Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds and Antioxidant Potentials
2.2. Phenolic Compounds
| Extracts | Yield (%) | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoids Content (mg CE/g Extract) |
|---|---|---|---|
| Di-SPT | 13.47 | 4.10 ± 0.09 | 2.36 ± 0.04 |
| Di-BB3-CMU | 14.85 | 3.87 ± 0.07 | 5.73 ± 0.15 |
| Et-SPT | 7.32 | 6.40 ± 0.31 | 7.45 ± 0.15 |
| Et-BB3-CMU | 4.38 | 6.60 ± 0.22 | 10.42 ± 0.11 |
| Extracts | Compound Contents (mg/100 g Extract) | |||
|---|---|---|---|---|
| γ-Oryzanol | α-Tocopherol | β + γ-Tocopherol | δ-Tocopherol | |
| Di-SPT | 1322.10 ± 15.32 | 12.99 ± 0.02 | 8.93 ± 0.03 | 1.15 ± 0.01 |
| Di-BB3-CMU | 1752.75 ± 53.05 | 31.55 ± 0.74 | 9.06 ± 0.49 | 1.30 ± 0.01 |
| Et-SPT | 144.30 ± 2.88 | 5.08 ± 0.04 | 2.12 ± 0.02 | 0.63 ± 0.02 |
| Et-BB3-CMU | 152.49 ± 0.29 | 6.28 ± 0.04 | 3.35 ± 0.03 | 0.83 ± 0.01 |
| Extracts | DPPH Scavenging Activity (IC50, mg/mL) | ABTS Scavenging Activity (IC50, mg/mL) | FRAP Reducing Power (mM Fe2+/g Extract) |
|---|---|---|---|
| Di-SPT | 36.08 ± 0.32 | 79.39 ± 0.67 | 63.91 ± 4.74 |
| Di-BB3-CMU | 40.52 ± 0.39 | 66.08 ± 2.79 | 55.45 ± 2.83 |
| Et-SPT | 9.65 ± 0.45 | 11.53 ± 0.35 | 119.71 ± 3.63 |
| Et-BB3-CMU | 10.62 ± 0.85 | 6.58 ± 0.57 | 88.46 ± 0.63 |
| Items | Extracts (mg/g Extract) | |
|---|---|---|
| Et-SPT | Et-BB3-CMU | |
| Caffeic acid | 5.55 ± 0.01 | 6.90 ± 0.01 |
| Epicatechin | 2.31 ± 0.02 | 2.18 ± 0.00 |
| Gallocatechin gallate | 6.93 ± 0.14 | 7.47 ± 0.13 |
| p-oumaric acid | 3.70 ± 0.01 | 3.61 ± 0.08 |
| o-coumaric acid | 2.99 ± 0.01 | 2.76 ± 0.01 |
| Naringin | 0.45 ± 0.00 | 0.55 ± 0.01 |
| Rosmarinic acid | 1.92 ± 0.01 | ND |
| Quercetin | 4.87 ± 0.03 | 3.99 ± 0.05 |
| Rutin | 2.36 ± 0.05 | 2.19 ± 0.00 |
| Phytic acid | 10.91 ± 0.03 | 13.43 ± 0.14 |
| Ferulic acid | 3.02 ± 0.01 | 3.35 ± 0.02 |
| Chlorogenic acid | 0.95 ± 0.02 | 1.04 ± 0.05 |
| Hydroxybenzoic acid | 1.84 ± 0.01 | 2.39 ± 0.06 |
2.3. Effect of Rice Bran Extracts on Cell Viability
2.4. Effect of Rice Bran Extracts on Anti-Inflammatory Activities
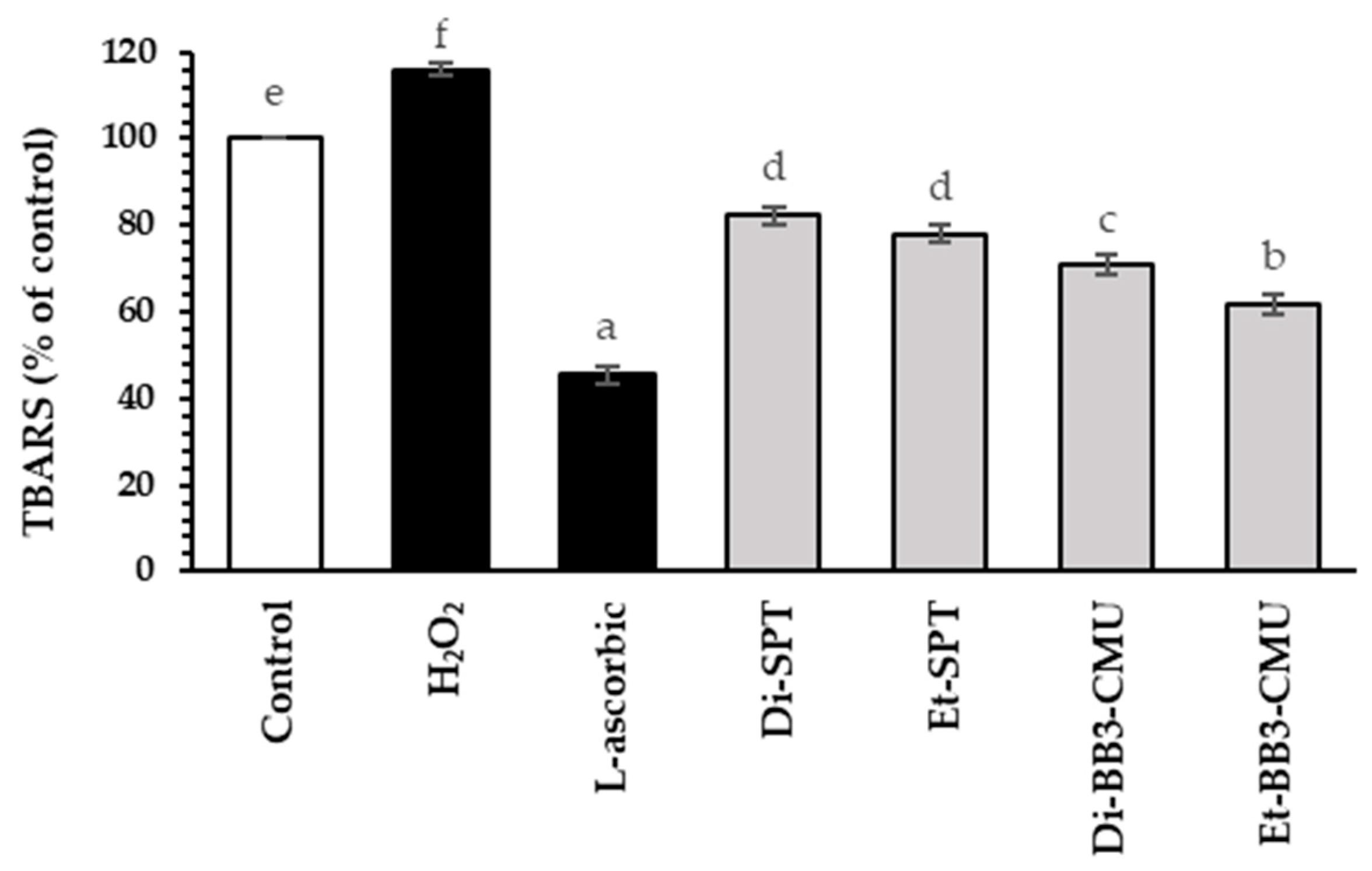
2.5. Effect of Rice Bran Extracts on Antioxidant Activities in HFDPC Cells
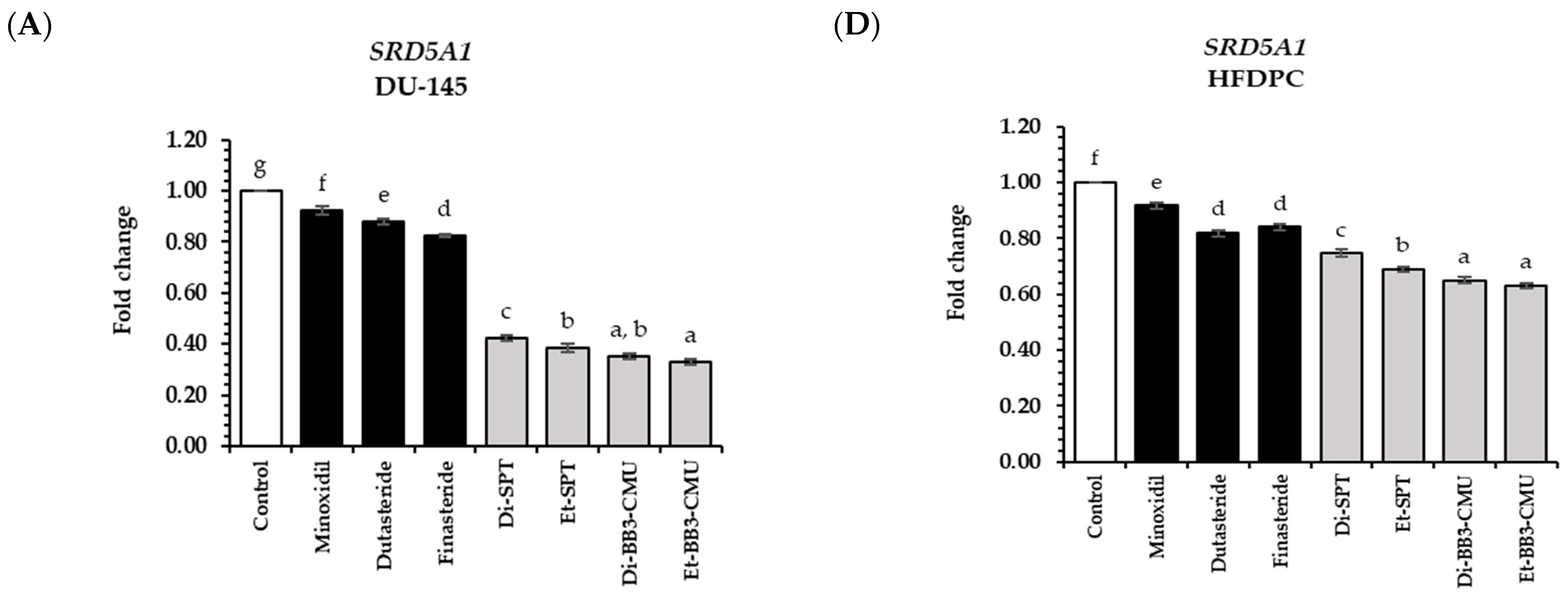
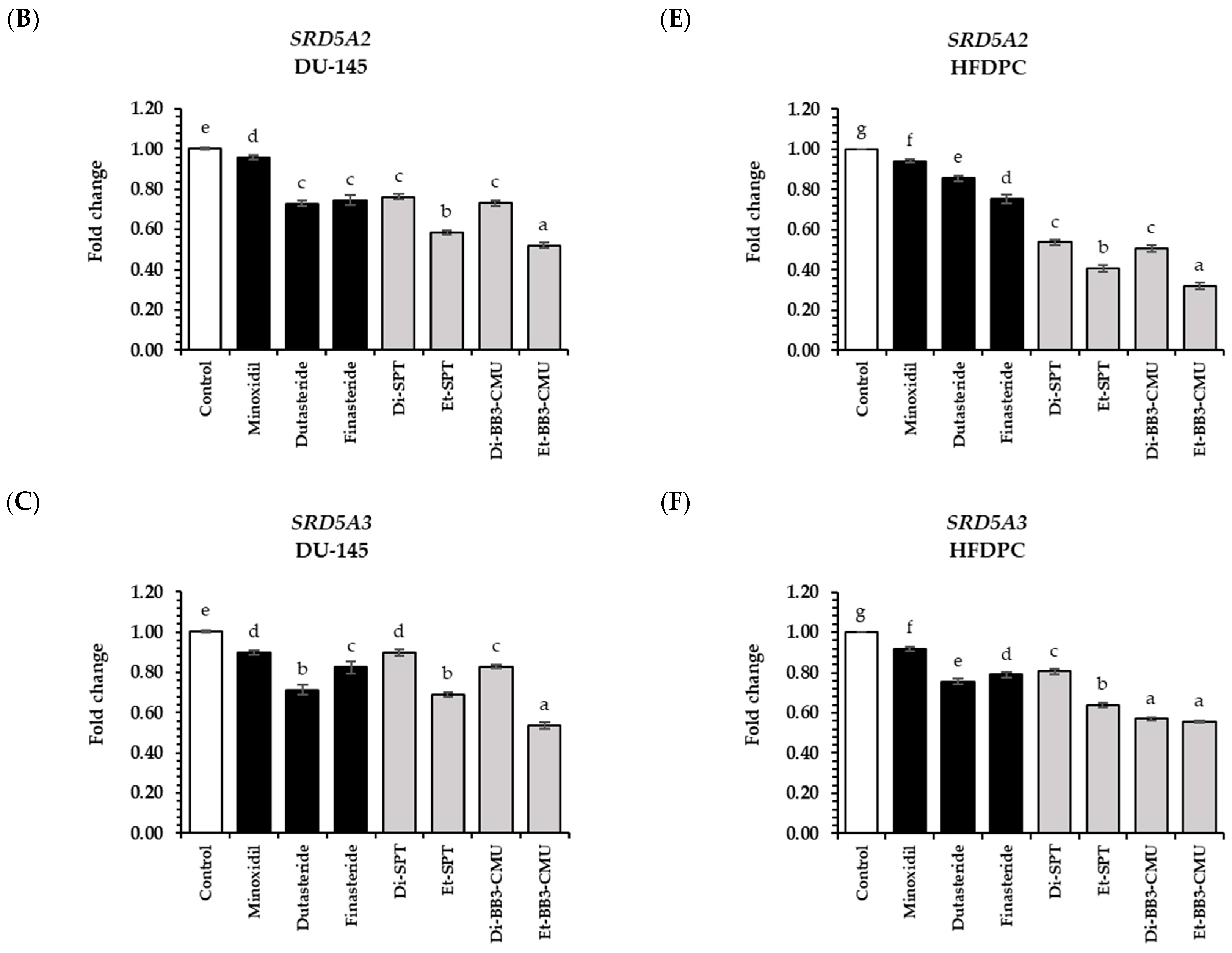
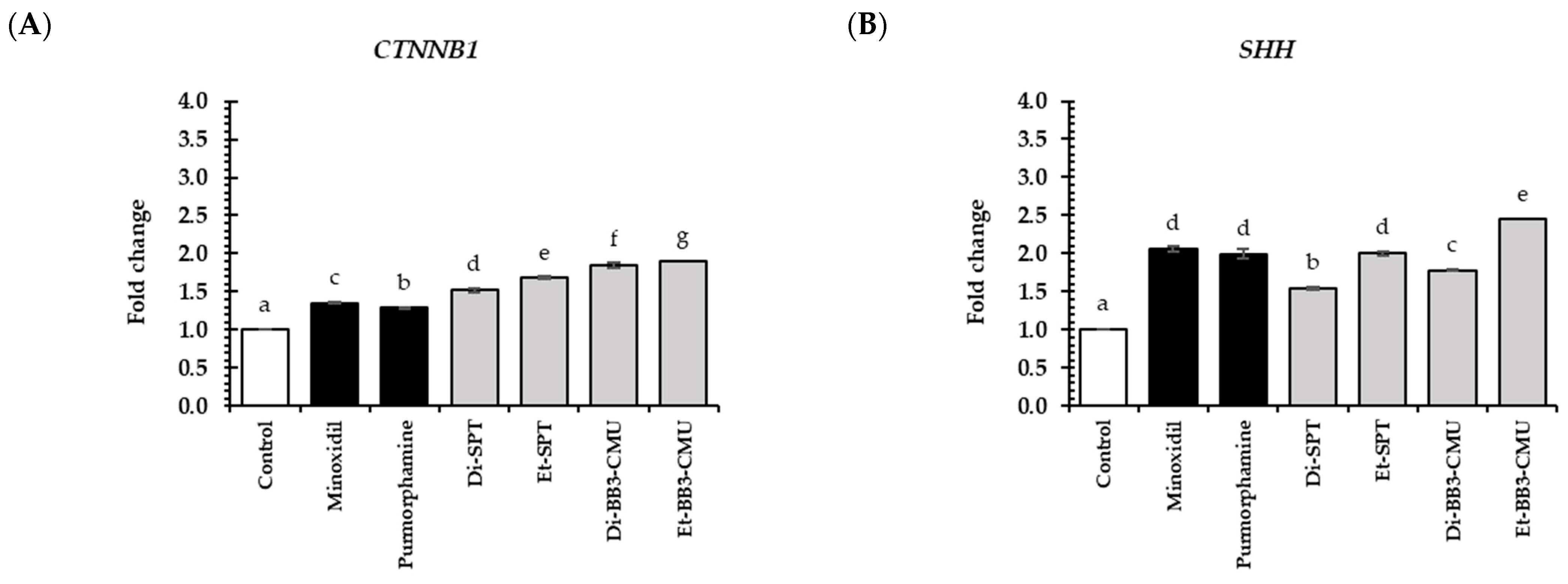
2.6. Effects of Rice Bran Extracts on Genes Expression
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Crude Extracts
3.3. Phytochemical Analysis and Antioxidant Activities
3.4. γ-Oryzanol and Tocopherol Analysis
3.5. Polyphenol Profile Analysis
3.6. In Vitro Cell Viability and Proliferation Assay
3.7. Anti-Inflammatory Activity Assay
3.8. Thiobarbituric Acid-Reactive Substances (TBARS) Assay
3.9. Semi-Quantitative Reverse Transcription and Polymerase Chain Reaction Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ring, C.M.; Finney, R.; Avram, M. Lasers, lights, and compounds for hair loss in aesthetics. Clin. Dermatol. 2022, 40, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.; Takeo, M.; Chou, W.; Myung, P.; Bosenberg, M.; Chin, L.; Taketo, M.M.; Ito, M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011, 145, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Mill, P.; Mo, R.; Fu, H.; Grachtchouk, M.; Kim, P.C.; Dlugosz, A.A.; Hui, C.-c. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003, 17, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Miao, Y.; Wang, J.; Wang, X.; Chen, C.-Y.; Hu, Z.-Q. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-β1 expression. Drug Des. Dev. Ther. 2015, 9, 5373–5383. [Google Scholar] [CrossRef]
- Kubanov, A.; Gallyamova, Y.A.; Korableva, O. The study of growth factors in patients with androgenic alopecia. Biomed. Pharmacol. J. 2017, 10, 1219–1228. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S.; Team, D.A.R. The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Williams, G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J. Dermatol. Treat. 2022, 33, 2946–2962. [Google Scholar] [CrossRef]
- Choochuen, N.; Jimtaisong, A. Physical stability and subjective efficacy study of liposome loaded with Clitoria ternatea (butterfly pea) flower extract and Eugenia caryophyllus (clove) oil. Pharm. Sci. Asia 2022, 49, 51–58. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Linsaenkart, P.; Khantham, C.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Prom-U-Thai, C.; Jamjod, S.; Yamuangmorn, S.; Arjin, C. Regulatory effects of thai rice by-product extracts from Oryza sativa L. cv. Bue Bang 3 CMU and Bue Bang 4 CMU on melanin production, nitric oxide secretion, and steroid 5α-reductase inhibition. Plants 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Pandey, M.K.; Sharma, A.; Prakash, J. Indian medicinal plants: For hair care and cosmetics. World J. Pharm. Sci. 2014, 2, 1552–1556. [Google Scholar]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-U-Thai, C. Antioxidation, anti-inflammation, and regulation of SRD5A gene expression of Oryza sativa cv. Bue Bang 3 CMU husk and bran extracts as androgenetic alopecia molecular treatment substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-u-Thai, C.; Sringarm, K. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front. Nutr. 2022, 9, 833730. [Google Scholar] [CrossRef]
- Prom-U-Thai, C.; Sanchai, C.; Rerkasem, B.; Jamjod, S.; Fukai, S.; Godwin, I.; Huang, L. Effect of grain morphology on degree of milling and iron loss in rice. Cereal Chem. 2007, 84, 384–388. [Google Scholar] [CrossRef]
- Reddy, C.K.; Kimi, L.; Haripriya, S.; Kang, N. Effects of polishing on proximate composition, physico-chemical characteristics, mineral composition and antioxidant properties of pigmented rice. Rice Sci. 2017, 24, 241–252. [Google Scholar] [CrossRef]
- Surin, S.; Seesuriyachan, P.; Thakeow, P.; You, S.; Phimolsiripol, Y. Antioxidant and antimicrobial properties of polysaccharides from rice brans. Chiang Mai J. Sci 2018, 45, 1372–1382. [Google Scholar]
- Khantham, C.; Ruksiriwanich, W.; Sringarm, K.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C.; Muangsanguan, A.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy 2023, 13, 295. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Hongsibsong, S.; Ruksiriwanich, W.; Niwat, C.; Tiyayon, P.; Jamjod, S.; Yamuangmorn, S.; Prom-U-Thai, C.; Sringarm, K. Antioxidant activities and characterization of polyphenols from selected Northern Thai rice husks: Relation with seed attributes. Rice Sci. 2023, 30, 148–159. [Google Scholar] [CrossRef]
- Negri, G.; Teixeira, E.W.; Florêncio Alves, M.L.T.M.; Moreti, A.C.d.C.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Chae, C.W.; Choi, G.E.; Shin, H.C.; Lim, J.R.; Chang, H.S.; Park, J.; Cho, J.H.; Park, M.R.; Lee, H.J. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity Geneve, Switzerland. International Organization for Standardization: Geneva, Switzerland, 2009.
- Choi, N.; Shin, S.; Song, S.U.; Sung, J.-H. Minoxidil promotes hair growth through stimulation of growth factor release from adipose-derived stem cells. Int. J. Mol. Sci. 2018, 19, 691. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Schönfelder, G.; Paul, M.; Blume-Peytavi, U. Nitric oxide in the human hair follicle: Constitutive and dihydrotestosterone-induced nitric oxide synthase expression and NO production in dermal papilla cells. J. Mol. Med. 2003, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The role of macrophages in skin homeostasis. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Figueiredo, C.P.; Passos, G.F.; Calixto, J.B. Reduced skin inflammatory response in mice lacking inducible nitric oxide synthase. Biochem. Pharmacol. 2009, 78, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sowden, H.; Naseem, K.; Tobin, D. Differential expression of nitric oxide synthases in human scalp epidermal and hair follicle pigmentary units: Implications for regulation of melanogenesis. Br. J. Dermatol. 2005, 153, 301–309. [Google Scholar] [CrossRef]
- Shin, K.; Kim, T.-S.; Kyung, J.; Kim, D.; Park, D.; Choi, E.-K.; Lee, S.-P.; Yang, W.-S.; Kang, M.-H.; Kim, Y.-B. Effectiveness of the combinational treatment of Laminaria japonica and Cistanche tubulosa extracts in hair growth. Lab. Anim. Res. 2015, 31, 24–32. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. The antioxidant and anti-inflammatory properties of rice bran phenolic extracts. Foods 2020, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.T.; Pham, T.-L.T.; Nguyen, K.T.; Le, P.H.; Kim, K.-H. Effect of extraction solvent on total phenol, flavonoid content, and antioxidant activity of Avicennia officinalis. Res. Appl. Chem. 2021, 12, 2678–2690. [Google Scholar]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, A.; Olszewska-Słonina, D.; Czajkowski, R.; Zegarska, B.; Białecka, A.; Męcińska-Jundziłł, K.; Piskorska, E.; Lampka, M. Investigation of oxidative stress in patients with alopecia areata by measuring the levels of malondialdehyde and ceruloplasmin in the blood. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2018, 35, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Muzolf-Panek, M. Predictive modeling of changes in TBARS in the intramuscular lipid fraction of raw ground beef enriched with plant extracts. Antioxidants 2021, 10, 736. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y.; Hu, R.-Z.; Chen, C.; Yao, X.-H.; Zhao, W.-G.; Liu, Z.-Y.; Li, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crops Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and mechanistic approach to the hair growth cycle and hair loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology 2021, 10, 319. [Google Scholar] [CrossRef]
- Muangsanguan, A.; Linsaenkart, P.; Chaitep, T.; Sangta, J.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Hair growth promotion and anti-hair loss effects of by-products arabica coffee pulp extracts using supercritical fluid extraction. Foods 2023, 12, 4116. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Kashimawo, A.J.; Ibe, A.C. Phytochemical evaluation and GC-MS profiling of the dichloromethane and ethanol extracts of Ocimum gratissimum L. and Lasianthera Africana. BEAUV. J. Phytomedicine Ther. 2022, 20, 640–655. [Google Scholar]
- Schiffer, L.; Arlt, W.; Storbeck, K.-H. 5α-reduction of epitestosterone is catalysed by human SRD5A1 and SRD5A2 and increases androgen receptor transactivation. J. Steroid Biochem. Mol. Biol. 2024, 241, 106516. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Kumar, S.; Choudhir, G.; Singh, G.; Gangwar, U.; Sharma, V.; Srivastava, R.K.; Sharma, S. Bioactive metabolites of edible mushrooms efficacious against androgenic alopecia: Targeting SRD5A2 using computational approach. J. Herb. Med. 2022, 36, 100611. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; He, J.; Wang, J.; Chen, X.; Yang, R. Regulation of signaling pathways in hair follicle stem cells. Burn. Trauma 2022, 10, tkac022. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Su, C.-H.; Chiang, C.-Y.; Wu, C.-N.; Kuan, Y.-H. Observation of the expression of vascular endothelial growth factor and the potential effect of promoting hair growth treated with Chinese herbal BeauTop. Evid.-Based Complement. Altern. Med. 2021, 2021, 6667011. [Google Scholar] [CrossRef]
- de Oliveira Formiga, R.; Júnior, E.B.A.; Vasconcelos, R.C.; Araújo, A.A.; de Carvalho, T.G.; de Araújo Junior, R.F.; Guerra, G.B.C.; Vieira, G.C.; de Oliveira, K.M.; Diniz, M.d.F.F.M. Effect of p-cymene and rosmarinic acid on gastric ulcer healing–Involvement of multiple endogenous curative mechanisms. Phytomedicine 2021, 86, 153497. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Pestana, V.R.; Zambiazi, R.C.; Mendonça, C.R.; Bruscatto, M.H.; Lerma-García, M.J.; Ramis-Ramos, G. Quality changes and tocopherols and γ-orizanol concentrations in rice bran oil during the refining process. J. Am. Oil Chem. Soc. 2008, 85, 1013–1019. [Google Scholar] [CrossRef]
- Mighri, H.; Akrout, A.; Bennour, N.; Eljeni, H.; Zammouri, T.; Neffati, M. LC/MS method development for the determination of the phenolic compounds of Tunisian Ephedra alata hydro-methanolic extract and its fractions and evaluation of their antioxidant activities. S. Afr. J. Bot. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Linsaenkart, P.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Arjin, C.; Sommano, S.R.; Phimolsiripol, Y.; Barba, F.J. Wound healing effect of supercritical carbon dioxide Datura metel L. leaves extracts: An in vitro study of anti-Inflammation, cell migration, MMP-2 Inhibition, and the modulation of the Sonic Hedgehog pathway in human fibroblasts. Plants 2023, 12, 2546. [Google Scholar] [CrossRef] [PubMed]


| Target Pathway | Primer Name | Gene Bank No. | Type of Sequence | Primer Sequence (5′-3′) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| Internal control | GAPDH | NM_001289745.3 | Forward | GGAAGGTGAAGGTCGGAGTC | 55 |
| Reverse | CTCAGCCTTGACGGTGCCATG | ||||
| 5α-reductase | SRD5A1 | NM_001047.4 | Forward | AGCCATTGTGCAGTGTATGC | 52 |
| Reverse | AGCCTCCCCTTGGTATTTTG | ||||
| SRD5A2 | NM_000348.4 | Forward | TGAATACCCTGATGGGTGG | 52 | |
| Reverse | CAAGCCACCTTGTGGAATC | ||||
| SRD5A3 | NM_024592.5 | Forward | TCCTTCTTTGCCCAAACATC | 50 | |
| Reverse | TCCTTCTTTGCCCAAACATC | ||||
| Wnt/β-catenin | CTNNB1 | NM_001330729.2 | Forward | CCCACTAATGTCCAGCGTTT | 55 |
| Reverse | AACCAAGCATTTTCACCAGG | ||||
| Sonic Hedgehog | SHH | NM_000193.4 | Forward | AAAAGCTGACCCCTTTAGCC | 51 |
| Reverse | GCTCCGGTGTTTTCTTCATC | ||||
| SMO | NM_005631.5 | Forward | GAAGTGCCCTTGGTTCGGACA | 57 | |
| Reverse | CCGCCAGTCAGCCACGAAT | ||||
| GLI1 | NM_005269.3 | Forward | GCAGGGAGTCAGCCAATACAG | 56 | |
| Reverse | GAGCGGCGGCTGACAGTATA | ||||
| Angiogenesis | VEGF | NM_001025366.3 | Forward | CTACCTCCACCATGCCAAGT | 55 |
| Reverse | GCGAGTCTGTGTTTTTGCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangsanguan, A.; Ruksiriwanich, W.; Arjin, C.; Jamjod, S.; Prom-u-Thai, C.; Jantrawut, P.; Rachtanapun, P.; Hnorkaew, P.; Satsook, A.; Sainakham, M.; et al. Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong. Plants 2024, 13, 3079. https://doi.org/10.3390/plants13213079
Muangsanguan A, Ruksiriwanich W, Arjin C, Jamjod S, Prom-u-Thai C, Jantrawut P, Rachtanapun P, Hnorkaew P, Satsook A, Sainakham M, et al. Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong. Plants. 2024; 13(21):3079. https://doi.org/10.3390/plants13213079
Chicago/Turabian StyleMuangsanguan, Anurak, Warintorn Ruksiriwanich, Chaiwat Arjin, Sansanee Jamjod, Chanakan Prom-u-Thai, Pensak Jantrawut, Pornchai Rachtanapun, Patipan Hnorkaew, Apinya Satsook, Mathukorn Sainakham, and et al. 2024. "Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong" Plants 13, no. 21: 3079. https://doi.org/10.3390/plants13213079
APA StyleMuangsanguan, A., Ruksiriwanich, W., Arjin, C., Jamjod, S., Prom-u-Thai, C., Jantrawut, P., Rachtanapun, P., Hnorkaew, P., Satsook, A., Sainakham, M., Castagnini, J. M., & Sringarm, K. (2024). Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong. Plants, 13(21), 3079. https://doi.org/10.3390/plants13213079











