The Effects of the Selective Removal of Adjacent Trees on the Diversity of Oak-Hosted Epiphytes and Tree-Related Microhabitats
Abstract
:1. Introduction
2. Results
2.1. Tree and Stand Parameters
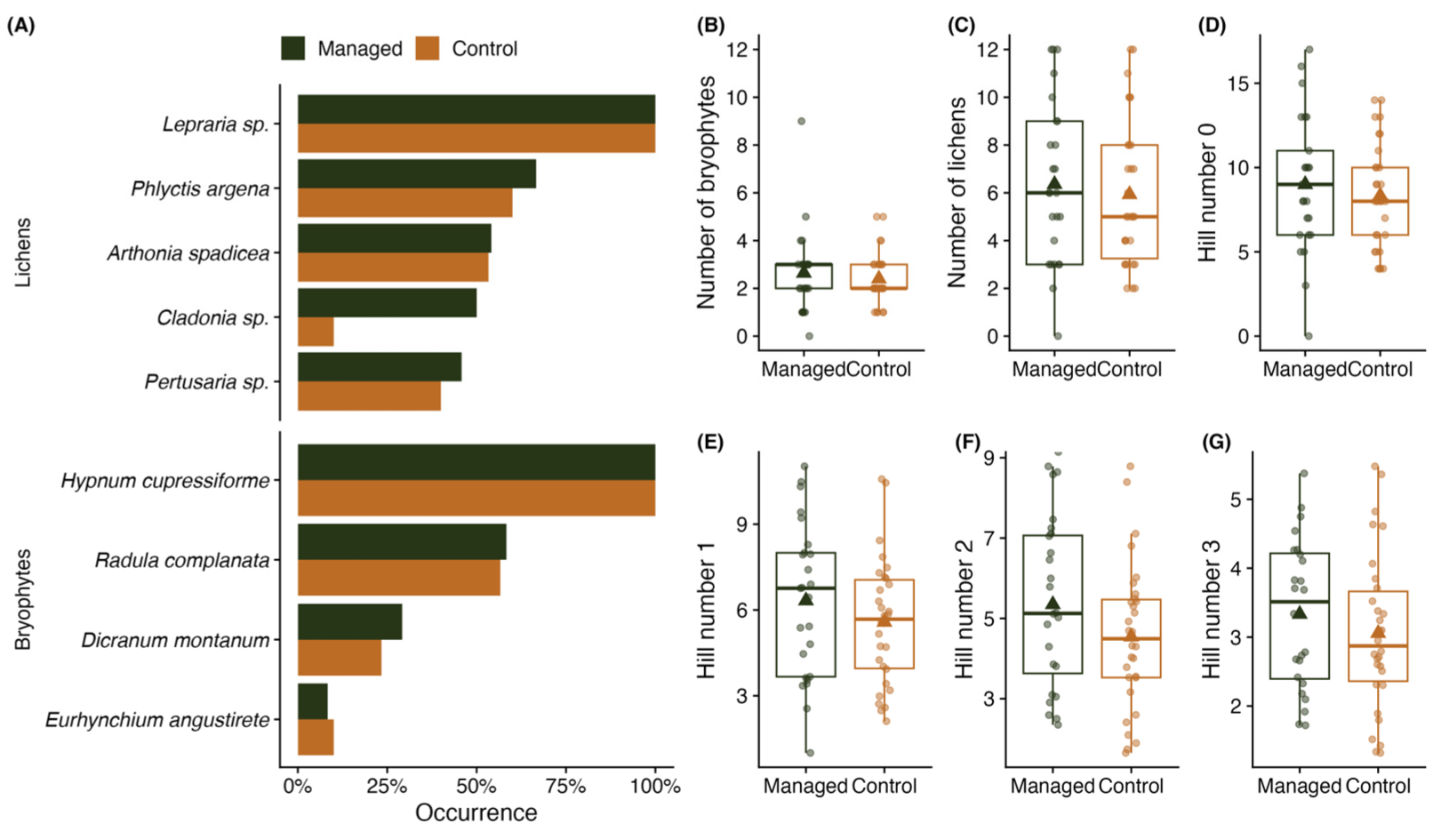
2.2. Epiphytes
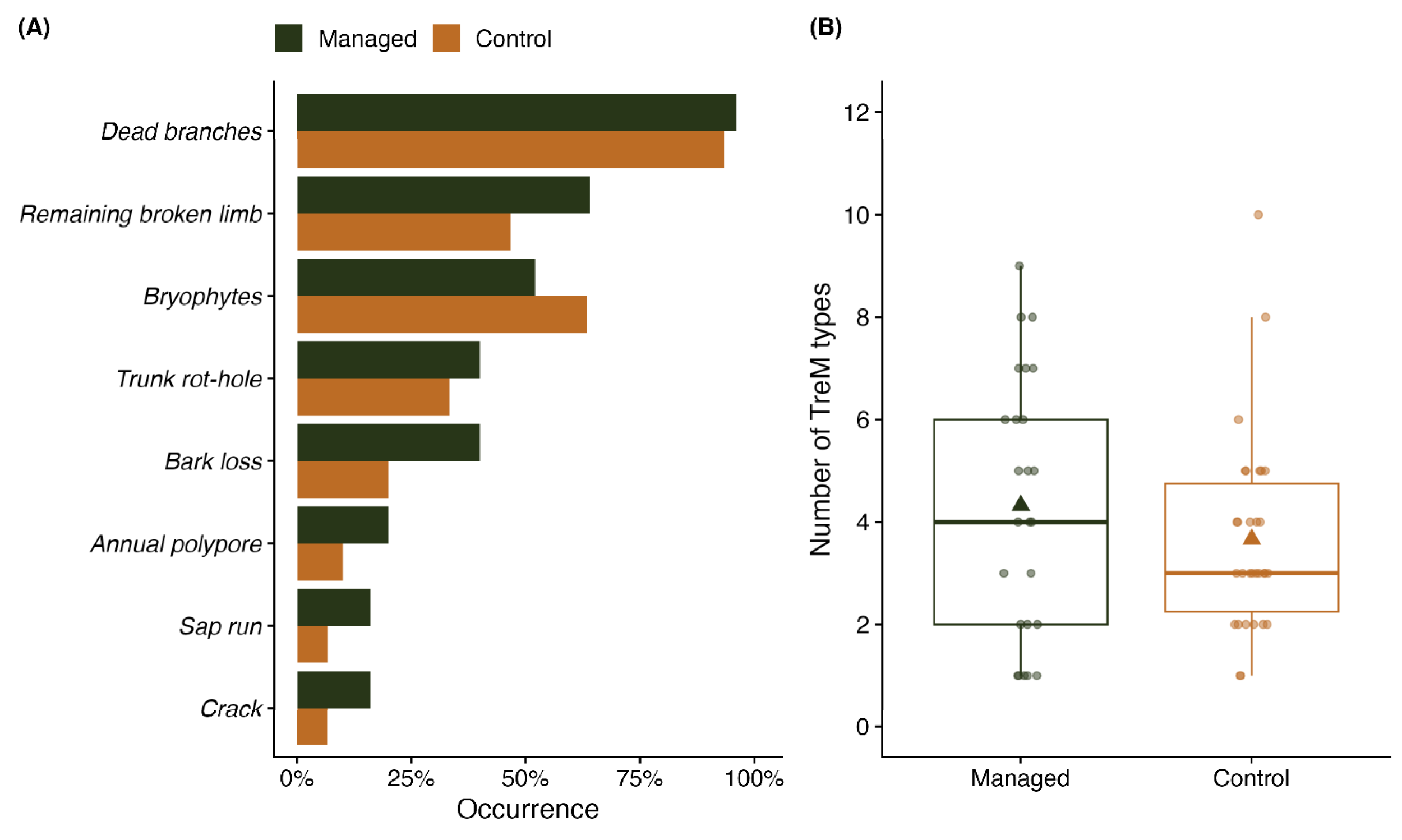
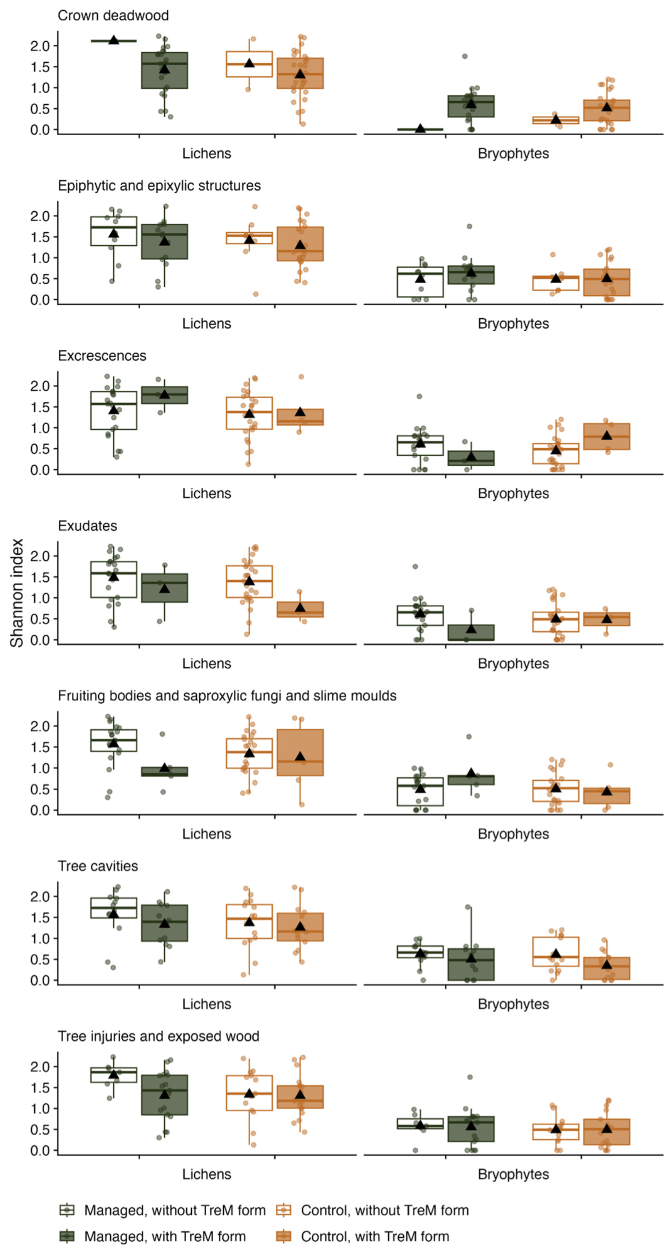
2.3. TreMs
3. Discussion
4. Materials and Methods
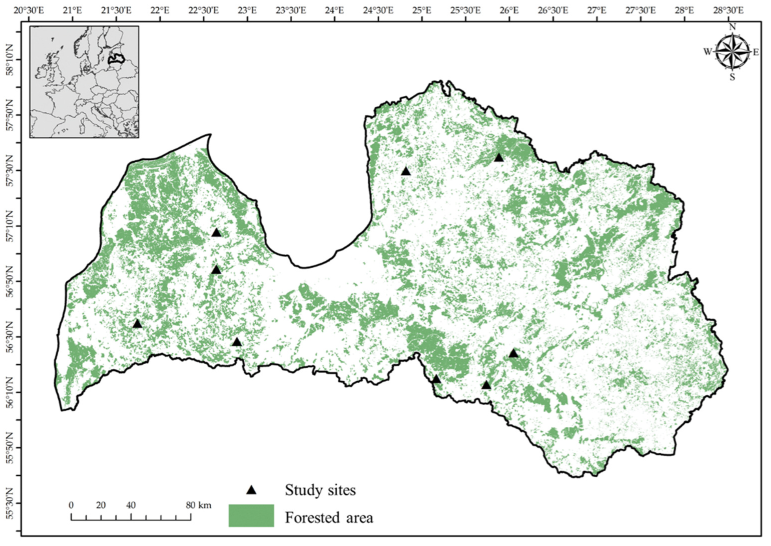
4.1. Description of the Study Sites
4.2. Data Collection and Measurements
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mölder, A.; Meyer, P.; Nagel, R.V. Integrative Management to Sustain Biodiversity and Ecological Continuity in Central European Temperate Oak (Quercus robur, Q. petraea) Forests: An Overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Piechnik, Ł.; Holeksa, J.; Ledwoń, M.; Kurek, P.; Szarek-łukaszewska, G.; Żywiec, M. Stand Composition, Tree-Related Microhabitats and Birds—A Network of Relationships in a Managed Forest. Forests 2022, 13, 103. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Raisio, J.; Tuomenvirta, H. Oak Decline in a Southern Finnish Forest as Affected. Geochronometria 2013, 41, 92–103. [Google Scholar] [CrossRef]
- Skiadaresis, G. Effects of Groundwater Extraction and Extreme Drought Events on Vitality and Growth of Pedunculate Oak (Quercus robur L.) Trees. Doctoral Dissertation, Universität Freiburg, Breisgau, Germany, 2021. ISBN 2029792020. [Google Scholar]
- Bouwman, M.; Forrester, D.I.; den Ouden, J.; Nabuurs, G.J.; Mohren, G.M.J. Species Interactions under Climate Change in Mixed Stands of Scots Pine and Pedunculate Oak. For. Ecol. Manag. 2021, 481, 118615. [Google Scholar] [CrossRef]
- Gran, O.; Götmark, F. Long-Term Experimental Management in Swedish Mixed Oak-Rich Forests Has a Positive Efect on Saproxylic Beetles after 10 Years. Biodivers. Conserv. 2019, 28, 1451–1472. [Google Scholar] [CrossRef]
- Strandberg, B.; Kristiansen, S.M.; Tybirk, K. Dynamic Oak-Scrub to Forest Succession: Effects of Management on Understorey Vegetation, Humus Forms and Soils. For. Ecol. Manag. 2005, 211, 318–328. [Google Scholar] [CrossRef]
- Willoughby, I.H.; Stokes, V.J.; Connolly, T. Using Ecoplugs Containing Glyphosate Can Be an Effective Method of Killing Standing Trees. Forestry 2017, 90, 719–727. [Google Scholar] [CrossRef]
- Götmark, F.; Paltto, H.; Nordén, B.; Götmark, E. Evaluating Partial Cutting in Broadleaved Temperate Forest under Strong Experimental Control: Short-Term Effects on Herbaceous Plants. For. Ecol. Manag. 2005, 214, 124–141. [Google Scholar] [CrossRef]
- Nordén, B.; Götmark, F.; Ryberg, M.; Paltto, H.; Allmér, J. Partial Cutting Reduces Species Richness of Fungi on Woody Debris in Oak-Rich Forests. Can. J. For. Res. 2008, 38, 1807–1816. [Google Scholar] [CrossRef]
- Nordén, B.; Paltto, H.; Claesson, C.; Götmark, F. Partial Cutting Can Enhance Epiphyte Conservation in Temperate Oak-Rich Forests. For. Ecol. Manag. 2012, 270, 35–44. [Google Scholar] [CrossRef]
- Stuiver, B.M.; Wardle, D.A.; Gundale, M.J.; Nilsson, M.C. Seedling Responses to Changes in Canopy and Soil Properties during Stand Development Following Clear-Cutting. For. Ecol. Manag. 2016, 378, 31–43. [Google Scholar] [CrossRef]
- Wagner, K.; Mendieta-Leiva, G.; Zotz, G. Host Specificity in Vascular Epiphytes: A Review of Methodology, Empirical Evidence and Potential Mechanisms. AoB Plants 2015, 7, plu092. [Google Scholar] [CrossRef] [PubMed]
- Heithecker, T.D.; Halpern, C.B. Variation in Microclimate Associated with Dispersed-Retention Harvests in Coniferous Forests of Western Washington. For. Ecol. Manag. 2006, 226, 60–71. [Google Scholar] [CrossRef]
- Sola, G.; El Mujtar, V.; Attis Beltrán, H.; Chauchard, L.; Gallo, L. Mixed Nothofagus Forest Management: A Crucial Link between Regeneration, Site and Microsite Conditions. New For. 2020, 51, 435–452. [Google Scholar] [CrossRef]
- Hauck, M.; de Bruyn, U.; Leuschner, C. Dramatic Diversity Losses in Epiphytic Lichens in Temperate Broad-Leaved Forests during the Last 150 Years. Biol. Conserv. 2013, 157, 136–145. [Google Scholar] [CrossRef]
- Ódor, P.; Király, I.; Tinya, F.; Bortignon, F.; Nascimbene, J. Patterns and Drivers of Species Composition of Epiphytic Bryophytes and Lichens in Managed Temperate Forests. For. Ecol. Manag. 2013, 306, 256–265. [Google Scholar] [CrossRef]
- Humphrey, J.W. Benefits to Biodiversity from Developing Old-Growth Conditions in British Upland Spruce Plantations: A Review and Recommendations. Forestry 2005, 78, 33–53. [Google Scholar] [CrossRef]
- Dittrich, S.; Hauck, M.; Jacob, M.; Rommerskirchen, A.; Leuschner, C. Response of Ground Vegetation and Epiphyte Diversity to Natural Age Dynamics in a Central European Mountain Spruce Forest. J. Veg. Sci. 2013, 24, 675–687. [Google Scholar] [CrossRef]
- Kaufmann, S.; Hauck, M.; Leuschner, C. Effects of Natural Forest Dynamics on Vascular Plant, Bryophyte, and Lichen Diversity in Primeval Fagus Sylvatica Forests and Comparison with Production Forests. J. Ecol. 2018, 106, 2421–2434. [Google Scholar] [CrossRef]
- Woods, C.L. Primary Ecological Succession in Vascular Epiphytes: The Species Accumulation Model. Biotropica 2017, 49, 452–460. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree Related Microhabitats in Temperate and Mediterranean European Forests: A Hierarchical Typology for Inventory Standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Asbeck, T.; Großmann, J.; Paillet, Y.; Winiger, N.; Bauhus, J. The Use of Tree-Related Microhabitats as Forest Biodiversity Indicators and to Guide Integrated Forest Management. Curr. For. Rep. 2021, 7, 59–68. [Google Scholar] [CrossRef]
- Auniņš, A. Eiropas Savienības Aizsargājamie Biotopi Latvijā. Noteikšanas Rokasgrāmata. 2. Precizētais Izdevums; Latvijas Dabas Fonds, Vides Aizsardzības un Reģionālās Attīstības Ministrija: Rīga, Latvija, 2013; p. 320. [Google Scholar]
- Ministru kabinets. Nr.39: Noteikumi par īpaši Aizsargājamo sugu un Ierobežoti Izmantojamo īpaši Aizsargājamo sugu Saraksts. 1. Pielikums, Latvijas Vēstnesis. 2000. Available online: https://m.likumi.lv/ta/id/12821 (accessed on 24 September 2024).
- Guzmán-Jacob, V.; Zotz, G.; Craven, D.; Taylor, A.; Krömer, T.; Monge-González, M.L.; Kreft, H. Effects of Forest-Use Intensity on Vascular Epiphyte Diversity along an Elevational Gradient. Divers. Distrib. 2020, 26, 4–15. [Google Scholar] [CrossRef]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Responses of Boreal Epiphytic Bryophytes to Different Levels of Partial Canopy Harvest. Botany 2010, 88, 315–328. [Google Scholar] [CrossRef]
- Johansson, P. Consequences of Disturbance on Epiphytic Lichens in Boreal and near Boreal Forests. Biol. Conserv. 2008, 141, 1933–1944. [Google Scholar] [CrossRef]
- Marmor, L.; Tõrra, T.; Saag, L.; Randlane, T. Species Richness of Epiphytic Lichens in Coniferous Forests: The Effect of Canopy Openness. Ann. Bot. Fenn. 2012, 49, 352–358. [Google Scholar] [CrossRef]
- Asbeck, T.; Messier, C.; Bauhus, J. Retention of Tree-Related Microhabitats Is More Dependent on Selection of Habitat Trees than Their Spatial Distribution. Eur. J. For. Res. 2020, 139, 1015–1028. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A. Species, Live Status, and Diameter Are Important Tree Features for Diversity and Abundance of Tree Microhabitats in Subnatural Montane Beech-Fir Forests. Can. J. For. Res. 2012, 42, 1433–1445. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A.; Brin, A.; Bouget, C.; Deconchat, M. Tree Microhabitats at the Stand Scale in Montane Beech-Fir Forests: Practical Information for Taxa Conservation in Forestry. Eur. J. For. Res. 2014, 133, 355–367. [Google Scholar] [CrossRef]
- Przepióra, F.; Ciach, M. Profile of Tree-Related Microhabitats in the Primeval Białowieża Forest: A Benchmark for Temperate Woodlands. Sci. Total Environ. 2023, 905, 167273. [Google Scholar] [CrossRef]
- Jansone, D.; Matisons, R.; Gerra-Inohosa, L.; Lībiete, Z.; Jansons, Ā. Dead Better than Alive—The Case of Retention Trees and Tree-Related Microhabitats in Young Stands of Hemiboreal Forests in Latvia. Forests 2023, 14, 1949. [Google Scholar] [CrossRef]
- Elias, J.P.C.; Mortara, S.R.; Nunes-Freitas, A.F.; van den Berg, E.; Ramos, F.N. Host Tree Traits in Pasture Areas Affect Forest and Pasture Specialist Epiphyte Species Differently. Am. J. Bot. 2021, 108, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Paillet, Y.; Debaive, N.; Archaux, F.; Cateau, E.; Gilg, O.; Guilbert, E. Nothing Else Matters? Tree Diameter and Living Status Have More Effects than Biogeoclimatic Context on Microhabitat Number and Occurrence: An Analysis in French Forest Reserves. PLoS ONE 2019, 14, e0216500. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, L.; Gosselin, F.; Archaux, F.; Chevalier, R.; Corriol, G.; Dauffy-Richard, E.; Deconchat, M.; Gosselin, M.; Ladet, S.; Savoie, J.M.; et al. Assessing the Potential of Routine Stand Variables from Multi-Taxon Data as Habitat Surrogates in European Temperate Forests. Ecol. Indic. 2019, 104, 116–126. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Piervittori, R. Lichen-Plant Interactions. J. Plant Interact. 2010, 5, 163–177. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Garbin, M.L.; Muylaert, R.L.; Ruiz-Miranda, C.R. Low Modularity and Specialization in a Commensalistic Epiphyte–Phorophyte Network in a Tropical Cloud Forest. Biotropica 2019, 51, 509–518. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Gradstein, S.R. Vertical Stratification of Vascular Epiphytes in Submontane and Montane Forest of the Bolivian Andes: The Importance of the Understory. Plant Ecol. 2007, 189, 261–278. [Google Scholar] [CrossRef]
- Pedro Costa Elias, J.; Aparecida Borges e Silva, B.; Gonçalves de Carvalho, R.; Bonesso Sampaio, M.; Mendieta-Leiva, G.; Nunes Ramos, F. Tree Structure Instead of Microclimatic Zones Determines Differences in Vascular Epiphyte Assemblages between Forest and Pasture. For. Ecol. Manag. 2024, 552, 121567. [Google Scholar] [CrossRef]
- Latvian Environment, Geology and Meteorology Centre (LEGMC). Available online: https://videscentrs.lvgmc.lv/ (accessed on 21 September 2024).
- Smith, C.; Aptroot, A.; Coppins, B.; Fletcher, A.; Gilbert, O.; James, P.; Wolseley, P. The Lichens of Great Britain and Ireland; Great Britain; Cambridge University Press: Cambridge, UK, 2009; Volume 42, pp. 123–126. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An Annotated Checklist of Bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.; Jost, L.; Chao, A.; Chiu, C.; Jost, L. Phylogenetic Diversity Measures Based on Hill Numbers Phylogenetic Diversity Measures Based on Hill Numbers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.R.; Hartmann, D.; Mendoza, M. Diverse: An R Package to Analyze Diversity in Complex Systems. R J. 2016, 8, 60–78. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 16 October 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liepiņa, A.A.; Jansone, D.; Elferts, D.; Barone, I.; Donis, J.; Jansons, Ā. The Effects of the Selective Removal of Adjacent Trees on the Diversity of Oak-Hosted Epiphytes and Tree-Related Microhabitats. Plants 2024, 13, 3019. https://doi.org/10.3390/plants13213019
Liepiņa AA, Jansone D, Elferts D, Barone I, Donis J, Jansons Ā. The Effects of the Selective Removal of Adjacent Trees on the Diversity of Oak-Hosted Epiphytes and Tree-Related Microhabitats. Plants. 2024; 13(21):3019. https://doi.org/10.3390/plants13213019
Chicago/Turabian StyleLiepiņa, Agnese Anta, Diāna Jansone, Didzis Elferts, Ilze Barone, Jānis Donis, and Āris Jansons. 2024. "The Effects of the Selective Removal of Adjacent Trees on the Diversity of Oak-Hosted Epiphytes and Tree-Related Microhabitats" Plants 13, no. 21: 3019. https://doi.org/10.3390/plants13213019
APA StyleLiepiņa, A. A., Jansone, D., Elferts, D., Barone, I., Donis, J., & Jansons, Ā. (2024). The Effects of the Selective Removal of Adjacent Trees on the Diversity of Oak-Hosted Epiphytes and Tree-Related Microhabitats. Plants, 13(21), 3019. https://doi.org/10.3390/plants13213019







