Drought-Induced Alterations in Carbon and Water Dynamics of Chinese Fir Plantations at the Trunk Wood Stage
Abstract
1. Introduction
2. Results
2.1. Daily and Seasonal Dynamics of Environmental Factors
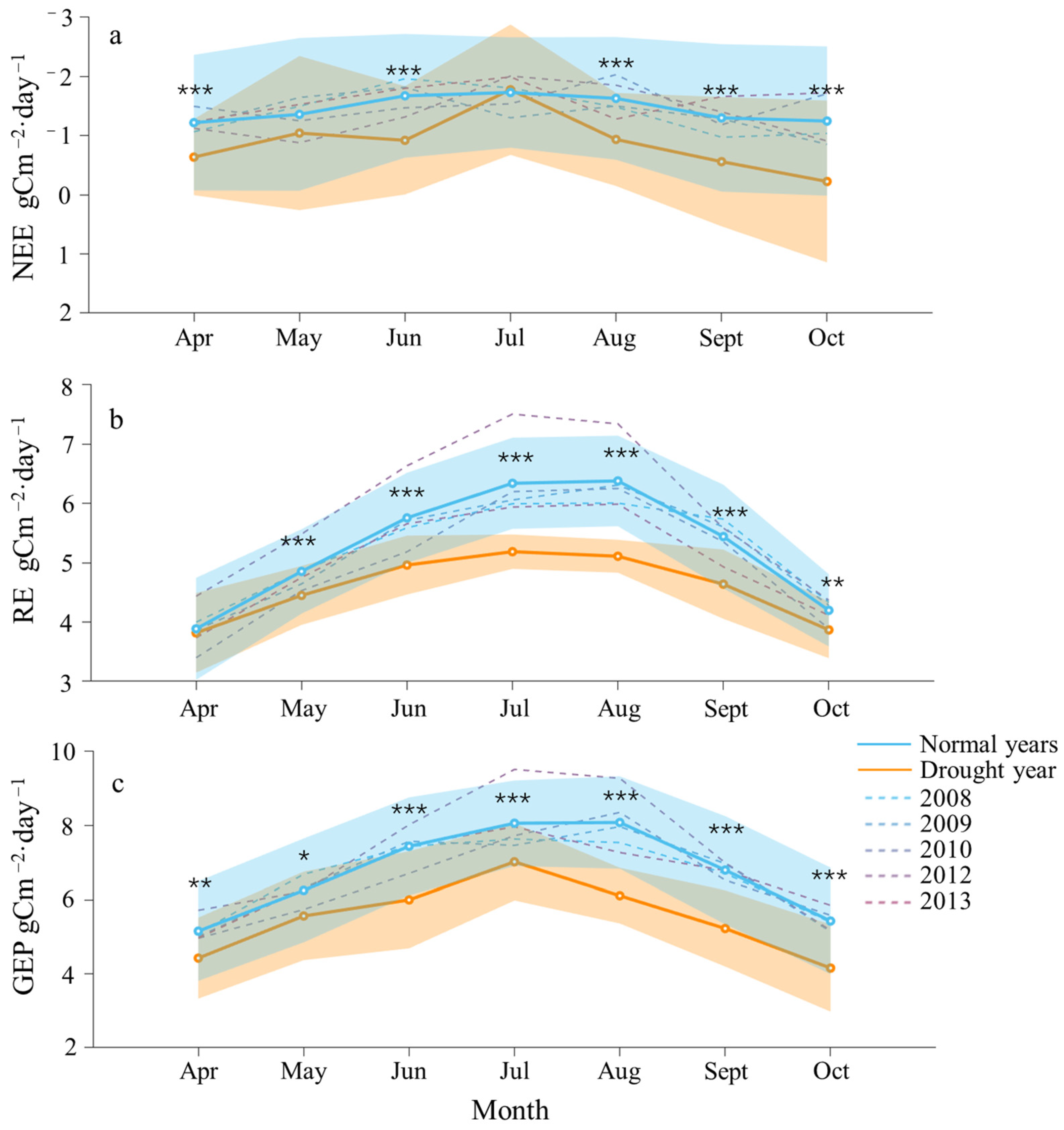
2.2. Seasonal Dynamics of Carbon and Water Fluxes
2.3. Differences in Carbon–Water Flux and Environmental Factors
3. Discussion
3.1. Carbon-Water Fluxes
3.2. Water Use Efficiency
3.3. The Water Use Efficiency and the Environmental Factors
4. Materials and Methods
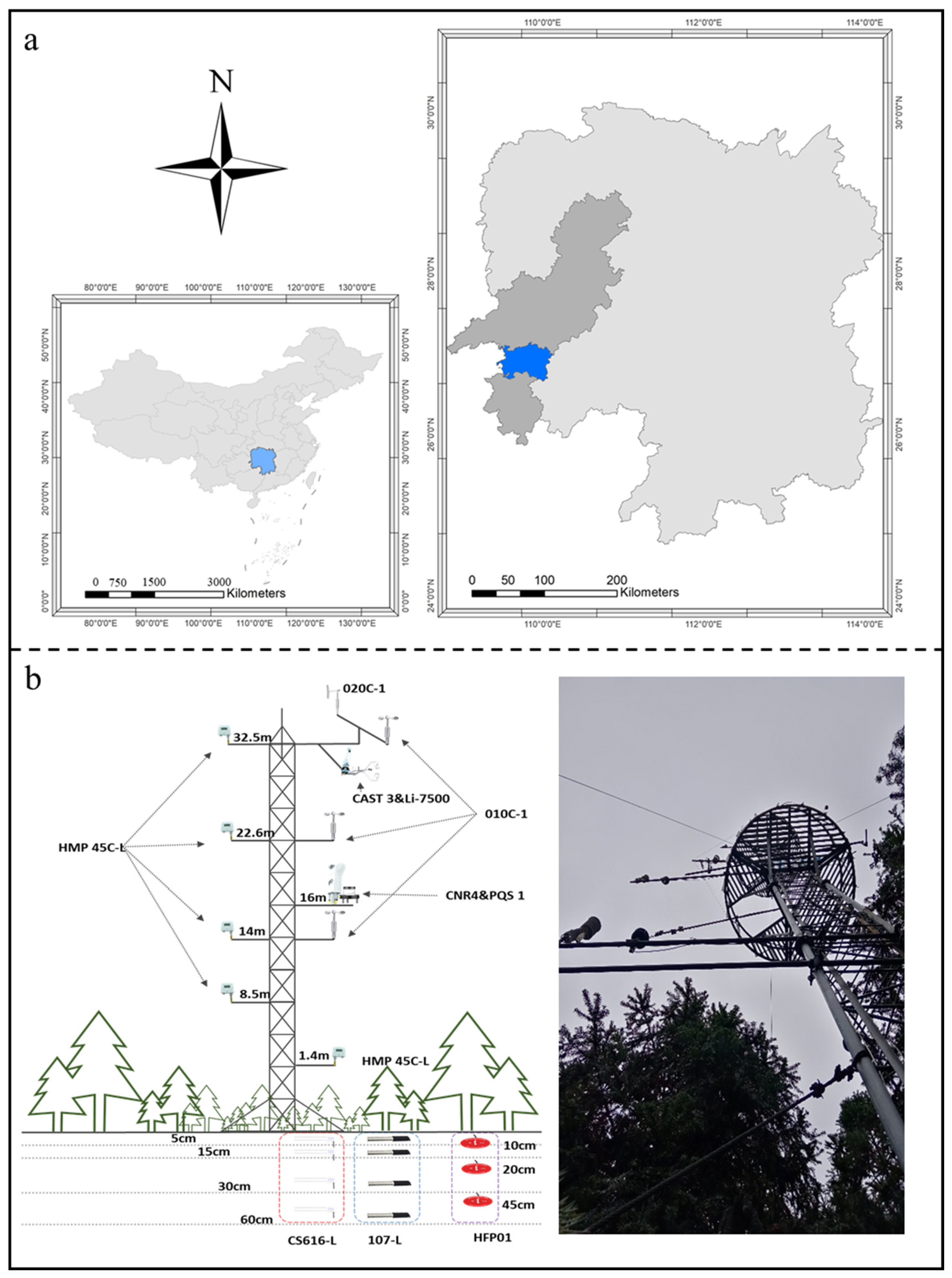
4.1. Study Area
4.2. Data Acquisition and Preprocessing
4.3. Calculation of Carbon Flux
4.4. Calculation of ET
4.5. Calculation of WUE
4.6. Average Values
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.H.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Summary for Policymakers. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate change and weather extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Maity, S.S.; Maity, R. Changing pattern of intensity-duration-frequency relationship of precipitation due to climate change. Water Resour. Manag. 2022, 36, 5371–5399. [Google Scholar] [CrossRef]
- Rahmani, F.; Fattahi, M.H. Investigation of alterations in droughts and floods patterns induced by climate change. Acta Geophys. 2024, 72, 405–418. [Google Scholar] [CrossRef]
- Van Ginkel, M.; Biradar, Ç.M. Drought early warning in agri-food systems. Climate 2021, 9, 134. [Google Scholar] [CrossRef]
- Locatelli, B.; Pavageau, C.; Pramova, E.; Di Gregorio, M. Integrating climate change mitigation and adaptation in agriculture and forestry: Opportunities and trade-offs. WIREs Clim. Chang. 2015, 6, 585–598. [Google Scholar] [CrossRef]
- Pradhan, K.; Ettinger, A.K.; Case, M.J.; Lambers, J. Applying climate change refugia to forest management and old-growth restoration. Glob. Chang. Biol. 2023, 29, 3692–3706. [Google Scholar] [CrossRef]
- Duncanson, L.; Liang, M.; Leitold, V.; Armston, J.; Moorthy, S.M.K.; Dubayah, R.; Costedoat, S.; Enquist, B.J.; Fatoyinbo, L.; Goetz, S.J.; et al. The effectiveness of global protected areas for climate change mitigation. Nat. Commun. 2023, 14, 2908. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Chiang, F.; Mazdiyasni, O.; AghaKouchak, A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity. Nat. Commun. 2021, 12, 2754. [Google Scholar] [CrossRef]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Berdugo, M.; Rocamora, B.; Solé, R.; Maestre, F. Ecological mechanisms underlying aridity thresholds in global drylands. Funct. Ecol. 2021, 36, 4–23. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Knauer, J.; Zaehle, S.; Medlyn, B.; Reichstein, M.; Williams, C.; Migliavacca, M.; De Kauwe, M.; Werner, C.; Keitel, C.; Kolari, P.; et al. Towards physiologically meaningful water-use efficiency estimates from eddy covariance data. Glob. Chang. Biol. 2018, 24, 694–710. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Fernández, J.; Coleman, M.; Hu, W.; Di, N.; Zou, S.; Liu, Y.; Xi, B.; Clothier, B. Variations in water-balance components and carbon stocks in poplar plantations with differing water inputs over a whole rotation: Implications for sustainable forest management under climate change. Agric. For. Meteorol. 2022, 320, 108958. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Wang, G.; Hu, Z.; Song, C.; Lin, S.; Sun, J.; Sun, S. An increasing effect of soil moisture on semiempirical water-use efficiency models from wet to dry climate regions. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007347. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Lavergne, A.; Graven, H.; De Kauwe, M.; Keenan, T.; Medlyn, B.; Prentice, I. Observed and modelled historical trends in the water-use efficiency of plants and ecosystems. Glob. Chang. Biol. 2019, 25, 2242–2257. [Google Scholar] [CrossRef]
- Lin, E.; Zhuang, H.; Yu, J.; Liu, X.; Huang, H.; Zhu, M.; Tong, Z. Genome survey of Chinese fir (Cunninghamia lanceolata): Identification of genomic SSRs and demonstration of their utility in genetic diversity analysis. Sci. Rep. 2020, 10, 4698. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Zhang, B.; Gao, D.; Wang, T.; Xu, W.; Ren, R.; Wang, S. Contrasting water-use patterns of Chinese fir among different plantation types in a subtropical region of China. Front. Plant Sci. 2022, 13, 946508. [Google Scholar] [CrossRef]
- Camarero, J.; Gazol, A.; Linares, J.; Fajardo, A.; Colangelo, M.; Valeriano, C.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Granda, E.; Gimeno, T. Differences in temperature sensitivity and drought recovery between natural stands and plantations of conifers are species-specific. Sci. Total Environ. 2021, 796, 148930. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Cuesta-Valero, F.; Miralles, D.; Mahecha, M.; Quaas, J.; Reichstein, M.; Zscheischler, J.; Peng, J. Soil heat extremes can outpace air temperature extremes. Nat. Clim. Chang. 2023, 13, 1237–1241. [Google Scholar] [CrossRef]
- Cai, G.; Wankmüller, F.; Ahmed, M.; Carminati, A. How the interactions between atmospheric and soil drought affect the functionality of plant hydraulics. Plant Cell Environ. 2023, 46, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Metze, D.; Schnecker, J.; Canarini, A.; Fuchslueger, L.; Koch, B.; Stone, B.; Hungate, B.; Hausmann, B.; Schmidt, H.; Schaumberger, A.; et al. Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions. Nat. Commun. 2023, 14, 5895. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes, and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Dai, A.; Zhao, T.; Chen, J. Climate change and drought: A precipitation and evaporation perspective. Curr. Clim. Chang. Rep. 2018, 4, 301–312. [Google Scholar] [CrossRef]
- Hartick, C.; Furusho-Percot, C.; Clark, M.; Kollet, S. An interannual drought feedback loop affects the surface energy balance and cloud properties. Geophys. Res. Lett. 2022, 49, e2022GL100924. [Google Scholar] [CrossRef]
- Wan, L.; Bento, V.; Qu, Y.; Qiu, J.; Song, H.; Zhang, R.; Wu, X.; Xu, F.; Lu, J.; Wang, Q. Drought characteristics and dominant factors across China: Insights from high-resolution daily SPEI dataset between 1979 and 2018. Sci. Total Environ. 2023, 901, 166362. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, J.; Kang, S.; Slater, L.; Gu, X.; Volchak, A. Quantifying the drivers of terrestrial drought and water stress impacts on carbon uptake in China. Agric. For. Meteorol. 2024, 344, 109817. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Shan, B.; Xu, M.; Cong, N.; Chen, N.; Ji, X.; Zhao, G.; Zheng, Z.; Zhu, J.; et al. Drought-induced resource use efficiency responses in an alpine meadow ecosystem of northern Tibet. Agric. For. Meteorol. 2023, 342, 109745. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Jones, J.A.; Sun, G.; Wei, X.; Ellison, D.; Archer, E.R.M.; McNulty, S.; Asbjornsen, H.; Zhang, Z.; et al. Managing the forest-water nexus for climate change adaptation. For. Ecol. Manag. 2022, 525, 120545. [Google Scholar] [CrossRef]
- Mi, N.; Wen, X.; Cai, F.; Wang, Y.; Zhang, Y. Effects of Seasonal Drought on the Water Use Efficiency of Qianyanzhou Plantation. Sci. Silvae Sin. 2014, 50, 24–31. [Google Scholar]
- Yang, Y.; Guan, H.; Batelaan, O.; McVicar, T.; Long, D.; Piao, S.; Liang, W.; Liu, B.; Jin, Z.; Simmons, C. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 2016, 6, 23284. [Google Scholar] [CrossRef]
- Seneviratne, S.; Corti, T.; Davin, E.; Hirschi, M.; Jaeger, E.; Lehner, I.; Orlowsky, B.; Teuling, A. Investigating soil moisture–climate interactions in a changing climate: A review. Earth Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Trenberth, K.; Fasullo, J.; Kiehl, J. Earth’s global energy budget. Bull. Am. Meteorol. Soc. 2009, 90, 311–324. [Google Scholar] [CrossRef]
- Schaefer, K.; Schwalm, C.; Williams, C.; Arain, M.; Barr, A.; Chen, J.; Davis, K.; Dimitrov, D.; Hilton, T.; Hollinger, D.; et al. A model-data comparison of gross primary productivity: Results from the North American Carbon Program site synthesis. J. Geophys. Res. 2012, 117, G03010. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Guan, K.; Pan, M.; Li, H.; Wolf, A.; Wu, J.; Medvigy, D.; Caylor, K.; Sheffield, J.; Wood, E.; Malhi, Y.; et al. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nat. Geosci. 2015, 8, 284–289. [Google Scholar] [CrossRef]
- Baldocchi, D.D. ‘Breathing’ of the terrestrial biosphere: Lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 2008, 56, 1–26. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.; Seneviratne, S.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, H.; Wang, W.; Xue, J.; Wu, Y.; Yu, S. Diurnal and seasonal changes of fluxes over a poplar plantation in Hongze Lake basin. J. Nanjing For. Univ. 2019, 43, 113–120. [Google Scholar]
- Lin, S.; Sun, X.; Huang, K.; Song, C.; Sun, J.; Sun, S.; Wang, G.; Hu, Z. The seasonal variability of future evapotranspiration over China during the 21st century. Sci. Total Environ. 2024, 926, 171816. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sachdeva, S.; Bhat, K.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular Basis. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Anderegg, W.; Flint, A.; Huang, C.; Flint, L.; Berry, J.; Frank, D.; Sperry, J.; Field, C. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.; Allen, C.; Breshears, D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Medranoa, H.; Tomása, M.; Martorella, S.; Flexasa, J.; Hernándeza, E.; Rossellóa, J.; Poub, A.; Escalonaa, J.-M.; Botaa, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Jiang, X.; Zhuang, Q.; Liu, J. Impacts of climate variability on water use efficiency and crop yields in the Midwest United States. J. Clim. 2009, 22, 1510–1521. [Google Scholar]
- Benitez-Alfonso, Y.; Soanes, B.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.; Martin, A.; et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
- Ding, J. The impact of global change on plant water physiology and ecology. Bot. Res. 2024, 13, 110–118. [Google Scholar]
- Ponton, S.; Flanagan, L.; Alstad, K.; Johnson, B.; Morgenstern, K.; Kljun, N.; Black, T.; Barr, A. Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob. Chang. Biol. 2006, 12, 294–310. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Li, R.; Long, F.; Zhang, L.; Zhang, Q.; Li, J. Water-use efficiency of an old-growth forest in lower subtropical China. Sci. Rep. 2017, 7, 42761. [Google Scholar] [CrossRef] [PubMed]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon? -How trees may cope with more frequent climate change-type drought events. Glob. Chang. Biol. 2011, 17, 642–655. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Baldocchi, D. Measuring fluxes of trace gases and energy between ecosystems and the atmosphere—The state and future of the eddy covariance method. Glob. Chang. Biol. 2014, 20, 3600–3609. [Google Scholar] [CrossRef]
- Xu, W.; Xia, X.; Piao, S.; Wu, D.; Li, W.; Yang, S.; Yuan, W. Weakened increase in global near-surface water vapor pressure during the last 20 years. Geophys. Res. Lett. 2024, 51, e2023GL107909. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, S.; Chen, J.; Xiang, N.; Sun, L.; Chen, B.; Fu, Z.; Zhu, K.; He, X. Divergent impacts of VPD and SWC on ecosystem carbon-water coupling under different dryness conditions. Sci. Total Environ. 2023, 905, 167007. [Google Scholar] [CrossRef]
- Rosie, F.; Koven, C.; Anderegg, W.; Christoffersen, B.; Dietze, M.; Farrior, C.; Holm, J.; Hurtt, G.; Knox, R.; Lawrence, P.; et al. Vegetation demographics in earth system models: A review of progress and priorities. Glob. Chang. Biol. 2017, 24, 35–54. [Google Scholar]
- Gonsamo, A.; Gonsamo, A.; Croft, H.; Chen, J.M.; Wu, C.; Froelich, N.J.; Staebler, R.M. Radiation contributed more than temperature to increased decadal autumn and annual carbon uptake of two eastern North America mature forests. Agric. For. Meteorol. 2015, 201, 8–16. [Google Scholar] [CrossRef]
- Pei, T.; Hou, Q.; Chen, Y.; Ji, Z.; Wu, H.; Xie, B.; Qi, P.; Zhang, J. Vegetation in Arid Areas of the Loess Plateau Showed More Sensitivity of Water-Use Efficiency to Seasonal Drought. Forests 2022, 13, 634. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, Y.; Liu, S.; Deng, D.; Zhu, L.; Zhao, M.; Wang, Z. Simulating the Impacts of Drought and Warming in Summer and Autumn on the Productivity of Subtropical Coniferous Forests. Forests 2022, 13, 2147. [Google Scholar] [CrossRef]
- Sun, X.; Wen, X.; Yu, G.; Liu, Y.; Liu, Q. Seasonal drought effects on carbon sequestration of a mid-subtropical planted forest of southeastern China. Sci. China Ser. D Earth Sci. 2006, 49, 110–118. [Google Scholar] [CrossRef]
- Buotte, P.C.; Law, B.E.; Ripple, W.J.; Berner, L.T. Carbon sequestration and biodiversity co-benefits of preserving forests in the western United States. Ecol. Appl. 2020, 30, e02039. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: 782 review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Sun, G.; Zha, T.; Zhu, J.; Shen, L.; Chen, J.; Fang, X.; Chen, J. Quantifying evapotranspiration and biophysical regulations of a poplar plantation assessed by eddy covariance and sap-flow methods. Chin. J. Plant Ecol. 2009, 33, 706–718. [Google Scholar] [CrossRef]





| Items/Unit | The Drought Year | The Normal Years | Relative Change |
|---|---|---|---|
| Ta/°C | 21.87 a | 21.96 a | −0.09 °C |
| Ts/°C | 21.29 a | 22.08 b | −0.79 °C |
| VPD/hPa | 0.87 a | 0.73 b | 19.18% |
| SWC/% | 0.23 a | 0.25 b | −8.00% |
| P/mm | 3.39 a | 4.22 a | −18.45% |
| RH/% | 73.85 a | 77.82 b | −5.10% |
| Rn W·m−2 | 123.55 a | 113.99 b | 8.39% |
| NEE/gCm−2·day−1 | 0.87 a | 1.45 b | −40.00% |
| RE/gCm−2·day−1 | 4.58 a | 5.27 b | −13.09% |
| GEP/gCm−2·day−1 | 5.50 a | 6.75 b | −18.52% |
| ET/mm | 2.03 a | 2.32 b | −12.50% |
| WUE/gC·kg−1H2O | 3.07 a | 3.26 b | −5.83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, L.; Yan, W.; Peng, Y.; Sun, H.; Chen, X. Drought-Induced Alterations in Carbon and Water Dynamics of Chinese Fir Plantations at the Trunk Wood Stage. Plants 2024, 13, 2937. https://doi.org/10.3390/plants13202937
Liu Y, Zhang L, Yan W, Peng Y, Sun H, Chen X. Drought-Induced Alterations in Carbon and Water Dynamics of Chinese Fir Plantations at the Trunk Wood Stage. Plants. 2024; 13(20):2937. https://doi.org/10.3390/plants13202937
Chicago/Turabian StyleLiu, Yijun, Li Zhang, Wende Yan, Yuanying Peng, Hua Sun, and Xiaoyong Chen. 2024. "Drought-Induced Alterations in Carbon and Water Dynamics of Chinese Fir Plantations at the Trunk Wood Stage" Plants 13, no. 20: 2937. https://doi.org/10.3390/plants13202937
APA StyleLiu, Y., Zhang, L., Yan, W., Peng, Y., Sun, H., & Chen, X. (2024). Drought-Induced Alterations in Carbon and Water Dynamics of Chinese Fir Plantations at the Trunk Wood Stage. Plants, 13(20), 2937. https://doi.org/10.3390/plants13202937







