294–1305. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Bacterial release is accompanied by ectopic accumulation of cell wall material around the vacuole in nodules of Pisum sativum sym33-3 allele encoding transcription factor PsCYCLOPS/PsIPD3. Protoplasma 2019, 256, 1449–1453. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Ivanova, K.A.; Tsyganov, V.E. Histological and ultrastructural nodule organization of the pea (Pisum sativum) mutant SGEFix−-5 in the Sym33 gene encoding the transcription factor PsCYCLOPS/PsIPD3. Ekol. Genet. 2019, 17, 65–70. [Google Scholar] [CrossRef]
- Borisov, A.Y.; Rozov, S.M.; Tsyganov, V.E.; Morzhina, E.V.; Lebsky, V.K.; Tikhonovich, I.A. Sequential functioning of Sym-13 and Sym-31, two genes affecting symbiosome development in root nodules of pea (Pisum sativum L). Mol. Gen. Genet. 1997, 254, 592–598. [Google Scholar] [CrossRef]
- Novák, K.; Pešina, K.; Nebesářová, J.; Škrdleta, V.; Lisá, L.; Našinec, V. Symbiotic tissue degradation pattern in the ineffective nodules of three nodulation mutants of pea (Pisum sativum L.). Ann. Bot. 1995, 76, 303–313. [Google Scholar] [CrossRef]
- Morzhina, E.V.; Tsyganov, V.E.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. Four developmental stages identified by genetic dissection of pea (Pisum sativum L.) root nodule morphogenesis. Plant Sci. 2000, 155, 75–83. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Foyer, C.H.; Mann, G.E. Reactive oxygen species are crucial “pro-life“ survival signals in plants. Free Radic. Biol. Med. 2018, 122, 1–3. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Bernards, M.A.; Summerhurst, D.K.; Razem, F.A. Oxidases, peroxidases and hydrogen peroxide: The suberin connection. Phytochem. Rev. 2004, 3, 113–126. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Clarke, A.; Hurst, R.D.; Neill, S.J. Cell signalling following plant/pathogen interactions involves the generation of reactive oxygen and reactive nitrogen species. Plant Physiol. Biochem. 2002, 40, 611–617. [Google Scholar] [CrossRef]
- Hérouart, D.; Sigaud, S.; Moreau, S.; Frendo, P.; Touati, D.; Puppo, A. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J. Bacteriol. 1996, 178, 6802–6809. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, T.; Shirakawa, Y.; Kusumoto, M.; Masuda, H.; Sato, T. Susceptibility to hydrogen peroxide and catalase activity of root nodule bacteria. Biosci. Biotechnol. Biochem. 1999, 63, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef]
- Kosterin, O.E.; Rozov, S.M. Mapping of the new mutation blb and the problem of integrity of linkage group I. Pisum Genet. 1993, 25, 27–31. [Google Scholar]
- Tsyganov, V.E.; Seliverstova, E.; Voroshilova, V.; Tsyganova, A.; Pavlova, Z.; Lebskii, V.; Borisov, A.Y.; Brewin, N.; Tikhonovich, I. Double mutant analysis of sequential functioning of pea (Pisum sativum L.) genes Sym13, Sym33, and Sym40 during symbiotic nodule development. Russ. J. Genet. Appl. Res. 2011, 1, 343–348. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V.; Voroshilova, V.A.; Borisov, A.Y.; Tikhonovich, I.A. Analysis of the interaction of pea (Pisum sativum L.) symbiotic genes Sym33 and Sym42 whose mutations result in abnormalities during infection thread development. Russ. J. Genet. Appl. Res. 2014, 4, 83–87. [Google Scholar] [CrossRef]
- Berdnikov, V.A.; Rosov, S.M.; Bogdanova, V.S. Construction of a series of laboratory pea lines. In Proceedings of the Conference on Genetics, Kiev, USSR, 23–25 May 1989; Volume 2, pp. 26–27. [Google Scholar]
- Kneen, B.E.; LaRue, T.A. Induced symbiosis mutants of pea (Pisum sativum) and sweetclover (Melilotus alba annua). Plant Sci. 1988, 58, 177–182. [Google Scholar] [CrossRef]
- Engvild, K.C. Nodulation and nitrogen fixation mutants of pea, Pisum sativum. Theor. Appl. Genet. 1987, 74, 711–713. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V. Symbiotic regulatory genes controlling nodule development in Pisum sativum L. Plants 2020, 9, 1741. [Google Scholar] [CrossRef]
- Young, J.P.W.; Jorrin, B.; Moeskjær, S.; James, E.K. Rhizobium brockwellii sp. nov., Rhizobium johnstonii sp. nov. and Rhizobium beringeri sp. nov., three genospecies within the Rhizobium leguminosarum species complex. Int. J. Syst. Evol. Microbiol. 2023, 73, 005979. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef][Green Version]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Bestwick, C.S.; Brown, I.R.; Bennett, M.H.; Mansfield, J.W. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 1997, 9, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]



| Genotype | Nodule Phenotype | References |
|---|---|---|
| SGE | Wild type | [52,67] |
| SGEFix–-1 (sym40-1) 1 | Hypertrophied infection droplets and infection threads, abnormal bacteroids, early nodule senescence | [52] |
| SGEFix–-2 (sym33-3) 2 | “Locked” infection threads, absence of bacterial release into the host cell cytoplasm of most infected cells 3 | [52] |
| RBT3 (sym33-3, sym40-1) | “Locked” infection threads, absence of bacterial release | [68] |
| RBT4 (sym33-3, sym42) | “Locked” infection threads, absence of bacterial release | [69] |
| Sprint-2 | Wild type | [70] |
| Sprint-2Fix– (sym31) | Undifferentiated bacteroids | [56] |
| ‘Sparkle’ | Wild type | [71] |
| ‘Finale’ | Wild type | [57,58,72] |
| RisFixV (sym42) | Early nodule senescence, thickening of the infection thread wall | [53,57,58,72,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganova, A.V.; Gorshkov, A.P.; Vorobiev, M.G.; Tikhonovich, I.A.; Brewin, N.J.; Tsyganov, V.E. Dynamics of Hydrogen Peroxide Accumulation During Tip Growth of Infection Thread in Nodules and Cell Differentiation in Pea (Pisum sativum L.) Symbiotic Nodules. Plants 2024, 13, 2923. https://doi.org/10.3390/plants13202923
Tsyganova AV, Gorshkov AP, Vorobiev MG, Tikhonovich IA, Brewin NJ, Tsyganov VE. Dynamics of Hydrogen Peroxide Accumulation During Tip Growth of Infection Thread in Nodules and Cell Differentiation in Pea (Pisum sativum L.) Symbiotic Nodules. Plants. 2024; 13(20):2923. https://doi.org/10.3390/plants13202923
Chicago/Turabian StyleTsyganova, Anna V., Artemii P. Gorshkov, Maxim G. Vorobiev, Igor A. Tikhonovich, Nicholas J. Brewin, and Viktor E. Tsyganov. 2024. "Dynamics of Hydrogen Peroxide Accumulation During Tip Growth of Infection Thread in Nodules and Cell Differentiation in Pea (Pisum sativum L.) Symbiotic Nodules" Plants 13, no. 20: 2923. https://doi.org/10.3390/plants13202923
APA StyleTsyganova, A. V., Gorshkov, A. P., Vorobiev, M. G., Tikhonovich, I. A., Brewin, N. J., & Tsyganov, V. E. (2024). Dynamics of Hydrogen Peroxide Accumulation During Tip Growth of Infection Thread in Nodules and Cell Differentiation in Pea (Pisum sativum L.) Symbiotic Nodules. Plants, 13(20), 2923. https://doi.org/10.3390/plants13202923









