Insights into the Transcriptional Reprogramming of Peach Leaves Inoculated with Taphrina deformans
Abstract
:1. Introduction
2. Results
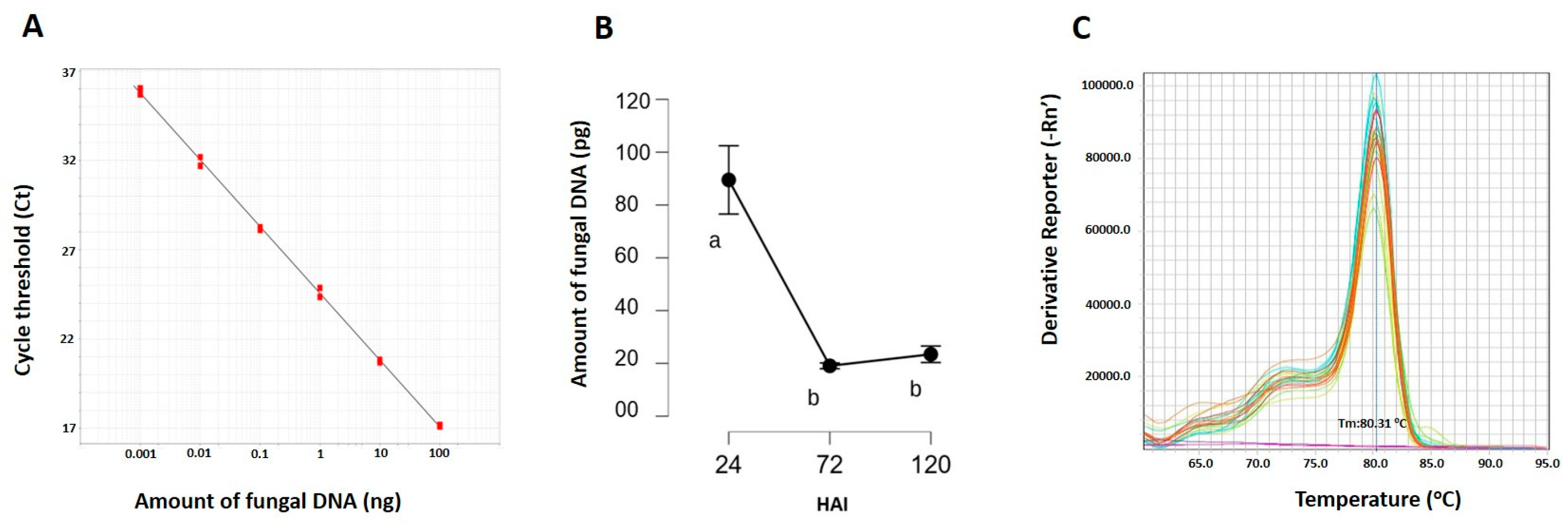
2.1. T. deformans Leaf Inoculation and Monitoring of Pathogen Growth
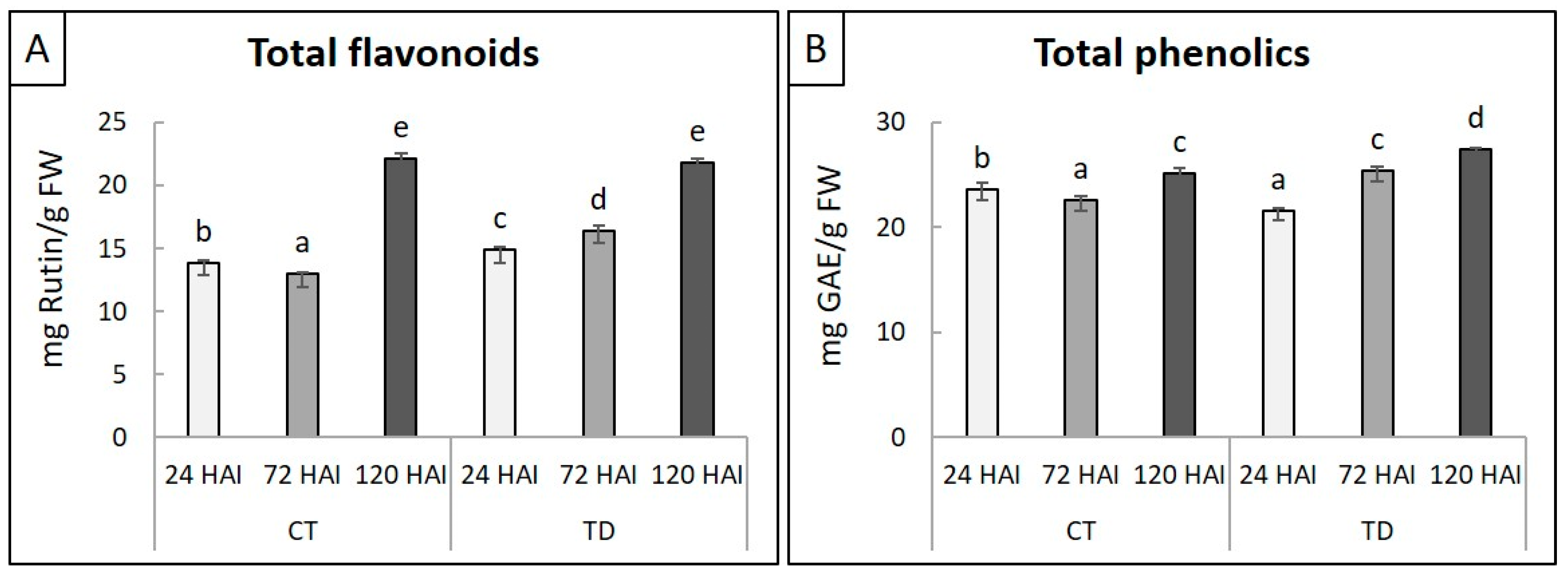
2.2. Physiological Changes in Leaves in Response to T. deformans Inoculation
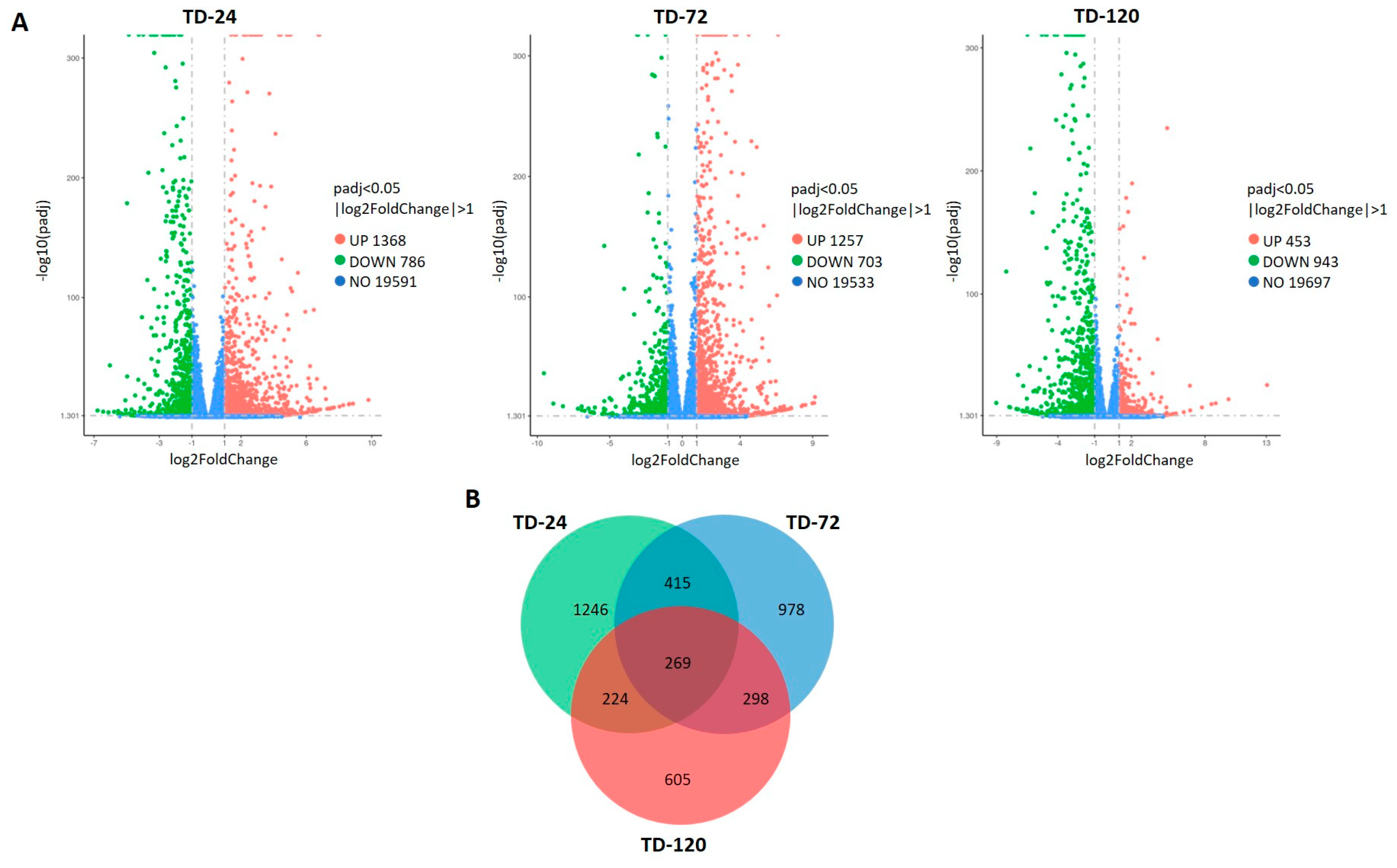
2.3. RNA-Seq Analysis of Leaves after Inoculation with T. deformans
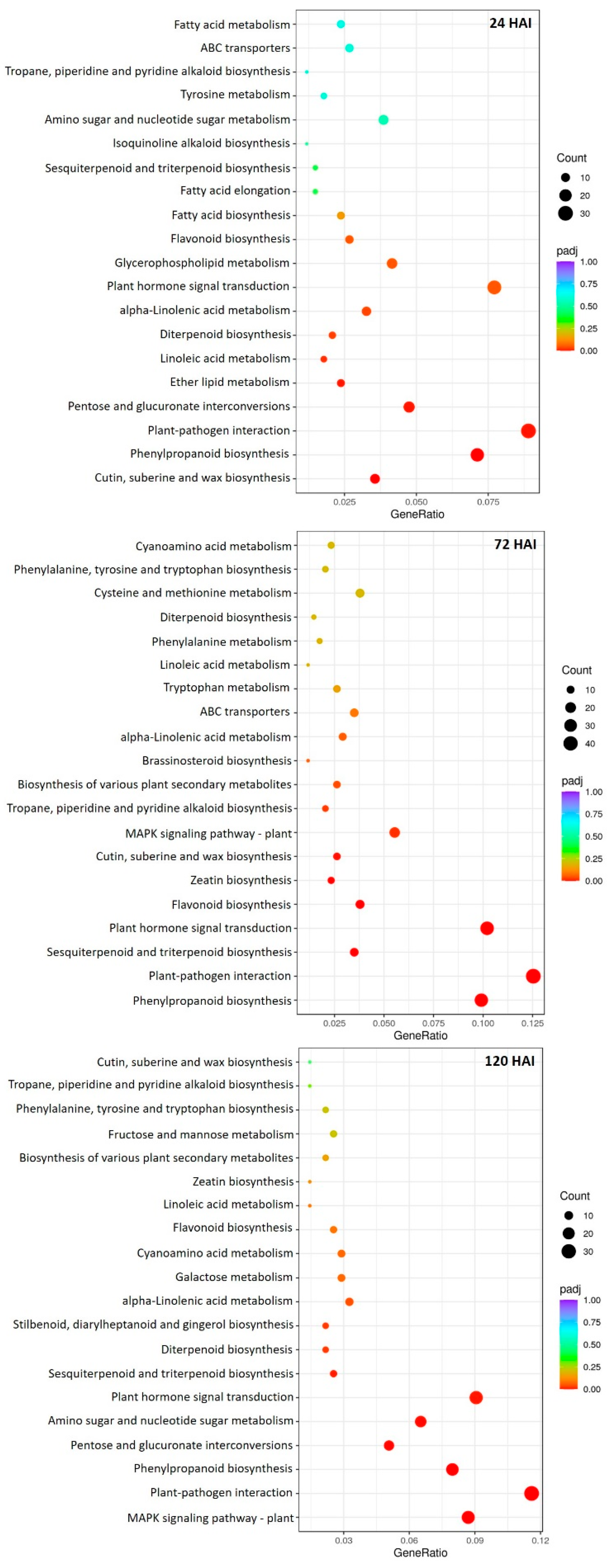
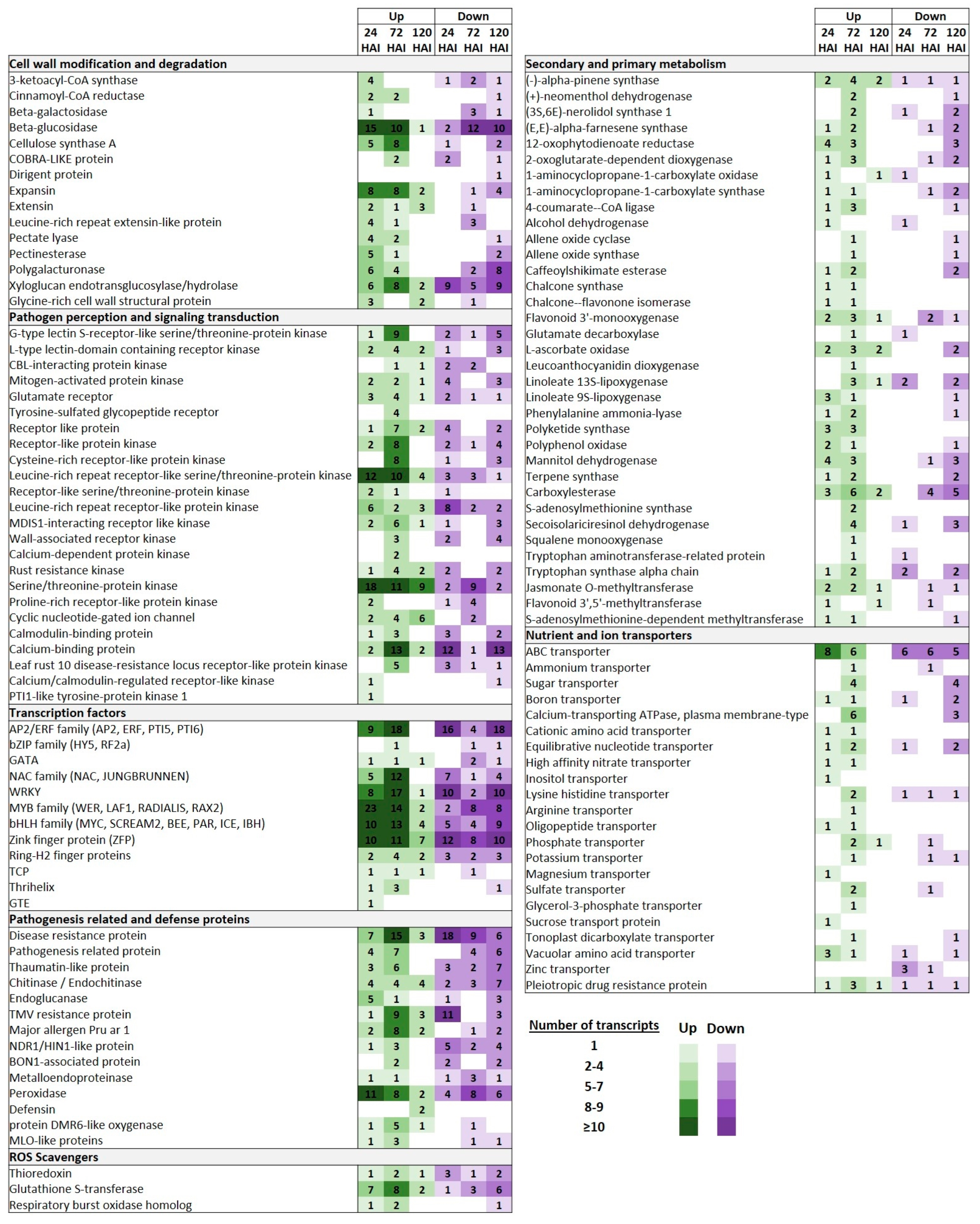
2.4. Functional Annotations and Classifications of Peach DEGs
2.5. DEGs Involved in Cell Wall Modification and Degradation
2.6. DEGs Involved in Pathogen Perception and Signaling Transduction
2.7. DEGs Encoding TFs
2.8. DEGs Encoding Pathogenesis-Related and Defense Proteins
2.9. DEGs Involved in Secondary and Primary Metabolism
2.10. DEGs Encoding Nutrient and Ion Transporters
2.11. Validation of RNA-Seq Data Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Pathogen Isolation
4.2. Plant Material and T. deformans Inoculation
4.3. Levels of Total Flavonoids and Phenolics on Peach Leaves
4.4. Development of a Diagnostic Assay for Pathogen Growth Rate
4.5. Transcriptome Sequencing and Bioinformatics Analysis
4.6. Quantitative Real-Time PCR (qRT-PCR) Validation of RNA-Seq Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cimen, I.; Ertugrul, B.B. Determination of Mycoflora in Almond Plantations under Drought Conditions in Southeastern Anatolia Project Region, Turkey. Plant Pathol. J. 2007, 6, 82–86. [Google Scholar] [CrossRef]
- Frisullo, S.; Rana, G.L.; Crescenzi, A. Apricot leaf curl in Campania and Apulia (Italy). Petria 2000, 10, 11–14. [Google Scholar]
- Cissé, O.H.; Almeida, J.M.; Fonseca, Á.; Kumar, A.A.; Salojärvi, J.; Overmyer, K.; Hauser, P.M.; Pagni, M. Genome sequencing of the plant pathogen Taphrina deformans, the causal agent of peach leaf curl. mBio 2013, 4, 10-1128. [Google Scholar] [CrossRef]
- Fonseca, Á.; Rodrigues, M.G. Taphrina fries (1832). In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 823–858. [Google Scholar]
- Rossi, V.; Bolognesi, M.; Giosuè, S. Influence of Weather Conditions on Infection of Peach Fruit by Taphrina deformans. Phytopathology 2007, 97, 1625–1633. [Google Scholar] [CrossRef]
- Rodrigues, M.G.; Fonseca, Á. Molecular Systematics of the Dimorphic Ascomycete Genus Taphrina. Int. J. Syst. Evol. Microbiol. 2003, 53, 607–616. [Google Scholar] [CrossRef]
- Svetaz, L.A.; Bustamante, C.A.; Goldy, C.; Rivero, N.; Müller, G.L.; Valentini, G.H.; Fernie, A.R.; Drincovich, M.F.; Lara, M.V. Unravelling early events in the Taphrina deformans–Prunus persica interaction: An insight into the differential responses in resistant and susceptible genotypes. Plant Cell Environ. 2017, 40, 1456–1473. [Google Scholar] [CrossRef]
- Tsai, I.J.; Tanaka, E.; Masuya, H.; Tanaka, R.; Hirooka, Y.; Endoh, R.; Sahashi, N.; Kikuchi, T. Comparative genomics of Taphrina fungi causing varying degrees of tumorous deformity in plants. Genome Biol. Evol. 2014, 6, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Giordani, E.; Padula, G.; Radice, S. Compared Anatomy of Young Leaves of Prunus persica (L.) Batsch with Different Degrees of Susceptibility to Taphrina deformans (Berk.) Tul. J. Phytopathol. 2013, 161, 190–196. [Google Scholar] [CrossRef]
- Bassi, M.; Conti, G.G.; Barbieri, N. Cell wall degradation by Taphrina deformans in host leaf cells: Ultrastructural and cytochemical observations. Mycopathologia 1984, 88, 115–125. [Google Scholar] [CrossRef]
- Doehlemann, G.; Wahl, R.; Horst, R.J.; Voll, L.M.; Usadel, B.; Poree, F.; Stitt, M.; Pons-Kühnemann, J.; Sonnewald, U.; Kahmann, R.; et al. Reprogramming a maize plant: Transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008, 56, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.M.; Zehr, E.I.; Biggs, A.R. Brown rot. In Compendium of Stone Fruit Diseases; American Phytopathological Society: Annapolis, MD, USA, 1995; pp. 7–10. [Google Scholar]
- Padula, G.; Bellini, E.; Giordani, E.; Ferri, A. Further investigations on the resistance to leaf curl (Taphrina deformans Berk. Tul.) of peach cultivars and F1 progenies. In Atti del VI Convegno Nazionale Sulla Peschicoltura Meridionale; Di Vaio, C., Damiano, C., Fideghelli, C., Eds.; Imago Media Editrice: Dragoni, Italy, 2008; pp. 113–119. [Google Scholar]
- Nibedita, C.; Jolly, B. Transcriptomics: A successful approach to unravel the molecular mechanism of plant-pathogen interaction in post-genomic era. Res. J. Biotechnol. 2017, 8, 90–100. [Google Scholar]
- Tyagi, P.; Singh, D.; Mathur, S.; Singh, A.; Ranjan, R. Upcoming progress of transcriptomics studies on plants: An overview. Front. Plant Sci. 2022, 13, 1030890. [Google Scholar] [CrossRef]
- Naidoo, S.; Visser, E.A.; Zwart, L.; du Toit, Y.; Bhadauria, V.; Shuey, L.S. Dual RNA-seq to elucidate the plant–pathogen duel. Curr. Issues Mol. Biol. 2018, 27, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Li, S.X.; Li, X.S.; Li, C.Y. Transcriptome studies with the third-generation sequencing technology. Life Sci. Instrum. 2018, 16, 114–121. [Google Scholar]
- Agudelo-Romero, P.; Erban, A.; Rego, C.; Carbonell-Bejerano, P.; Nascimento, T.; Sousa, L.; Martínez-Zapater, J.M.; Kopka, J.; Fortes, A.M. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 2015, 66, 1769–1785. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, X.; Shi, S.; Zhao, N.; Li, D.; Fang, P.; Chen, X.; Qi, W.; Zhang, Z. Comparative RNA-Seq analysis reveals a critical role for brassinosteroids in rose (Rosa hybrida) petal defense against Botrytis cinerea infection. BMC Genet. 2018, 19, 62. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, G.; He, Z.; Yan, M.; Zou, M.; Jiang, J. Transcriptome analysis of Actinidia chinensis in response to Botryosphaeria dothidea infection. PLoS ONE 2020, 15, e0227303. [Google Scholar] [CrossRef] [PubMed]
- Zambounis, A.; Ganopoulos, I.; Valasiadis, D.; Karapetsi, L.; Madesis, P. RNA sequencing-based transcriptome analysis of kiwifruit infected by Botrytis cinerea. Physiol. Mol. Plant Pathol. 2020, 111, 101514. [Google Scholar] [CrossRef]
- AbuQamar, S. Expansins: Cell wall remodeling proteins with a potential function in plant defense. J. Plant Biochem. Physiol. 2014, 2, 1000e118. [Google Scholar] [CrossRef]
- Yang, L.; Huang, W.; Xiong, F.; Xian, Z.; Su, D.; Ren, M.; Li, Z. Silencing of Sl PL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef]
- Lannoo, N.; Van Damme, E.J. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 108308. [Google Scholar] [CrossRef]
- Delteil, A.; Gobbato, E.; Cayrol, B.; Estevan, J.; Michel-Romiti, C.; Dievart, A.; Kroj, T.; Morel, J.B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016, 16, 17. [Google Scholar] [CrossRef]
- Haile, Z.M.; Guzman, N.D.; Grace, E.; Moretto, M.; Sonego, P.; Engelen, K.; Moser, C.; Baraldi, E. Transcriptome profiles of strawberry (Fragaria vesca) fruit interacting with Botrytis cinerea at different ripening stages. Front. Plant Sci. 2019, 10, 1131. [Google Scholar] [CrossRef]
- De Cremer, K.; Mathys, J.; Vos, C.; Froenicke, L.; Michelmore, R.W.; CAMMUE, B.P.A.; De Coninck, B. RNA seq-based transcriptome analysis of Lactuca sativa infected by the fungal necrotroph Botrytis cinerea. Plant Cell Environ. 2013, 36, 1992–2007. [Google Scholar] [CrossRef]
- Smith, J.E.; Mengesha, B.; Tang, H.; Mengiste, T.; Bluhm, B.H. Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genom. 2014, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M.; Khalid, N.; Islam, W.; Sanaullah, T.; Anwar, M.; Khan, S.; Ye, W.; Lou, Y. Zinc finger protein transcription factors: Integrated line of action for plant antimicrobial activity. Microb. Pathog. 2019, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Gain, H.; Mandal, A. MYB transcription factors in biotic stress tolerance. Plant Stress 2023, 10, 100252. [Google Scholar] [CrossRef]
- Vargas, W.A.; Martín, J.M.; Rech, G.E.; Rivera, L.P.; Benito, E.P.; Díaz-Mínguez, J.M.; Thon, M.R.; Sukno, S.A. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol. 2012, 158, 1342–1358. [Google Scholar] [CrossRef]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef]
- Giacomelli, L.; Zeilmaker, T.; Giovannini, O.; Salvagnin, U.; Masuero, D.; Franceschi, P.; Vrhovsek, U.; Scintilla, S.; Rouppe Van Der Voort, J.; Moser, C. Simultaneous editing of two DMR6 genes in grapevine results in reduced susceptibility to downy mildew. Front. Plant Sci. 2023, 14, 1242240. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.S.; Zhu, H.Y.; Bai, Y.B.; Liu, H.; Cheng, Z.M. RNA sequencing-based transcriptome analysis of mature strawberry fruit infected by necrotrophic fungal pathogen Botrytis cinerea. Physiol. Mol. Plant Pathol. 2018, 104, 77–85. [Google Scholar] [CrossRef]
- Lanver, D.; Berndt, P.; Tollot, M.; Naik, V.; Vranes, M.; Warmann, T.; Münch, K.; Rössel, N.; Kahmann, R. Plant surface cues prime Ustilago maydis for biotrophic development. PLoS Pathog. 2014, 10, e1004272. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Moreno-Rico, O.; Luna-Ruíz, J.J.; Sosa-Ramírez, J.; Moreno-Manzano, C.E. AISLAMIENTO E IDENTIFICACIÓN DE Taphrina caerulescens EN Quercus eduardii EN AGUASCALIENTES, MÉXICO. Agrociencia 2019, 53, 781–795. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine Homeostasis in Tomato Biotic/Abiotic Stress Cross-Tolerance. Gene 2020, 727, 144230. [Google Scholar] [CrossRef] [PubMed]
- Chatzistathis, T.; Fanourakis, D.; Aliniaeifard, S.; Kotsiras, A.; Delis, C.; Tsaniklidis, G. Leaf age-dependent effects of boron toxicity in two Cucumis melo varieties. Agronomy 2021, 11, 759. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- International Peach Genome Initiative; Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniatis, E.I.; Karamichali, I.; Stefanidou, E.; Boutsika, A.; Tsitsigiannis, D.I.; Paplomatas, E.; Madesis, P.; Zambounis, A. Insights into the Transcriptional Reprogramming of Peach Leaves Inoculated with Taphrina deformans. Plants 2024, 13, 861. https://doi.org/10.3390/plants13060861
Maniatis EI, Karamichali I, Stefanidou E, Boutsika A, Tsitsigiannis DI, Paplomatas E, Madesis P, Zambounis A. Insights into the Transcriptional Reprogramming of Peach Leaves Inoculated with Taphrina deformans. Plants. 2024; 13(6):861. https://doi.org/10.3390/plants13060861
Chicago/Turabian StyleManiatis, Elissaios I., Ioanna Karamichali, Eleni Stefanidou, Anastasia Boutsika, Dimitrios I. Tsitsigiannis, Epaminondas Paplomatas, Panagiotis Madesis, and Antonios Zambounis. 2024. "Insights into the Transcriptional Reprogramming of Peach Leaves Inoculated with Taphrina deformans" Plants 13, no. 6: 861. https://doi.org/10.3390/plants13060861




