Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variation
2.2. Trait Correlations
2.3. Principal Component Analysis
2.4. Classification of 46 Rice Genotypes for Salinity Tolerance
3. Discussion
3.1. Identification of Salinity-Tolerant Resources
3.2. Salinity Stress Responsive Mechanisms in Rice
3.3. Phenotyping Methodology
4. Materials and Methods
4.1. Plant Materials
4.2. Evaluation of Morphological and Physiological Traits for Salinity Tolerance
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kota, S.; Flowers, T.J. Salt tolerance in rice: Seedling and reproductive stage QTL mapping come of age. Theor. Appl. Genet. 2021, 134, 3495–3533. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D. Screening Rice for Salinity Tolerance; International Rice Research Institute: Manila, Philippines, 1997. [Google Scholar]
- Lin, H.X.; Zhu, M.Z.; Yano, M.; Gao, J.P.; Liang, Z.W.; Su, W.A.; Hu, X.H.; Ren, Z.H.; Chao, D.Y. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 2004, 108, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Chen, Z.; Newhnam, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Cuin, T.A.; Betts, S.A.; Chalmandrier, R.; Shabala, S. A root’s ability to retain K+ correlates with salt tolerance in wheat. J. Exp. Bot. 2008, 59, 2697–2706. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Shabala, L.; Zhou, M.; Meinke, H.; Venkataraman, G.; Chen, Z.; Zeng, F.; Zhao, Q. Tissue-Specific Regulation of Na+ and K+ Transporters Explains Genotypic Differences in Salinity Stress Tolerance in Rice. Front. Plant Sci. 2019, 10, 486909. [Google Scholar] [CrossRef] [PubMed]
- Solis, C.A.; Yong, M.-T.; Venkataraman, G.; Milham, P.; Zhou, M.; Shabala, L.; Holford, P.; Shabala, S.; Chen, Z.-H. Sodium sequestration confers salinity tolerance in an ancestral wild rice. Physiol. Plant. 2021, 172, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Rajakani, R.; Sellamuthu, G.; Ishikawa, T.; Ahmed, H.A.I.; Bharathan, S.; Kumari, K.; Shabala, L.; Zhou, M.; Chen, Z.-H.; Shabala, S.; et al. Reduced apoplastic barriers in tissues of shoot-proximal rhizomes of Oryza coarctata are associated with Na+ sequestration. J. Exp. Bot. 2021, 73, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt Tolerance in Rice: Focus on Mechanisms and Approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, H.; Meng, L.; Chen, J. Gene Mapping, Cloning and Association Analysis for Salt Tolerance in Rice. Int. J. Mol. Sci. 2021, 22, 11674. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shabala, S.; Niu, Y.; Chen, Z.-H.; Shabala, L.; Meinke, H.; Venkataraman, G.; Pareek, A.; Xu, J.; Zhou, M. Molecular mechanisms of salinity tolerance in rice. Crop J. 2021, 9, 506–520. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Han, S.; Van Handel, B.; Dong, L.; Li, X.; Xie, X. Whole genome sequencing and comparative transcriptome analysis of a novel seawater adapted, salt-resistant rice cultivar—Sea rice 86. BMC Genom. 2017, 18, 655. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, Y.; Zhou, J.L.; Wang, G.; Mo, Y.; Ling, Y.; Huang, Y.; Zhang, Y.; Hu, H.; Wang, Y. RL-WG26 mediated salt stress tolerance in rice seedlings: A new insight into molecular mechanisms. Plant Stress 2024, 11, 100306. [Google Scholar] [CrossRef]
- Gao, Q.; Yin, X.; Wang, F.; Zhang, C.; Xiao, F.; Wang, H.; Hu, S.; Liu, W.; Zhou, S.; Chen, L.; et al. Jacalin-related lectin 45 (OsJRL45) isolated from ‘sea rice 86′ enhances rice salt tolerance at the seedling and reproductive stages. BMC Plant Biol. 2023, 23, 553. [Google Scholar] [CrossRef]
- Yeo, A.R.; Flowers, T.J. Selection for physiological characters—Examples from breeding for salt tolerance. In Plants under Stress: Biochemistry, Physiology and Ecology and their Application to Plant Improvement; Jones, H.G., Jones, M.B., Flowers, T.J., Eds.; Society for Experimental Biology Seminar Series; Cambridge University Press: Cambridge, UK, 1989; pp. 217–234. [Google Scholar]
- Prusty, M.R.; Kim, S.-R.; Vinarao, R.; Entila, F.; Egdane, J.; Diaz, M.G.Q.; Jena, K.K. Newly identified wild rice accessions conferring high salt tolerance might use a tissue tolerance mechanism in leaf. Front. Plant Sci. 2018, 9, 325180. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Raghavan, C.; Zhou, B.; Oliva, R.; Choi, I.R.; Lacorte, V.; Jubay, M.L.; Cruz, C.V.; Gregorio, G.; Singh, R.K.; et al. Allele mining and enhanced genetic recombination for rice breeding. Rice 2015, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Zhou, M.; Su, N.; Wu, Q.; Ul-Haq, T.; Zhu, J.; Mancuso, S.; Azzarello, E.; Shabala, S. Root vacuolar Na+ sequestration but not exclusion from uptake correlates with barley salt tolerance. Plant J. 2019, 100, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Solis, C.A.; Yong, M.T.; Vinarao, R.; Jena, K.; Holford, P.; Shabala, L.; Zhou, M.; Shabala, S.; Chen, Z.-H. Back to the Wild: On a Quest for Donors Toward Salinity Tolerant Rice. Front. Plant Sci. 2020, 11, 524985. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yu, H.; Wang, B.; Li, J. Retrospective and perspective of rice breeding in China. J. Genet. Genom. 2018, 45, 603–612. [Google Scholar] [CrossRef] [PubMed]
- De Leon, T.B.; Linscombe, S.; Subudhi, P.K. Molecular dissection of seedling salinity tolerance in rice (Oryza sativa L.) using a high-density GBS-based SNP linkage map. Rice 2016, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Thomson, M.J.; De Ocampo, M.; Egdane, J.A.; Salam, M.A.; Shah-E-Alam, M.; Ismail, A.M. Assessing trait contribution and mapping novel QTL for salinity tolerance using the Bangladeshi rice landrace Capsule. Rice 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- De Leon, T.B.; Linscombe, S.; Gregorio, G.; Subudhi, P.K. Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 2015, 6, 145635. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Opportunity and challenges of phenotyping plant salt tolerance. Trends Plant Sci. 2023, 28, 552–566. [Google Scholar] [CrossRef]
- Wang, H.; Shabala, L.; Zhou, M.; Shabala, S. Hydrogen peroxide-Induced root Ca2+ and K+ fluxes correlate with salt tolerance in cereals: Towards the cell-based phenotyping. Int. J. Mol. Sci. 2018, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pan, T.; Allakhverdiev, S.I.; Yu, M.; Shabala, S. Crop Halophytism: An Environmentally Sustainable Solution for Global Food Security. Trends Plant Sci. 2020, 25, 630–634. [Google Scholar] [CrossRef] [PubMed]
- 3000 Rice Genomes Project. The 3000 rice genomes project. Gigascience 2014, 3, 7. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Y.; Chen, K.; Shen, C.; Zhao, X.; Shabala, S.; Shabala, L.; Meinke, H.; Venkataraman, G.; Chen, Z.-H.; et al. Identification of new QTL for salt tolerance from rice variety Pokkali. J. Agron. Crop Sci. 2020, 206, 202–213. [Google Scholar] [CrossRef]
- Naveed, S.A.; Zhang, F.; Zhang, J.; Zheng, T.-Q.; Meng, L.-J.; Pang, Y.-L.; Xu, J.-L.; Li, Z.-K. Identification of QTN and candidate genes for Salinity Tolerance at the Germination and Seedling Stages in Rice by Genome-Wide Association Analyses. Sci. Rep. 2018, 8, 6505. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Lê, S.; Mazet, J. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
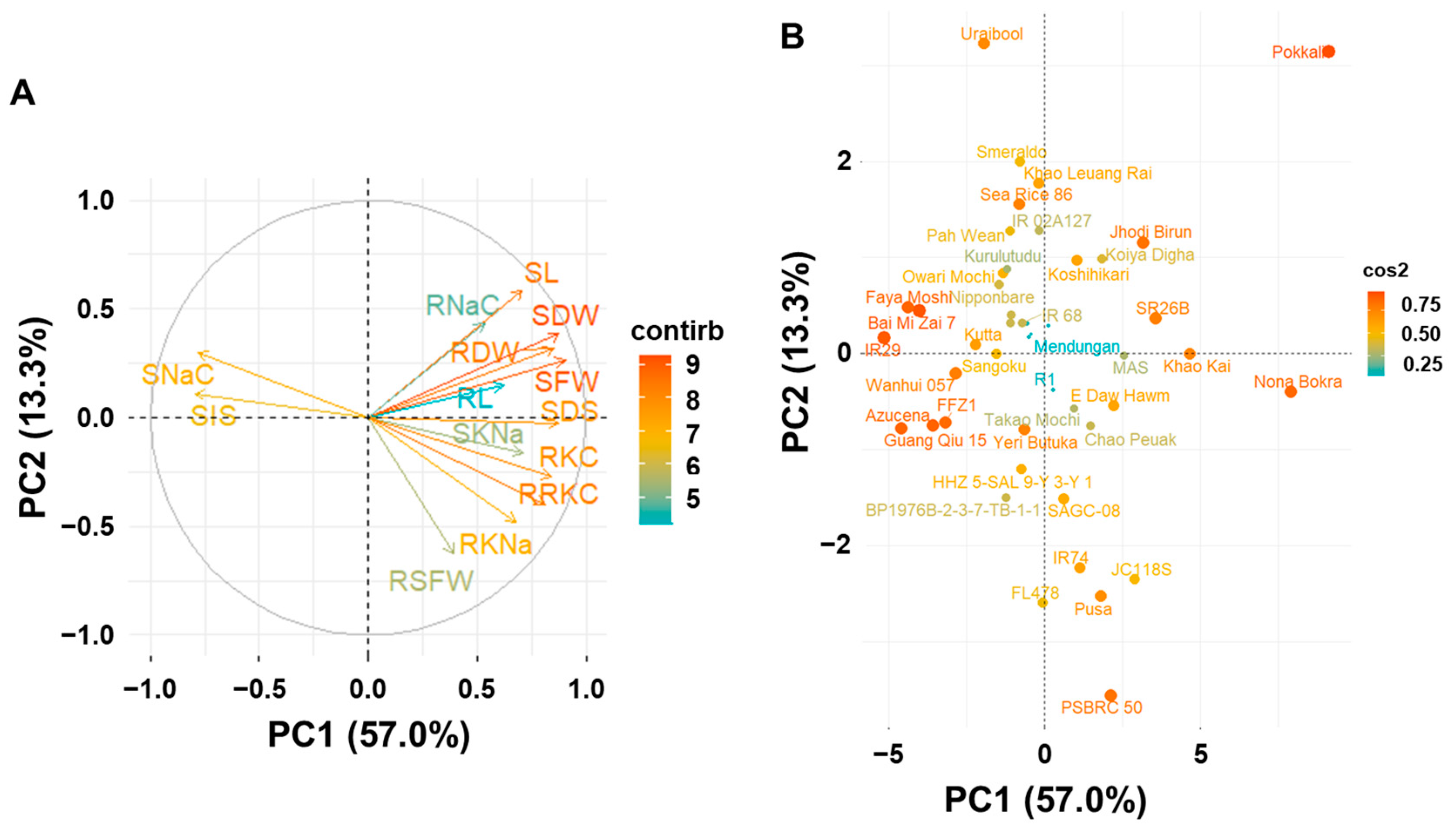
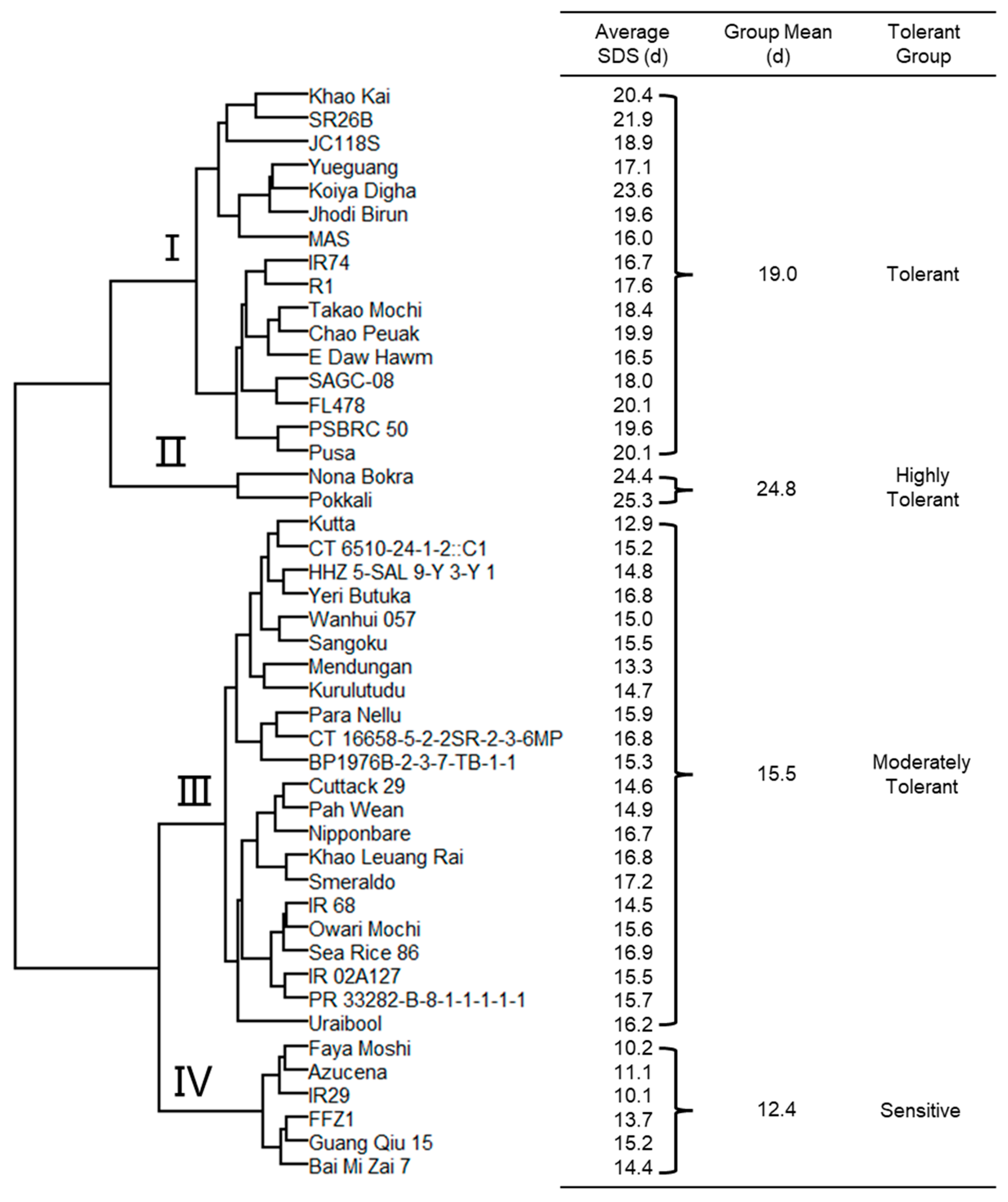
| Trial | Trait | Range | Mean ± SD |
|---|---|---|---|
| Control | SFW (mg) | 96.10–777.40 | 256.17 ± 129.96 |
| SDW (mg) | 15.83–107.40 | 38.43 ± 17.50 | |
| RDW (mg) | 4.23–16.93 | 7.65 ± 2.48 | |
| SL (cm) | 22.93–59.94 | 38.29 ± 9.47 | |
| RL (cm) | 5.29–15.57 | 8.87 ± 2.42 | |
| SKC (μmol mg−1) | 0.84–1.29 | 1.06 ± 0.10 | |
| RKC (μmol mg−1) | 0.35–0.72 | 0.51 ± 0.09 | |
| Salinity stress | SDS (d) | 10.08–25.30 | 16.73 ± 3.25 |
| SIS | 2.33–9.00 | 5.25 ± 1.92 | |
| SFW (mg) | 34.47–381.73 | 112.60 ± 62.32 | |
| SDW (mg) | 10.85–74.60 | 27.08 ± 10.95 | |
| RDW (mg) | 3.35–11.47 | 5.61 ± 1.57 | |
| SL (cm) | 12.84–43.71 | 24.93 ± 5.89 | |
| RL (cm) | 4.54–16.18 | 8.49 ± 2.17 | |
| SKC (μmol mg−1) | 0.28–0.71 | 0.56 ± 0.08 | |
| SNaC (μmol mg−1) | 0.70–2.44 | 1.74 ± 0.41 | |
| SKNa | 0.20–0.98 | 0.35 ± 0.14 | |
| RKC (μmol mg−1) | 0.04–0.19 | 0.10 ± 0.03 | |
| RNaC (μmol mg−1) | 0.48–0.87 | 0.64 ± 0.09 | |
| RKNa | 0.07–0.29 | 0.16 ± 0.05 | |
| Relative value | RSFW (%) | 23.45–76.72 | 44.98 ± 12.71 |
| RSDW (%) | 45.44–106.47 | 72.74 ± 14.88 | |
| RRDW (%) | 51.08–102.15 | 74.49 ± 13.81 | |
| RSL (%) | 50.72–86.18 | 65.70 ± 6.88 | |
| RRL (%) | 73.31–170.63 | 97.76 ± 20.38 | |
| RSKC (%) | 24.99–68.44 | 53.54 ± 9.36 | |
| RRKC (%) | 6.24–37.47 | 20.58 ± 6.49 |
| SDS | SIS | SFW | SDW | RDW | SL | RL | RSFW | RSDW | RRDW | RSL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIS | −0.87 *** | 1 | |||||||||
| SFW | 0.80 *** | −0.62 *** | 1 | ||||||||
| SDW | 0.76 *** | −0.59 *** | 0.98 *** | 1 | |||||||
| RDW | 0.65 *** | −0.54 *** | 0.90 *** | 0.91 *** | 1 | ||||||
| SL | 0.60 *** | −0.44 ** | 0.81 *** | 0.86 *** | 0.77 *** | 1 | |||||
| RL | 0.40 ** | −0.46 ** | 0.47 *** | 0.48 *** | 0.54 *** | 0.50 ** | 1 | ||||
| RSFW | 0.39 ** | −0.44 ** | 0.28 | 0.18 | 0.17 | −0.1 | 0.1 | 1 | |||
| RSDW | 0.17 | −0.23 | 0.05 | −0.02 | 0 | −0.29 * | −0.05 | 0.92 *** | 1 | ||
| RRDW | 0.12 | −0.15 | 0.10 | 0.03 | 0.12 | −0.23 | 0 | 0.83 *** | 0.85 *** | 1 | |
| RSL | 0.21 | −0.24 | 0.19 | 0.19 | 0.12 | 0.05 | −0.12 | 0.59 *** | 0.66 *** | 0.51 *** | 1 |
| RRL | 0.06 | −0.07 | 0.04 | −0.01 | 0.03 | −0.14 | 0.29 | 0.6 *** | 0.63 *** | 0.70 *** | 0.39 ** |
| SDS | SIS | SNaC | SKC | SKNa | RNaC | RKC | RKNa | RSKC | |
|---|---|---|---|---|---|---|---|---|---|
| SIS | −0.87 *** | 1 | |||||||
| SNaC | −0.66 *** | 0.61 *** | 1 | ||||||
| SKC | −0.03 | −0.11 | −0.09 | 1 | |||||
| SKNa | 0.59 *** | −0.43 ** | −0.81 *** | 0.53 *** | 1 | ||||
| RNaC | 0.46 ** | −0.45 ** | −0.31 * | 0 | 0.3 * | 1 | |||
| RKC | 0.69 *** | −0.66 *** | −0.63 *** | −0.14 | 0.51 *** | 0.40 ** | 1 | ||
| RKNa | 0.55 *** | −0.54 *** | −0.54 *** | −0.16 | 0.39 ** | 0.01 | 0.91 *** | 1 | |
| RSKC | −0.26 | 0.34 * | 0.04 | 0.82 *** | 0.34 * | −0.12 | −0.27 | −0.27 | 1 |
| RRKC | 0.65 *** | −0.58 *** | −0.68 *** | 0 | 0.62 *** | 0.30 * | 0.85 *** | 0.78 *** | −0.07 |
| Accession | Origin | Accession | Origin |
|---|---|---|---|
| Jhodi Birun | Bangladesh | Yueguang | Japan |
| Koiya Digha | Bangladesh | Takao Mochi | Japan |
| FFZ1 | China | Sangoku | Japan |
| SAGC-08 | China | Owari Mochi | Japan |
| R1 | China | Chao Peuak | Laos |
| JC118S | China | Yeri Butuka | Nigeria |
| Sea Rice 86 | China | Pusa | Pakistan |
| Wanhui 057 | China | PR 33282-B-8-1-1-1-1-1 | Philippines |
| Guang Qiu 15 | China | IR 02A127 | Philippines |
| Bai Mi Zai 7 | China | IR 68 | Philippines |
| CT 16658-5-2-2SR-2-3-6MP | Colombia | HHZ 5-SAL 9-Y 3-Y 1 | Philippines |
| CT 6510-24-1-2::C1 | Colombia | IR 29 | Philippines |
| Nona Bokra | India | FL478 | Philippines |
| Cuttack 29 | India | Azucena | Philippines |
| SR26B | India | IR 74 | Philippines |
| Para Nellu | India | PSBRC 50 | Philippines |
| Uraibool | India | POKKALI | Sri Lanka |
| Kutta | India | Kurulutudu | Sri Lanka |
| BP1976B-2-3-7-TB-1-1 | Indonesia | Faya Moshi | Tanzania |
| MAS | Indonesia | Khao Kai | Thailand |
| Mendungan | Indonesia | Khao Leuang Rai | Thailand |
| Smeraldo | Italy | Pah Wean | Thailand |
| Nipponbare | Japan | E Daw Hawm | Thailand |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Niu, Y.; Yang, C.; Liang, Y.; Xu, J. Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism. Plants 2024, 13, 1036. https://doi.org/10.3390/plants13071036
Chen T, Niu Y, Yang C, Liang Y, Xu J. Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism. Plants. 2024; 13(7):1036. https://doi.org/10.3390/plants13071036
Chicago/Turabian StyleChen, Tianxiao, Yanan Niu, Changdeng Yang, Yan Liang, and Jianlong Xu. 2024. "Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism" Plants 13, no. 7: 1036. https://doi.org/10.3390/plants13071036
APA StyleChen, T., Niu, Y., Yang, C., Liang, Y., & Xu, J. (2024). Screening of Rice (Oryza sativa L.) Genotypes for Salinity Tolerance and Dissecting Determinants of Tolerance Mechanism. Plants, 13(7), 1036. https://doi.org/10.3390/plants13071036








