Response of Arabidopsis thaliana to Flooding with Physical Flow
Abstract
:1. Introduction
2. Results
2.1. Effects of Flooding on Growth of Arabidopsis thaliana Plants
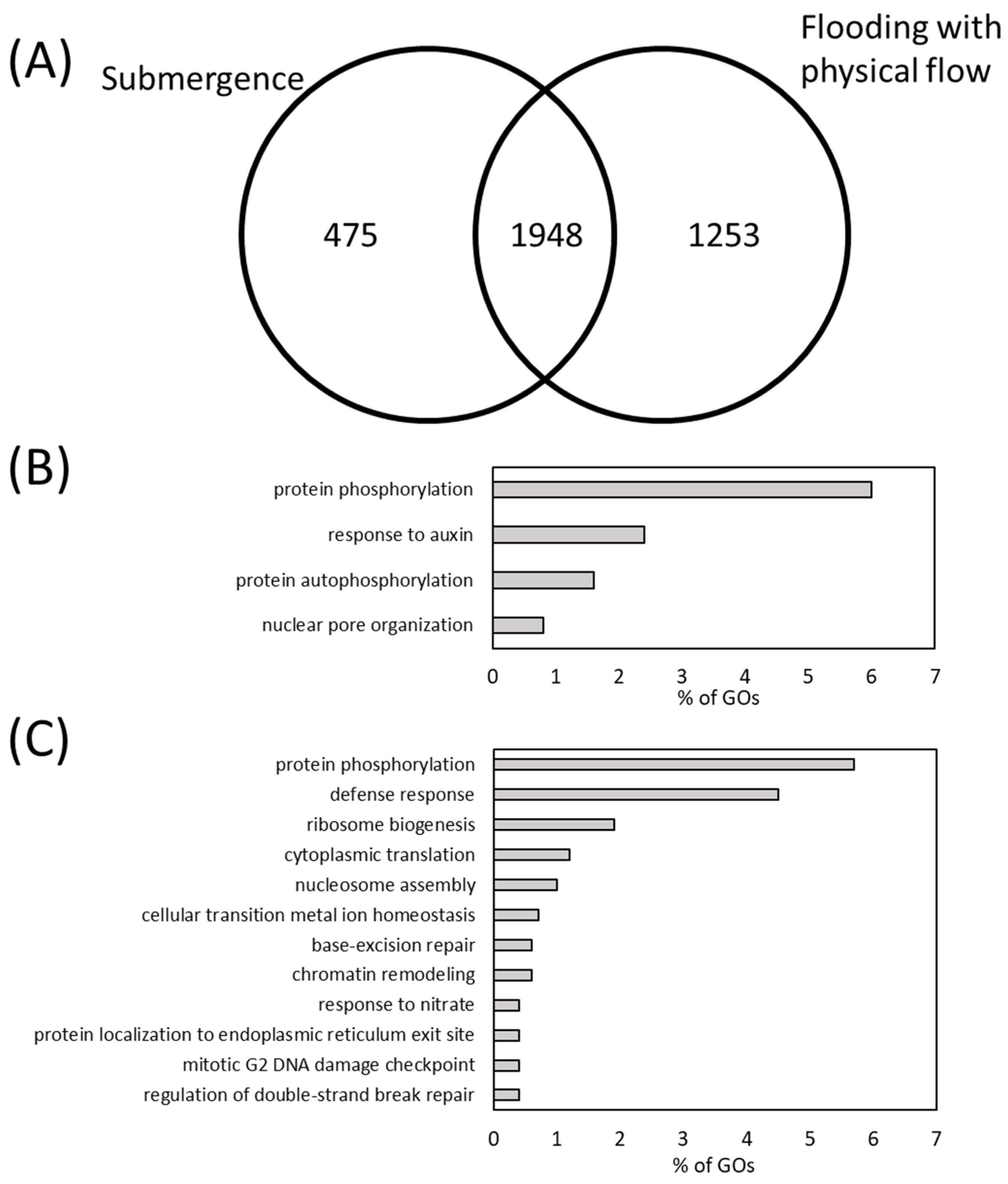
2.2. Transcriptome Analysis of Arabidopsis thaliana Plants Exposed to Flooding with Physical Flow
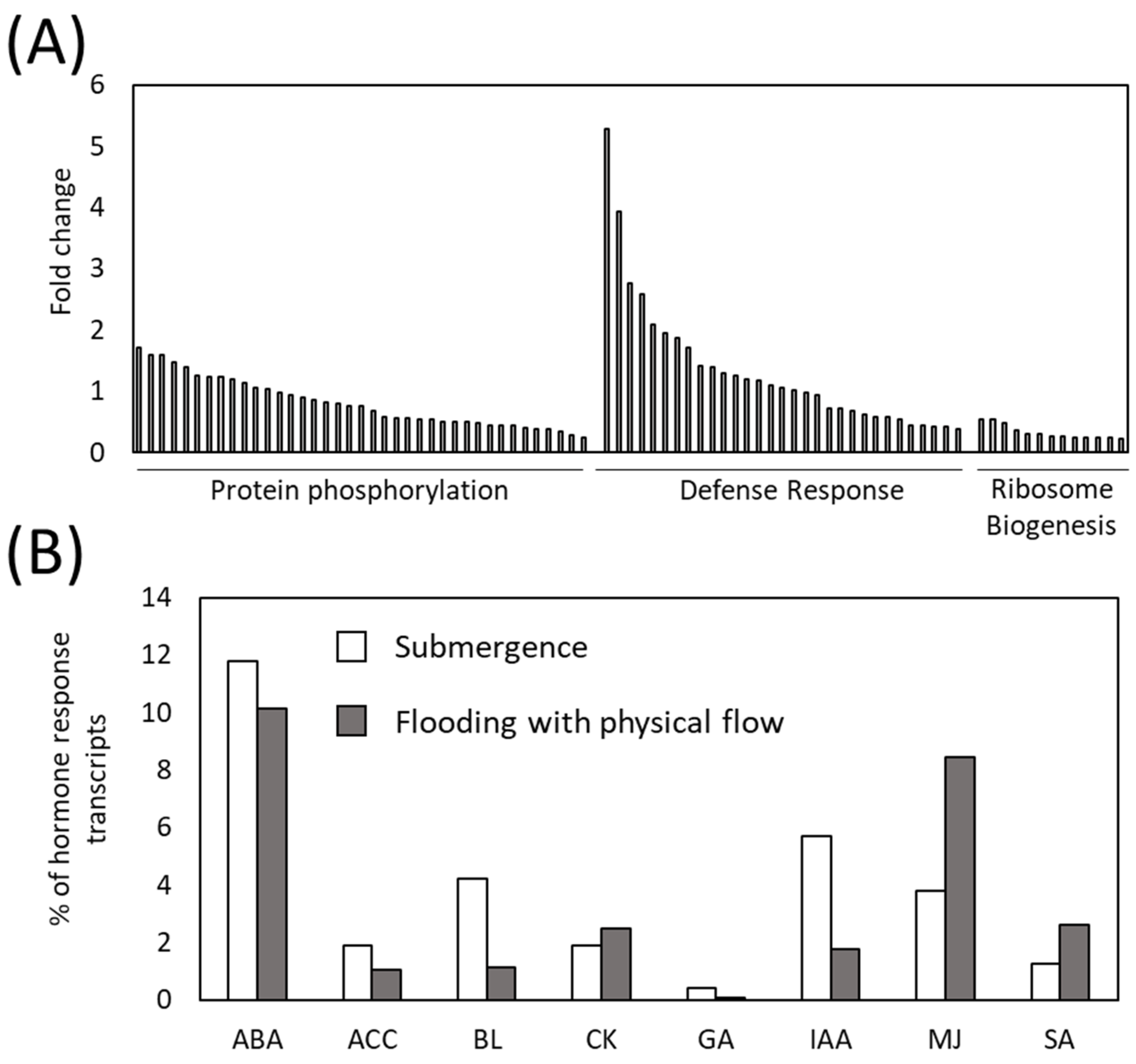
2.3. Involvement of Defense Mechanisms and Its Regulatory Plant Hormone in the Response of Arabidopsis thaliana to Flooding with Physical Flow
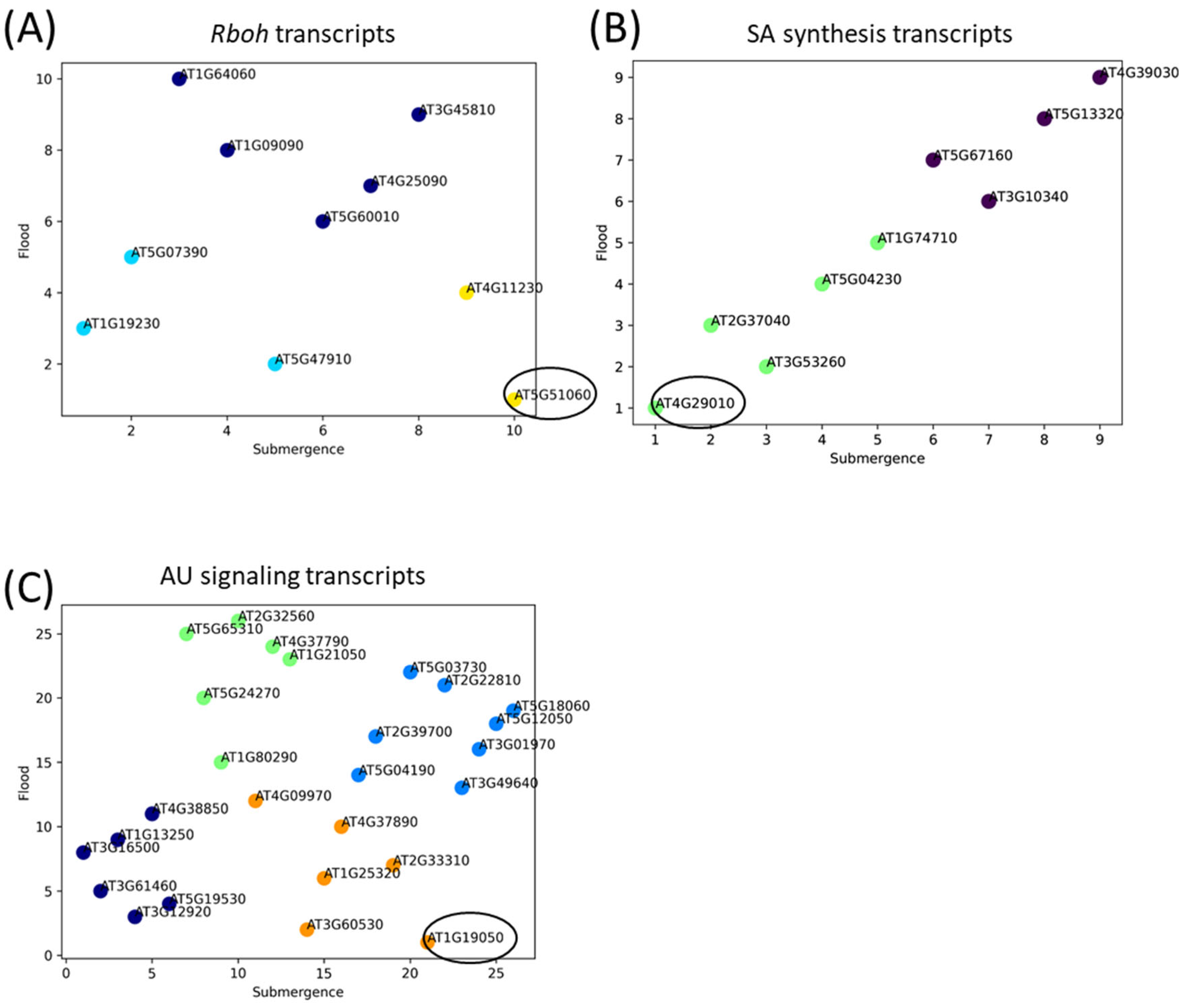
2.4. Up-Regulation of Transcripts Involved in JA or SA Synthesis and ROS Production
2.5. Up-Regulation of AP2/Ethylene Response Factors in Response to Submergence and Flooding
2.6. Multiple Regression Analysis of Transcripts Involved in ROS Production, SA Synthesis and AU Signals
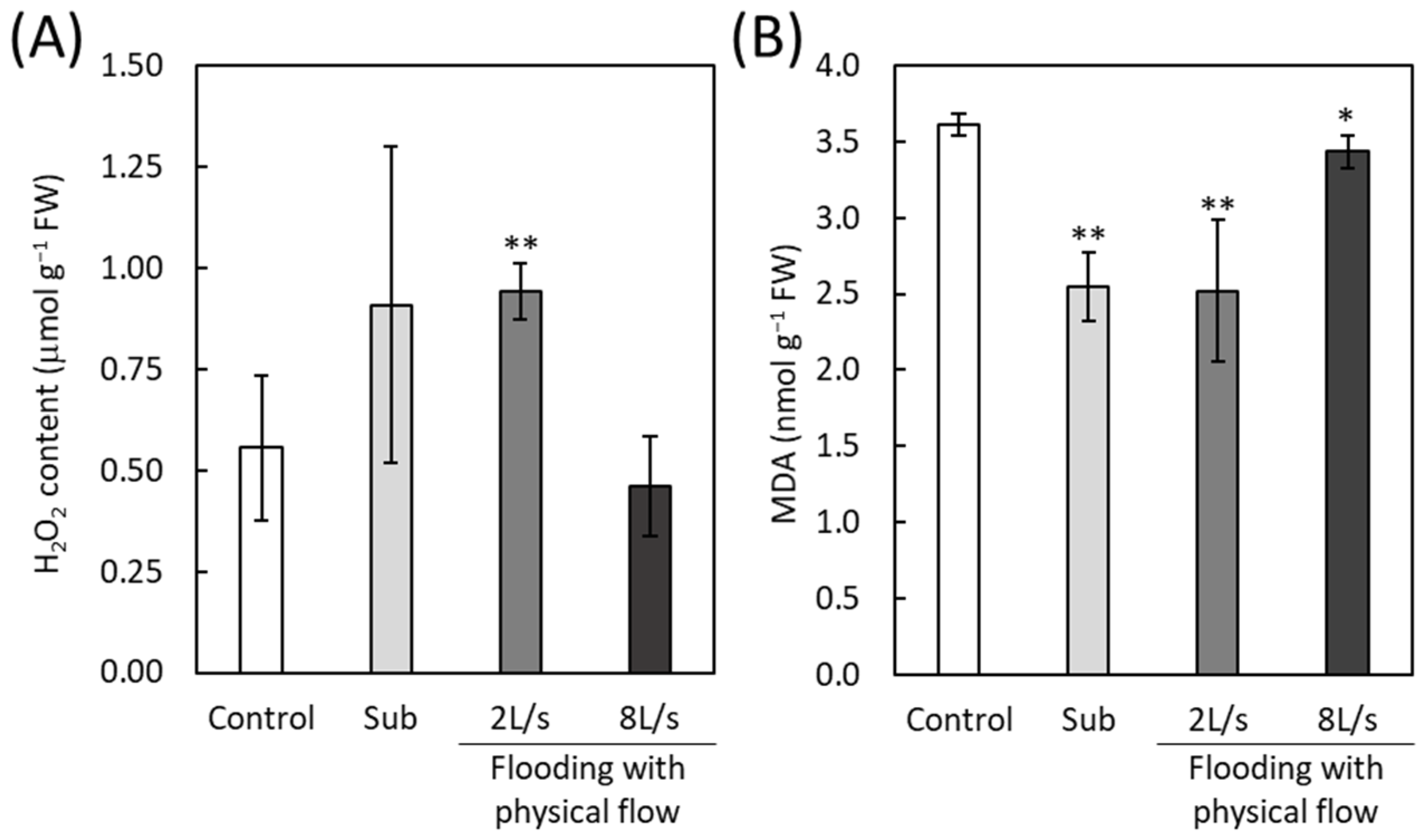
2.7. Accumulation of H2O2 and Malondialdehyde in Plants Exposed to Submergence or Flooding with Physical Flow
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Stress Treatment and Measurement of Growth Parameters
4.3. RNA-Seq Analysis and Quantitative Real-Time PCR
4.4. Measurement of H2O2 and MDA Contents
4.5. Database and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, A.; Mahmood, A.; Ur-Rehman, H.; Li, C.; Liang, X.; Shao, J.; Negm, S.; Moustafa, M.; Aamer, M.; Hassan, M.U. Plant Adaptation to Flooding Stress under Changing Climate Conditions: Ongoing Breakthroughs and Future Challenges. Plants 2023, 12, 3824. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Setter, T.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Westra, S.; Fowler, H.J.; Evans, J.P.; Alexander, L.V.; Berg, P.; Johnson, F.; Kendon, E.J.; Lenderink, G.; Roberts, N. Future changes to the intensity and frequency of short-duration extreme rainfall. Rev. Geophys. 2014, 52, 522–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Visser, E.J.; Cohen, J.D.; Barendse, G.W.; Blom, C.W.; Voesenek, L.A. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Zabalza, A.; Van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmalzlin, E.; Igal, M.; Orcaray, L.; Royuela, M. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef]
- Caruso, P.; Baldoni, E.; Mattana, M.; Pietro Paolo, D.; Genga, A.; Coraggio, I.; Russo, G.; Picchi, V.; Reforgiato Recupero, G.; Locatelli, F. Ectopic expression of a rice transcription factor, Mybleu, enhances tolerance of transgenic plants of Carrizo citrange to low oxygen stress. Plant Cell Tissue Organ Cult. 2012, 109, 327–339. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef] [PubMed]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.D.S.C.D.; Braga, E.J.B.; Oliveira, A.C.B.D.; Amarante, L.D. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Renziehausen, T.; Frings, S.; Romy Schmidt-Schippers, R. ‘Against all floods’: Plant adaptation to flooding stress and combined abiotic stresses. Plant J. 2024, 117, 1836–1855. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Sun, D.; Han, Z.; Ni, D.; Norris, A.; Jiang, C.-Z. PhERF2, an ethylene-responsive element binding factor, plays an essential role in waterlogging tolerance of petunia. Hortic. Res. 2019, 6, 83. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Mignolli, F.; Todaro, J.S.; Vidoz, M.L. Internal aeration and respiration of submerged tomato hypocotyls are enhanced by ethylene-mediated aerenchyma formation and hypertrophy. Physiol. Plant. 2020, 169, 49–63. [Google Scholar] [CrossRef]
- Wang, J.; Han, M.; Huang, Y.; Zhao, J.; Liu, C.; Ma, Y. Flooding Tolerance of Rice: Regulatory Pathways and Adaptive Mechanisms. Plants 2024, 13, 1178. [Google Scholar] [CrossRef]
- Hong, B.; Zhou, B.; Peng, Z.; Yao, M.; Wu, J.; Wu, X.; Guan, C.; Guan, M. Tissue-specific transcriptome and metabolome analysis reveals the response mechanism of Brassica napus to waterlogging stress. Int. J. Mol. Sci. 2023, 24, 6015. [Google Scholar] [CrossRef]
- Gomes, G.; Scortecci, K. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Alaguero-Cordovilla, A.; Sánchez-García, A.B.; Ibáñez, S.; Albacete, A.; Cano, A.; Acosta, M.; Pérez-Pérez, J.M. An auxin-mediated regulatory framework for wound-induced adventitious root formation in tomato shoot explants. Plant Cell Environ. 2021, 44, 1642–1662. [Google Scholar] [CrossRef]
- Finkelsteina, R.R.; Rockb, C.D. Abscisic acid biosynthesis and response. Arab. Book 2002, 11, e0166. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Franceschi, V.R. Methyl Jasmonate-Induced Ethylene Production Is Responsible for Conifer Phloem Defense Responses and Reprogramming of Stem Cambial Zone for Traumatic Resin Duct Formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative Analysis of Endogenous Hormones Level in Two Soybean (Glycine max L.) Lines Differing in Waterlogging Tolerance. Front. Plant Sci. 2015, 6, 141434. [Google Scholar] [CrossRef] [PubMed]
- Maciag, T.; Kozieł, E.; Otulak-Kozieł, K.; Jafra, S.; Czajkowski, R. Looking for Resistance to Soft Rot Disease of Potatoes Facing Environmental Hypoxia. Int. J. Mol. Sci. 2024, 25, 3757. [Google Scholar] [CrossRef]
- Li, C.-X.; Wei, H.; Geng, Y.-H.; Schneider, R. Effects of submergence on photosynthesis and growth of Pterocarya stenoptera (Chinese wingnut) seedlings in the recently-created Three Gorges Reservoir region of China. Wetl. Ecol. Manag. 2010, 18, 485–494. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2009, 33, 453–467. [Google Scholar] [CrossRef]
- Suzuki, N. Fine Tuning of ROS, Redox and Energy Regulatory Systems Associated with the Functions of Chloroplasts and Mitochondria in Plants under Heat Stress. Int. J. Mol. Sci. 2023, 24, 1356. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kulshreshtha, N.; Kumar, A.; Yaduvanshi, N.; Singh, M.; Prasad, K.; Basak, N. Waterlogging effects on elemental composition of wheat genotypes in sodic soils. J. Plant Nutr. 2018, 41, 1252–1262. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for improving waterlogging tolerance in cereal crops—Physiological traits and genetic mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Hu, C.H.; Wang, P.Q.; Zhang, P.P.; Nie, X.M.; Li, B.B.; Tai, L.; Liu, W.T.; Li, W.Q.; Chen, K.M. NADPH Oxidases: The Vital Performers and Center Hubs during Plant Growth and Signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hong, C.-P. The NADPH oxidase Rboh D is involved in primary hypoxia signalling and modulates expression of hypoxia-inducible genes under hypoxic stress. Environ. Exp. Bot. 2015, 115, 63–72. [Google Scholar] [CrossRef]
- Yao, Y.; He, R.J.; Xie, Q.; Zhao, X.H.; Deng, X.M.; He, J.B.; Song, L.; He, J.; Marchant, A.; Chen, X.Y.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response to different stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef]
- Lee, J.; Hanh Nguyen, H.; Park, Y.; Lin, J.; Hwang, I. Spatial regulation of RBOHD via AtECA4-mediated recycling and clathrin-mediated endocytosis contributes to ROS accumulation during salt stress response but not flg22-induced immune response. Plant J. 2022, 109, 816–830. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Peláez-Vico, M.Á.; Tukuli, A.; Singh, P.; Mendoza-Cózatl, D.G.; Joshi, T.; Mittler, R. Rapid systemic responses of Arabidopsis to waterlogging stress. Plant Physiol. 2023, 193, 2215–2231. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Alché, J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, M.; Julien, J.-D.; Mirabet, V.; Boudaoud, A.; Hamant, O. Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, E4294–E4303. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gong, Y.-W.; Yuan, Y.-J. RGD-dependent mechanotransduction of suspension cultured Taxus cell in response to shear stress. Biotechnol. Prog. 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, H.; Akter, A.; Miyaji, N.; Miyazaki, J.; Shea, D.J.; Fujimoto, R.; Doullah, M.A.-U. Genetics of Clubroot and Fusarium Wilt Disease Resistance in Brassica Vegetables: The Application of Marker Assisted Breeding for Disease Resistance. Plants 2020, 9, 726. [Google Scholar] [CrossRef]
- Sett, S.; Prasad, A.; Prasad, M. Resistance genes on the verge of plant-virus interaction. Trends Plant Sci. 2022, 27, 1242–1252. [Google Scholar] [CrossRef]
- Thireault, C.; Shyu, C.; Yoshida, Y.; Aubin, B.S.; Campos, M.L.; Howe, G.A. Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J. 2015, 82, 669–679. [Google Scholar] [CrossRef]
- Woo, O.-G.; Kim, S.-H.; Cho, S.K.; Kim, S.-H.; Lee, H.N.; Chung, T.; Yang, S.W.; Lee, J.-H. BPH1, a novel substrate receptor of CRL3, plays a repressive role in ABA signal transduction. Plant Mol. Biol. 2018, 96, 593–606. [Google Scholar] [CrossRef]
- Serrano, L.; Gu, Y.; Qi, D.; Dubiella, U.; Innes, R.W. The Arabidopsis EDR1 protein kinase negatively regulates the ATL1 E3 ubiquitin ligase to suppress cell death. Plant Cell 2014, 26, 4532–4546. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Blanco, F.; Salinas, P.; Cecchini, N.M.; Jordana, X.; Van Hummelen, P.; Alvarez, M.E.; Holuigue, L. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol. Biol. 2009, 70, 79–102. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Ron Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Wawrzyńska, A.; Sirko, A. Sulfate Availability and Hormonal Signaling in the Coordination of Plant Growth and Development. Int. J. Mol. Sci. 2024, 25, 3978. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Strader, L.C. An auxin research odyssey: 1989–2023. Plant Cell 2024, 36, 1410–1428. [Google Scholar] [CrossRef]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An Ethylene Response Transcription Factor That Is Important for Hypoxia Survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Peláez-Vico, M.Á.; Sinha, R.; Pascual, L.S.; Mittler, R. The impact of multifactorial stress combination on plants, crops, and ecosystems: How should we prepare for what comes next? Plant J. 2024, 117, 1800–1814. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, A.; Li, T.; Ren, L.; Li, L.; Su, Y.; Zhang, Q. ROS and calcium oscillations are required for polarized root hair growth. Plant Signal Behav. 2022, 17, 2106410. [Google Scholar] [CrossRef]
- Fichman, T.; Miller, G.; Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Carmody, M.; Albrecht, V.; Pogson, B. Systemic and Local Responses to Repeated HL Stress-Induced Retrograde Signaling in Arabidopsis. Front. Plant Sci. 2013, 3, 303. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, V.N.; Gedam, P.; Pradhan, A.; Gaikwad, B.; Kale, R.; Gawande, S. Concurrent waterlogging and anthracnose-twister disease in rainy-season onions (Allium cepa): Impact and management. Front. Microbiol. 2022, 13, 1063472. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, X.; Bürger, M.; Wang, Y.; Chory, J. Two interacting ethylene response factors regulate heat stress response. Plant Cell 2021, 33, 338–357. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Eccersall, S.; Meisrimler, C.N. Shared signals, different fates: Calcium and ROS in plant PRR and NLR immunity. Cell Rep. 2024, 43, 114910. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Vonapartis, E.; Mohamed, D.; Li, J.; Pan, W.; Wu, J.; Gazzarrini, S. CBF4/DREB1D represses XERICO to attenuate ABA, osmotic and drought stress responses in Arabidopsis. Plant J. 2022, 110, 961–977. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yamoto, N.; Suzuki, Y.; Chiba, Y.; Yamazaki, K.; Sato, T.; Yamaguchi, J. The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci. 2013, 213, 79–87. [Google Scholar] [CrossRef]
- Cao, F.Y.; DeFalco, T.A.; Moeder, M.; Li, B.; Gong, Y.; Liu, X.-M.; Taniguchi, M.; Lumba, S.; Toh, S.; Shan, L.; et al. Arabidopsis ETHYLENE RESPONSE FACTOR 8 (ERF8) has dual functions in ABA signaling and immunity. BMC Plant Biol. 2018, 18, 211. [Google Scholar] [CrossRef]
- Hong, C.-P.; Wang, M.-C.; Yang, C.-Y. NADPH Oxidase RbohD and Ethylene Signaling are Involved in Modulating Seedling Growth and Survival Under Submergence Stress. Plants 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Wienkoop, S.; Lüthje, S. Hypoxia-Induced Aquaporins and Regulation of Redox Homeostasis by a Trans-Plasma Membrane Electron Transport System in Maize Roots. Antioxidants 2022, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Zelinová, V.; Demecsová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Extracellular nitric oxide sustains root surface redox activity and growth under sudden flooding-induced hypoxic conditions in barley root tips. Planta 2023, 259, 3. [Google Scholar] [CrossRef]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Breusegem, F.V.; Karpiński, S.M.; Mittler, R. ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef]
- Katano, K.; Kataoka, R.; Fujii, M.; Suzuki, N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol. Biochem. 2018, 123, 288–296. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaji, M.; Katano, K.; Anee, T.I.; Nitta, H.; Yamaji, R.; Shimizu, R.; Shigaki, S.; Suzuki, H.; Suzuki, N. Response of Arabidopsis thaliana to Flooding with Physical Flow. Plants 2024, 13, 3508. https://doi.org/10.3390/plants13243508
Kaji M, Katano K, Anee TI, Nitta H, Yamaji R, Shimizu R, Shigaki S, Suzuki H, Suzuki N. Response of Arabidopsis thaliana to Flooding with Physical Flow. Plants. 2024; 13(24):3508. https://doi.org/10.3390/plants13243508
Chicago/Turabian StyleKaji, Momoko, Kazuma Katano, Taufika Islam Anee, Hiroshi Nitta, Ryotaro Yamaji, Rio Shimizu, Shunsuke Shigaki, Hiroyuki Suzuki, and Nobuhiro Suzuki. 2024. "Response of Arabidopsis thaliana to Flooding with Physical Flow" Plants 13, no. 24: 3508. https://doi.org/10.3390/plants13243508
APA StyleKaji, M., Katano, K., Anee, T. I., Nitta, H., Yamaji, R., Shimizu, R., Shigaki, S., Suzuki, H., & Suzuki, N. (2024). Response of Arabidopsis thaliana to Flooding with Physical Flow. Plants, 13(24), 3508. https://doi.org/10.3390/plants13243508








