The Specific Impacts of Allelopathy and Resource Competition from Artemisia frigida on the Growth of Three Plant Species in Northern China
Abstract
1. Introduction
2. Results
2.1. Interference Between A. frigida and Three Target Plant Species
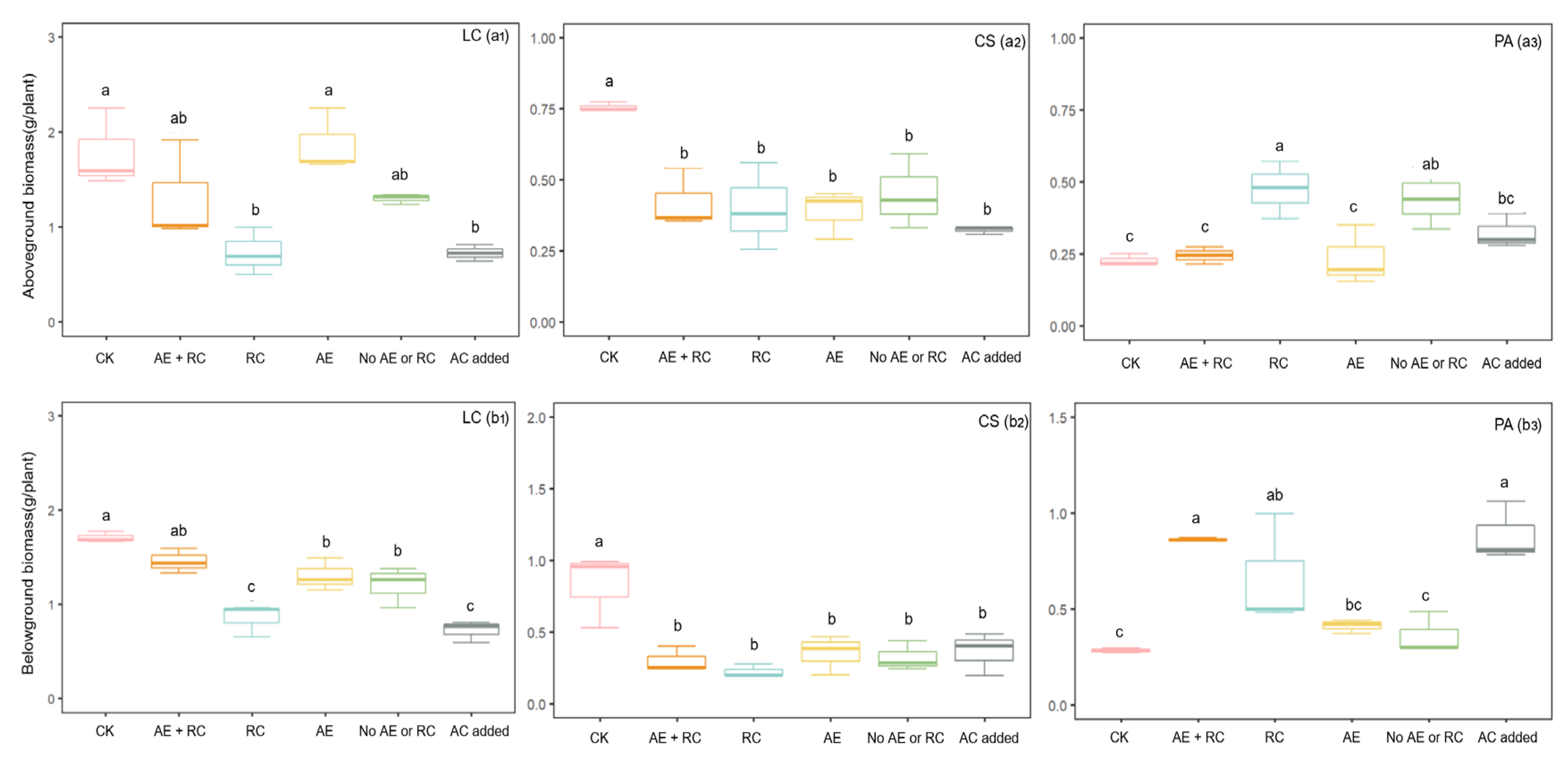
2.2. Specific Contributions of Allelopathy and Resource Competition from A. frigida to the Growth of Three Target Species
2.3. Effects of Allelopathy and Resource Competition and Their Coupling Effect on the Plant Growth of Three Target Species
3. Discussion
3.1. Interference of A. frigida with Three Target Plant Species
3.2. Separation of Allelopathy and Resource Competition from Plant Interference
3.3. The Impact of Allelopathy and Resource Competition and Their Coupling Effects on the Dominant Status of A. frigida in Moderate Grazing Grassland
4. Materials and Methods
4.1. Study Site
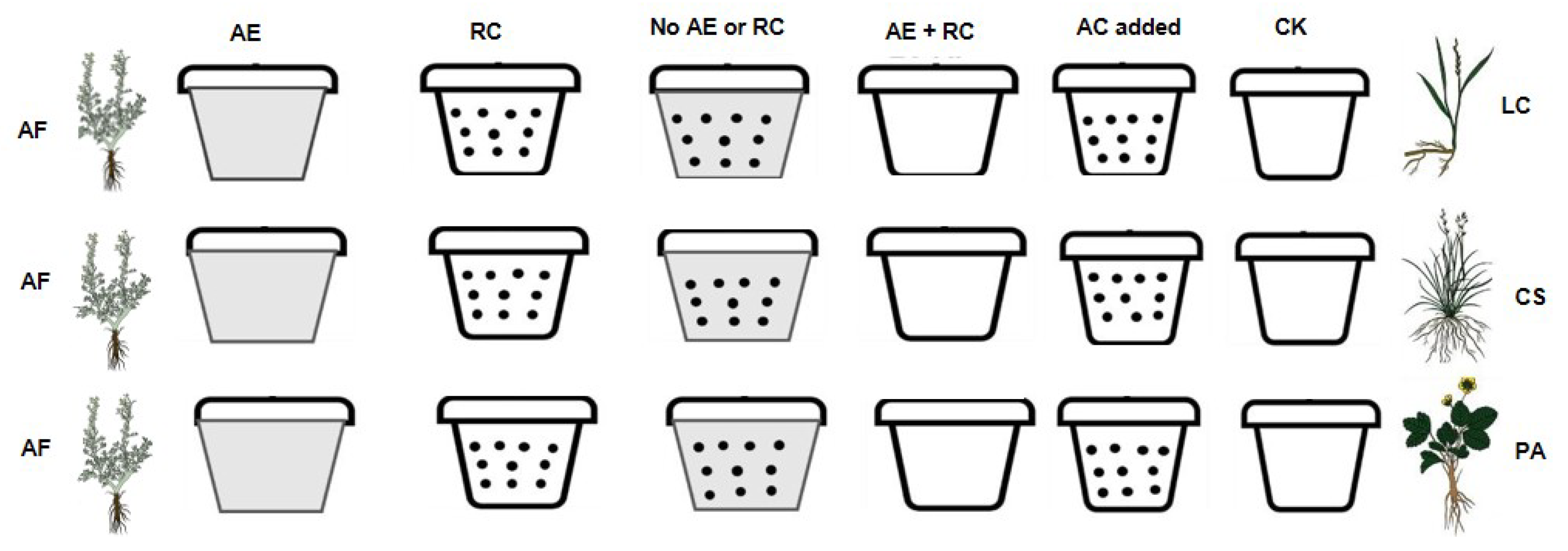
4.2. Experimental Design
4.3. Measurements
4.4. Data Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, C.H.; Li, Z.; Li, F.L.; Xia, X.X.; Wang, P. Chemically mediated plant-plant interactions: Allelopathy and allelobiosis. Plants 2024, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; An, M.; Johnson, I.R.; Lovett, J.V. Mathematical modelling of allelopathy: IV. Assessment of contributions of competition and allelopathy to interference by barley. Nonlinearity Biol. Toxicol. Med. 2005, 3, 213–224. [Google Scholar] [CrossRef] [PubMed]
- San Emeterio, L.; Damgaard, C.; Canals, R.M. Modelling the combined effect of chemical interference and resource competition on the individual growth of two herbaceous populations. Plant Soil 2007, 292, 95–103. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1221–1227. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Inderjit; del Moral, R. Is separating resource competition from allelopathy realistic? Bot. Rev. 1997, 63, 221–230. [Google Scholar] [CrossRef]
- He, H.B.; Wang, H.B.; Fang, C.X.; Lin, Z.H.; Yu, Z.M.; Lin, W.X. Separation of allelopathy from resource competition using rice/barnyardgrass mixed-cultures. PLoS ONE 2012, 7, e37201. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Mallik, A.U. Allelopathy: Advances, Challenges and Opportunities. In Allelopathy in Sustainable Agriculture and Forestry; Zeng, R.S., Mallik, A.U., Luo, S.M., Eds.; Springer: New York, NY, USA, 2008; pp. 25–38. [Google Scholar]
- Fernandez, C.; Monnier, Y.; Santonja, M.; Gallet, C.; Weaton, L.A.; Prevosto, B.; Saunier, A.; Baldy, V.; Bousquet-Melou, A. The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front. Plant Sci. 2016, 7, 594. [Google Scholar] [CrossRef]
- Qasem, J.R. A new technology separating allelopathy from competition in pot experiments. Int. J. Agric. Sci. Food Technol. 2017, 3, 19–25. [Google Scholar] [CrossRef]
- Nilsson, M.C. Separation of allelopathy and resource competition by the boreal dwarf shrub Empetrum hermaphroditum Hagerup. Oecologia 1994, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bieberich, J.; Lauerer, M.; Drachsler, M.; Heinrichs, J.; Müller, S.; Feldhaar, H. Species- and developmental stage-specific effects of allelopathy and competition of invasive Impatiens glandulifera on co-occurring plants. PLoS ONE 2018, 13, e0205843. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.; Fomsgaard, I.S.; Mathiassen, S.K.; Stuart, R.M.; Kudsk, P. Weed suppression by winter cereals: Relative contribution of competition for resources and allelopathy. Chemoecology 2018, 28, 109–121. [Google Scholar] [CrossRef]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.; Ishii, T.; Nakamura, I.; Asaduzzaman, M.; Bhowmik, P.C. Performance of Bangladesh indigenous rice in a weed infested field and separation of allelopathy from resource competition. Weed Biol. Manag. 2019, 19, 39–50. [Google Scholar] [CrossRef]
- Uddin, M.N.; Robinson, R.W. Allelopathy and resource competition: The effects of Phragmites australis invasion in plant communities. Bot. Stud. 2017, 58, 29. [Google Scholar] [CrossRef]
- Martines, I.P.; Kojouharov, H.V.; Grover, J.P. A chemostat model of resource competition and allelopathy. Appl. Math Comput. 2009, 215, 573–582. [Google Scholar] [CrossRef]
- Liu, C.; Bu, Z.J.; Mallik, A.; Rochefort, L.; Hu, X.F.; Yu, Z. Resource competition and allelopathy in two peat mosses: Implication for niche differentiation. Plant Soil 2020, 446, 229–242. [Google Scholar] [CrossRef]
- Mougi, A. Allelopathic adaptation can cause competitive coexistence. Theor. Ecol. 2013, 6, 165–171. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, J.; Huang, D.; Wang, L.X.; Wang, K. Allelopathic potential of Artemisia frigida and successional changes of plant communities in the northern China steppe. Plant Soil 2011, 341, 383–398. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.F.; Müller, K.; Lin, L.J.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Wang, S.P.; Han, X.G.; Patton, B.D.; Nyren, P.E. Competition between Artemisia frigida and Cleistogenes squarrosa under different clipping intensities in replacement series mixtures at different nitrogen levels. Grass Forage Sci. 2005, 60, 119–127. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Yang, Q.; Wang, T.M.; Zhang, Z.W.; Liu, J.L.; Shi, M.M.; Ping, X.Y. The impact of grazing intensity on the allelopathic effect of Artemisia frigida in a temperate grassland in northern China. Flora 2022, 288, 152005. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Liu, K.; Li, X.; Huang, D.; Wang, K. The allelopathic effect of Potentilla acaulis on the changes of plant community in grassland, northern China. Ecol. Res. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Li, J.H.; Li, Z.; Ren, J.Z. Effect of grazing intensity on clonal morphological plasticity and biomass allocation patterns of Artemisia frigida and Potentilla acaulis in the Inner Mongolia steppe. New Zealand J. Agric. Res. 2005, 48, 57–61. [Google Scholar]
- Li, F.R.; Zhao, A.F.; Zhou, H.Y.; Zhang, T.H.; Zhao, X. Effects of simulated grazing on growth and persistence of Artemisia frigida in a semiarid sandy rangeland. Grass Forage Sci. 2002, 57, 239–246. [Google Scholar] [CrossRef]
- Zhang, R.M.; Zuo, Z.J.; Gao, P.J.; Hou, P.; Wen, G.S.; Gao, Y. Allelopathic effects of VOCs of Artemisia frigida Willd. on the regeneration of pasture grasses in Inner Mongolia. J. Arid Environ. 2012, 87, 212–218. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Zuo, Z.J.; Li, R.; Wu, J.; Gao, Y. Inhibition effects of volatile organic compounds from Artemisia frigida Willd: On the pasture grass intake by lambs. Small Rumin. Res. 2014, 121, 248–254. [Google Scholar] [CrossRef]
- Padilla, F.M.; Mommer, L.; de Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.M.; Ouborg, N.J.; de Kroon, H. Early root overproduction not triggered by nutrients decisive for competitive success belowground. PLoS ONE 2013, 8, e55805. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C.; Richards, C.L. Phenotypic plasticity and interactions among plants. Ecology 2003, 84, 1115–1128. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Brooker, R.; Atwater, D.Z.; Maron, J.L.; Callaway, R.M. The mechanisms and consequences of interspecific competition among plants. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 263–281. [Google Scholar] [CrossRef]
- Wang, S.; Callaway, R.M. Plasticity in response to plant-plant interactions and water availability. Ecology 2021, 102, e03361. [Google Scholar] [CrossRef] [PubMed]
- Freckleton, R.P.; Watkinson, A.R. Asymmetric competition between plant species. Funct. Ecol. 2001, 15, 615–623. [Google Scholar] [CrossRef]
- Wang, L.; Delgado-Baquerizo, M.; Wang, D.; Isbell, F.; Liu, J.; Feng, C.; Liu, J.; Zhong, Z.; Zhu, H.; Yuan, X.; et al. Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 6187. [Google Scholar] [CrossRef]
- Falquet, B.; Roux, D.; Henriet, L.; Tschuy, F.; Wirth, J. Simple method to separate resource competition from allelopathic root interactions. Allelopath. J. 2014, 34, 227–240. [Google Scholar]
- Inderjit, K.M.; Foy, C.L. On the significance of field studies in allelopathy. Weed Technol. 2001, 15, 792–797. [Google Scholar] [CrossRef]
- Ridenour, W.M.; Callaway, R.M. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 2001, 126, 444–450. [Google Scholar] [CrossRef]
- Lau, J.A.; Puliafico, K.P.; Kopshever, J.A.; Setltzer, H.; Jarvis, E.P.; Schwarzländer, M.; Strauss, S.Y.; Hufbauer, R.A. Inference of allelopathy is complicated by effects of activated carbon on plant growth. New Phytol. 2008, 178, 412–423. [Google Scholar] [CrossRef]
- Meynet, P.; Hale, S.E.; Davenport, R.J.; Cornelissen, G.; Breedveld, G.D.; Werner, D. Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environ. Sci. Technol. 2012, 46, 5057–5066. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Y.; Grenier-Héon, D. Effects of allelopathy and availability of nutrients and water resources on the survival and growth of plant species in a natural Dacrydium forest. Can. J. For. Res. 2021, 52, 100–108. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003, 54, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.J.; Zhang, R.M.; Gao, P.J.; Wen, G.S.; Gao, Y. Allelopathic effects of Artemisia frigida Willd. on growth of pasture grasses in Inner Mongolia, China. Biochem. Syst. Ecol. 2011, 39, 377–383. [Google Scholar]
- Xu, Y.; Hao, Y. A review of ecological research on Cleistogenes squarrosa. Acta Agrestia Sin. 2016, 24, 726–730. (In Chinese) [Google Scholar]
- Qin, F.; Liu, S.; Yu, S. Effects of allelopathy and competition for water and nutrients on survival and growth of tree species in Eucalyptus urophylla plantations. For. Ecol. Manag. 2018, 424, 387–395. [Google Scholar] [CrossRef]
- Callaway, R.M.; Kikodze, D.; Chiboshvili, M.; Khetsuriani, L. Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology 2005, 86, 1856–1862. [Google Scholar] [CrossRef]
- Maestre, F.; Bautista, S.; Cortina, J. Positive, negative, and net effects in grass-shrub interactions in Mediterranean semiarid grasslands. Ecology 2003, 84, 3186–3197. [Google Scholar] [CrossRef]
- Weston, L.A.; Barney, J.N.; DiTommaso, A. A review of the biology and ecology of three invasive perennials in New York State: Japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow-wort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69. [Google Scholar] [CrossRef]
- Parepa, M.; Bossdorf, O. Testing for allelopathy in invasive plants: It all depends on the substrate! Biol. Invasions 2016, 18, 2975–2982. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.; Michalet, R.; Paolini, L.; Pugnaire, F.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef]


| Indexes of Plant Interference | RNE | ||
|---|---|---|---|
| Aboveground Biomass | Belowground Biomass | Root/Shoot Ratio | |
| AF-LC | 0.28 ± 0.10 a | 0.15 ± 0.04 b | −0.16 ± 0.08 b |
| AF-CS | 0.45 ± 0.10 a | 0.64 ± 0.07 a | 0.30 ± 0.08 a |
| AF-PA | −0.08 ± 0.04 b | −0.67 ± 0.01 c | −0.64 ± 0.05 c |
| LC-AF | −0.27 ± 0.07 b | −0.40 ± 0.07 a | −0.18 ± 0.05 b |
| CS-AF | −0.64 ± 0.02 c | −0.52 ± 0.04 b | 0.23 ± 0.09 a |
| PA-AF | 0.30 ± 0.06 a | −0.59 ± 0.04 b | −0.71 ± 0.02 c |
| Plant Species | Dry Matter Proportion (%) | |||
|---|---|---|---|---|
| No Grazing | Light Grazing | Medium Grazing | Extreme Grazing | |
| Leymus chinensis | 35.34 ± 1.45 a | 26.32 ± 1.89 b | 15.53 ± 1.23 c | 6.12 ± 0.78 d |
| Cleistogenes squarrosa | 5.61 ± 0.32 d | 43.64 ± 3.27 a | 25.25 ± 2.32 b | 13.42 ± 1.62 c |
| Artemisia frigida | 3.26 ± 0.25 c | 10.25 ± 1.67 b | 38.21 ± 2.87 a | 28.02 ± 2.98 a |
| Potentilla acaulis | 0.08 ± 0.03 c | 2.35 ± 0.08 bc | 7.30 ± 1.12 b | 32.75 ± 4.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Kong, M.; Wang, J.; Gao, B.; Ping, X. The Specific Impacts of Allelopathy and Resource Competition from Artemisia frigida on the Growth of Three Plant Species in Northern China. Plants 2024, 13, 3286. https://doi.org/10.3390/plants13233286
Wang Q, Kong M, Wang J, Gao B, Ping X. The Specific Impacts of Allelopathy and Resource Competition from Artemisia frigida on the Growth of Three Plant Species in Northern China. Plants. 2024; 13(23):3286. https://doi.org/10.3390/plants13233286
Chicago/Turabian StyleWang, Qing, Mengqiao Kong, Junwen Wang, Bin Gao, and Xiaoyan Ping. 2024. "The Specific Impacts of Allelopathy and Resource Competition from Artemisia frigida on the Growth of Three Plant Species in Northern China" Plants 13, no. 23: 3286. https://doi.org/10.3390/plants13233286
APA StyleWang, Q., Kong, M., Wang, J., Gao, B., & Ping, X. (2024). The Specific Impacts of Allelopathy and Resource Competition from Artemisia frigida on the Growth of Three Plant Species in Northern China. Plants, 13(23), 3286. https://doi.org/10.3390/plants13233286





