Carotenoid Accumulation in the Rhododendron chrysanthum Is Mediated by Abscisic Acid Production Driven by UV-B Stress
Abstract
1. Introduction
2. Results
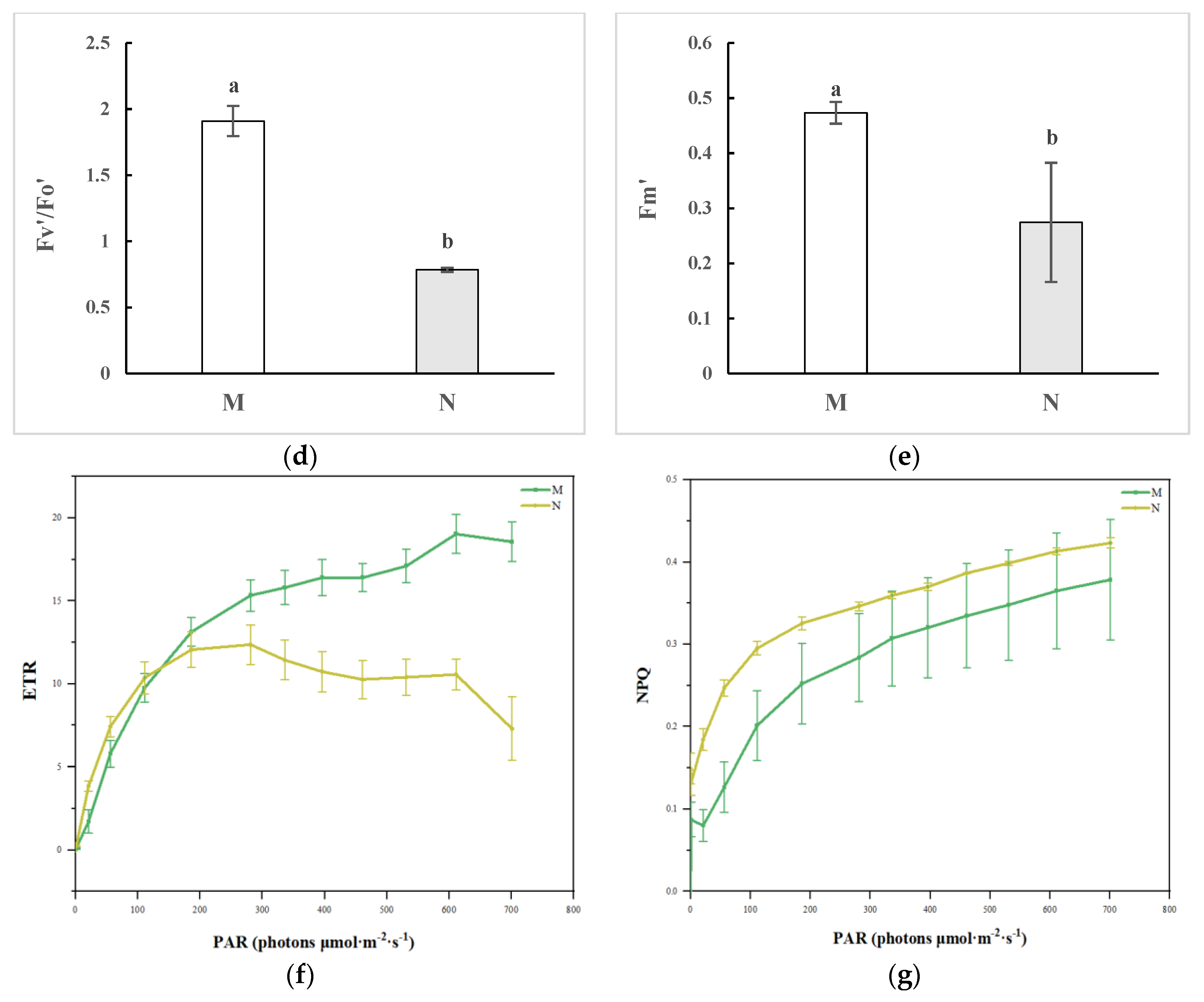
2.1. Chlorophyll Fluorescence Imaging and Alterations in the Kinetic Curve in R. chrysanthum Exposed to UV-B Radiation
2.2. R. chrysanthum’s Carotenoid Concentration and Its Relationship with ABA
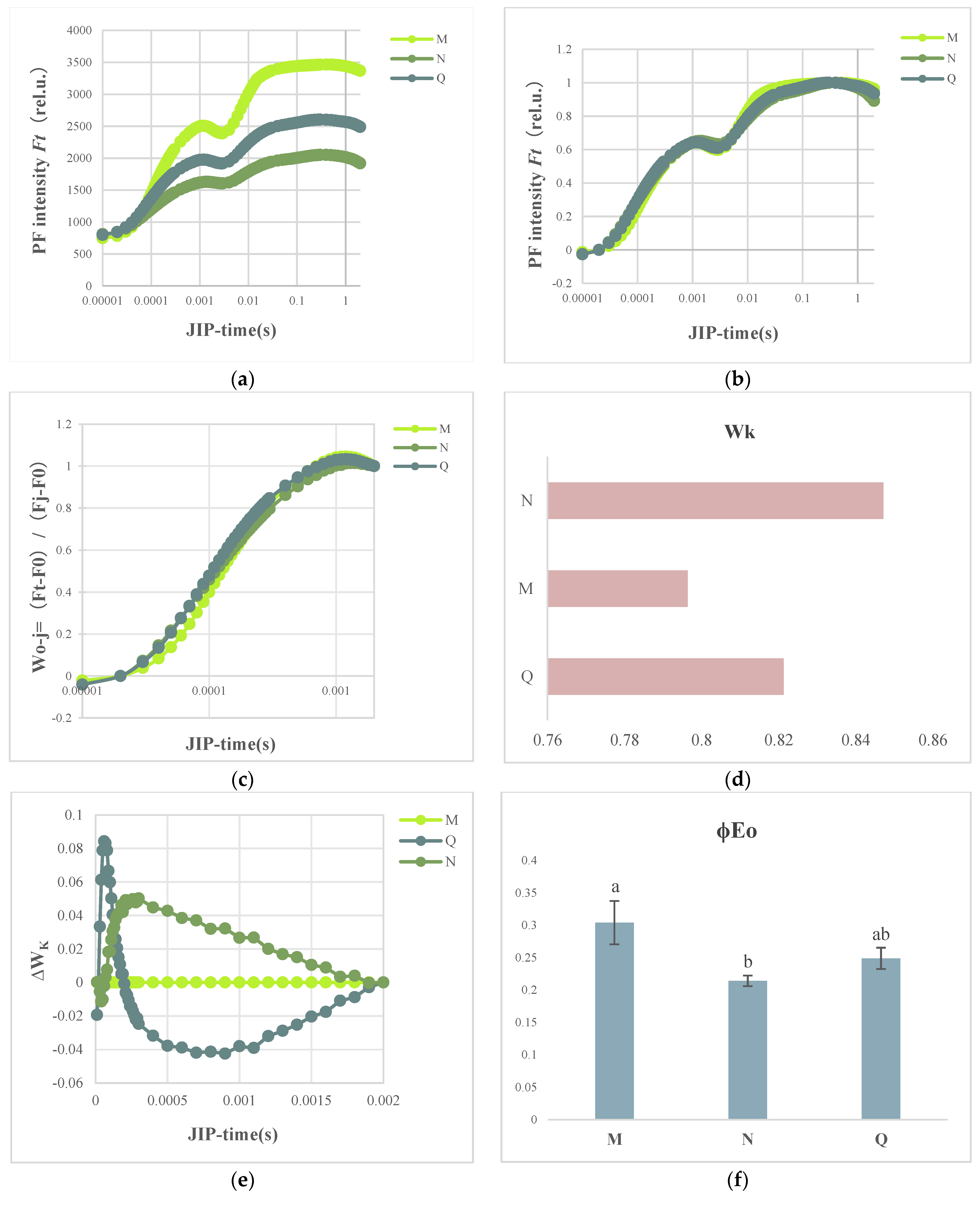
2.3. OJIP Transient Curve Modifications following External ABA Application
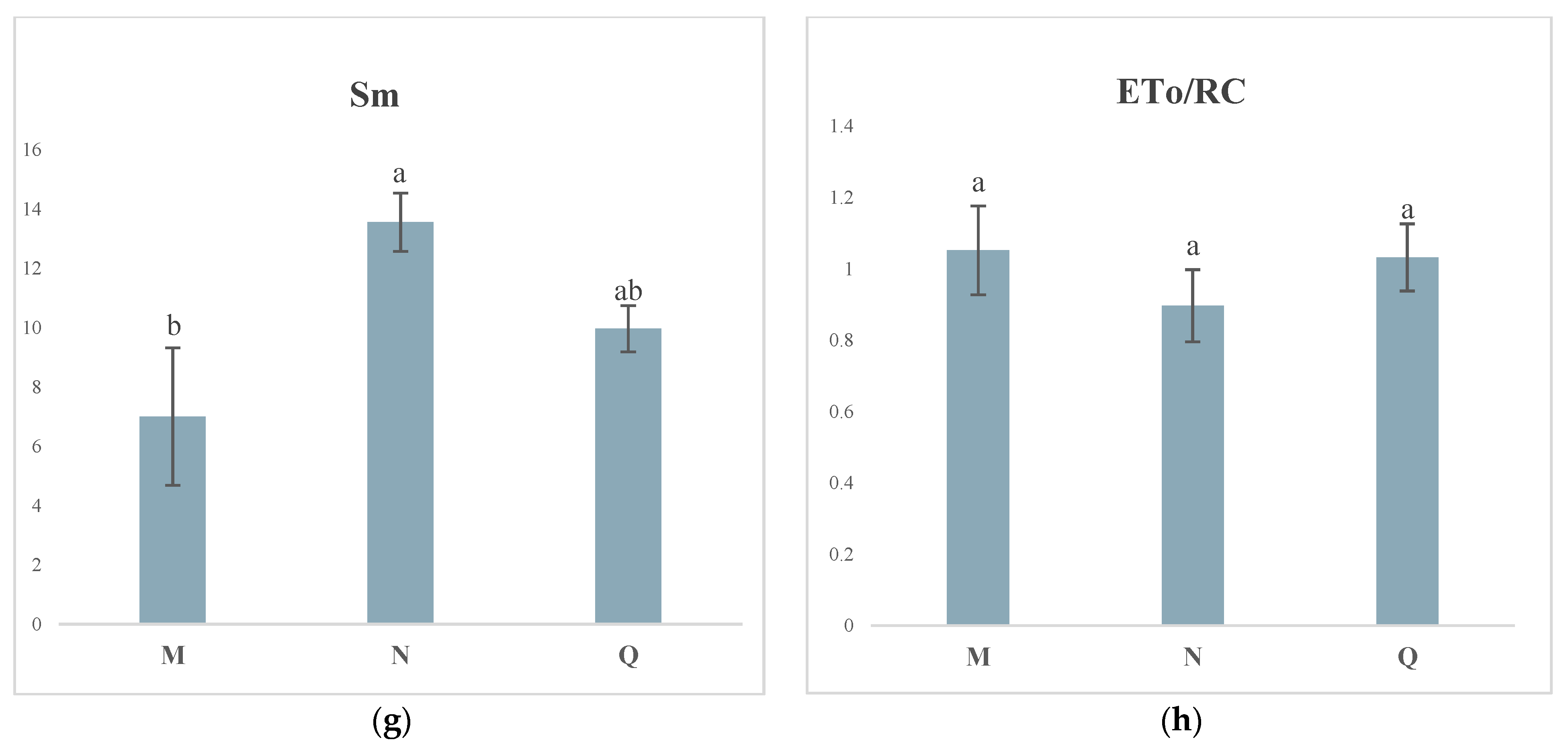
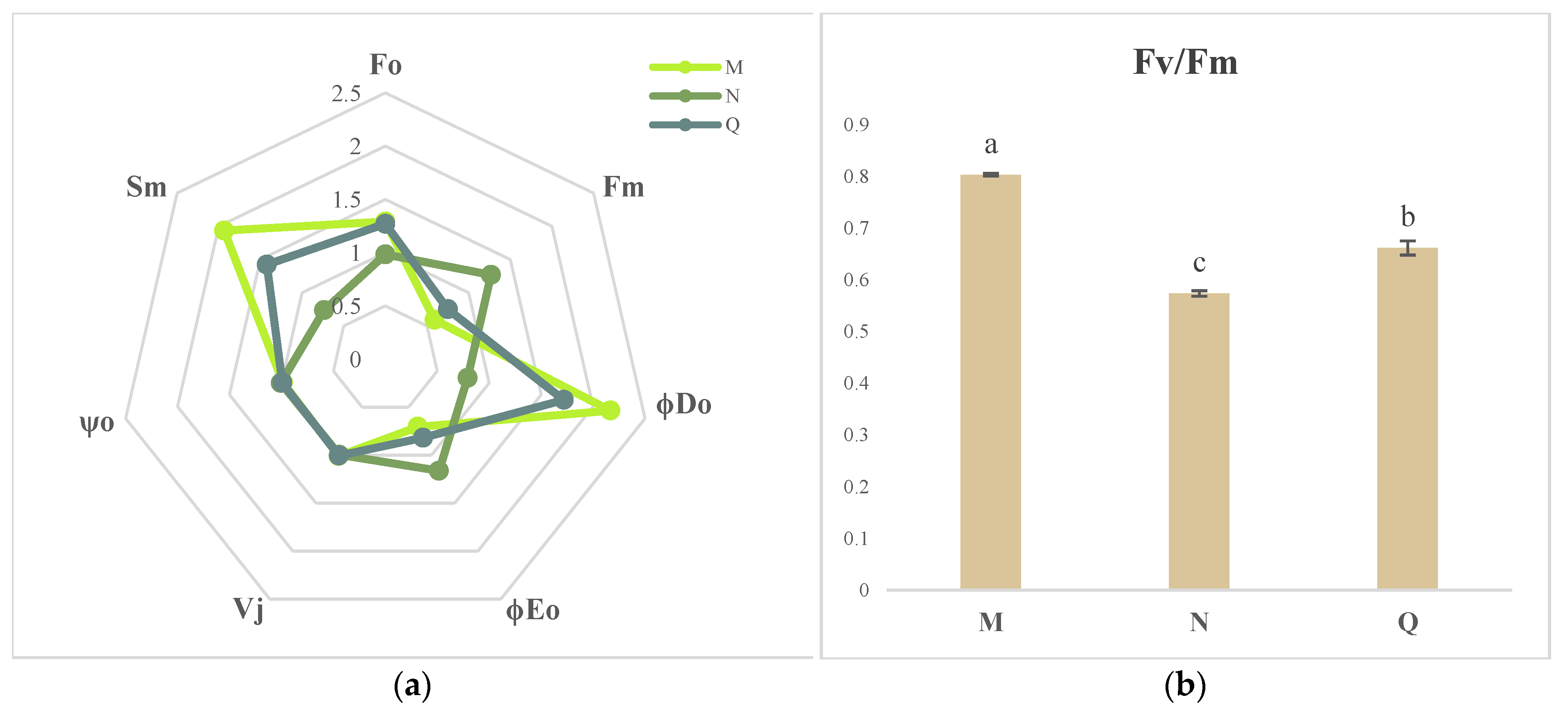
2.4. JIP Test Parameters
2.5. Finding the Main Pathways for the Differential Metabolite Enrichment of R. chrysanthum under UV-B Stress versus UV-B Stress When ABA Is Applied Externally
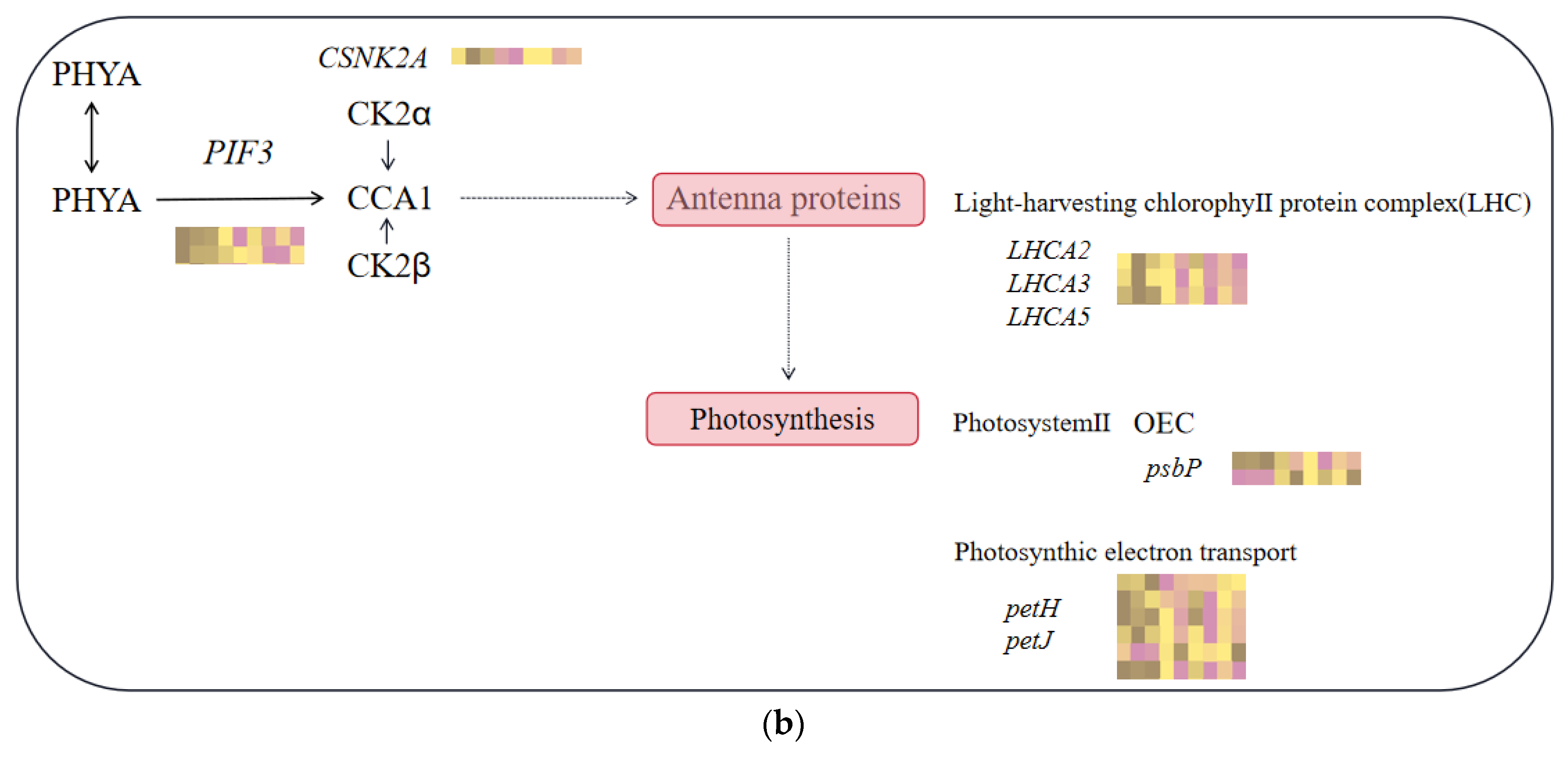
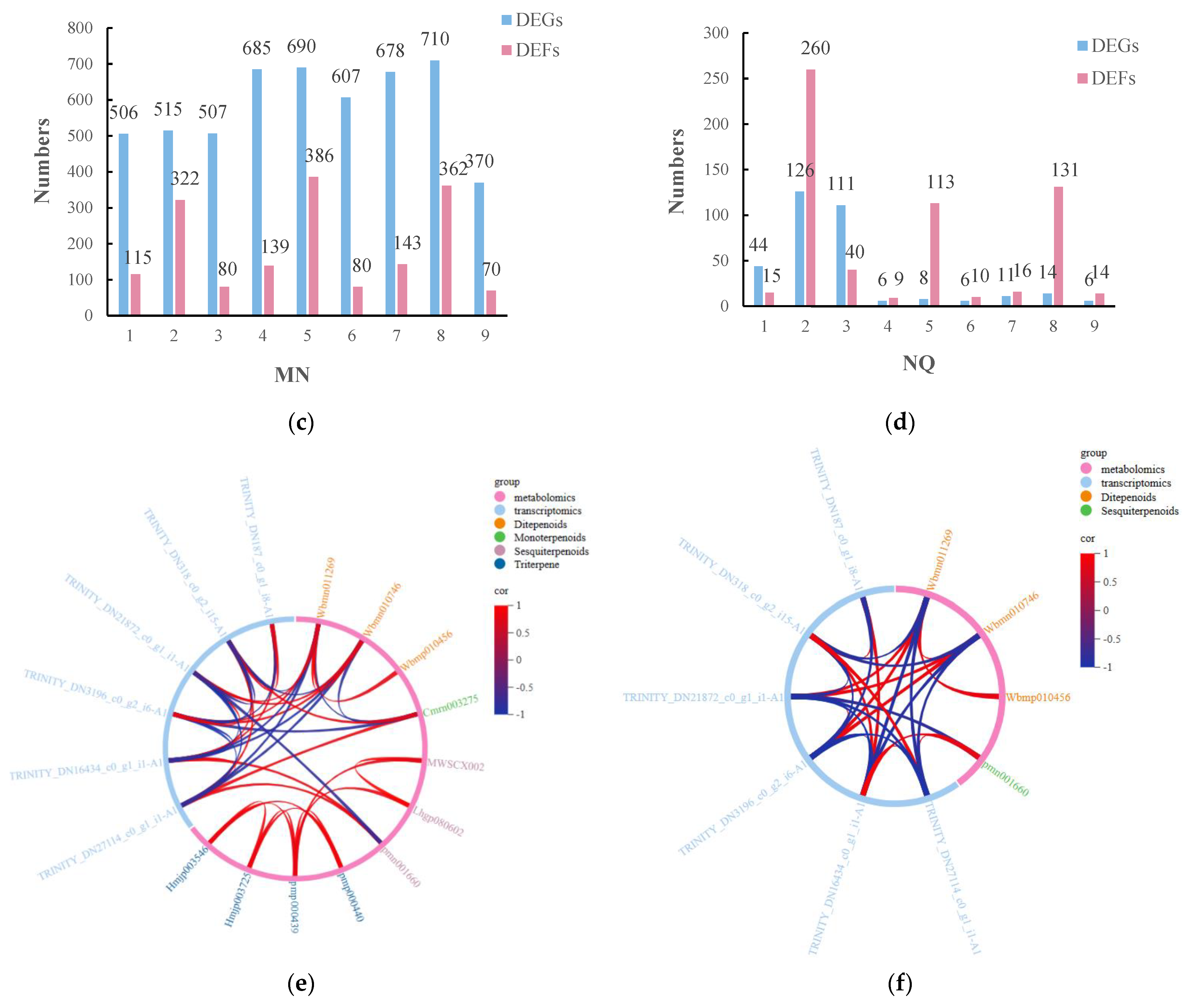
2.6. Differential Genes’ KEGG Enrichment Analysis and Critical Transcription Factor Family Searches
2.7. The Use of ABA Therapy Exogenously Encourages the Accumulation of Carotenoids
2.8. Analysis of DMs and DEGs Combined under UV-B Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growing Conditions, and Treatments
4.2. Chlorophyll and Carotenoid Content Determination
4.3. OJIP Transient Measurement and Rapid Fluorescence Induction Kinetics Analysis
4.4. Establishing Parameters for Chlorophyll Fluorescence
4.5. UPLC-MS/MS Investigation of R. chrysanthum Metabolites
4.6. Building cDNA Libraries and Analyzing Transcriptomics Data
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramzan, T.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Saman, R.U.; Lili, N.; Irshad, M.; Maqsood, S.; Haider, A.; Shahzad, B.; et al. Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Biochem. 2023, 201, 107828. [Google Scholar] [CrossRef]
- Lidon, F.C.; Ramalho, J.C. Impact of UV-B irradiation on photosynthetic performance and chloroplast membrane components in Oryza sativa L. J. Photochem. Photobiol. B 2011, 104, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Schmidt, É.C.; Felix, M.R.d.L.; Polo, L.K.; Rover, T.; Kreusch, M.; Pereira, D.T.; Chow, F.; Ramlov, F.; Maraschin, M.; et al. Effects of Ultraviolet Radiation (UVA+UVB) on Young Gametophytes of Gelidium floridanum: Growth Rate, Photosynthetic Pigments, Carotenoids, Photosynthetic Performance, and Ultrastructure. Photochem. Photobiol. 2014, 90, 1050–1060. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Q.; Cao, K.; Xu, H.; Zhou, X. Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells 2023, 12, 478. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Siefermann, D.; Yamamoto, H.Y. Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated chloroplasts: A transmembrane model for the violaxanthin cycle. Arch. Biochem. Biophys. 1975, 171, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhai, H.; Xue, L.; Zhao, N.; He, S.; Liu, Q. A lycopene β-cyclase gene, IbLCYB2, enhances carotenoid contents and abiotic stress tolerance in transgenic sweetpotato. Plant Sci. 2018, 272, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Li, T.; Teng, R.-M.; Han, M.-H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, M.; Cao, K.; Xu, H.; Zhou, X. UV-B Irradiation to Amino Acids and Carbohydrate Metabolism in Rhododendron chrysanthum Leaves by Coupling Deep Transcriptome and Metabolome Analysis. Plants 2022, 11, 2730. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Cao, K.; Xu, H.; Zhou, X. Transcriptome and proteome depth analysis indicate ABA, MAPK cascade and Ca2+ signaling co-regulate cold tolerance in Rhododendron chrysanthum Pall. Front. Plant Sci. 2023, 14, 1146663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Rodrigues, A.; Pizzio, G.A.; Rodriguez, P.L. Selective Inhibition of Clade A Phosphatases Type 2C by PYR/PYL/RCAR Abscisic Acid Receptors. Plant Physiol. 2012, 158, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Takahashi, Y.; Scavo, A.; Brandt, B.; Nguyen, D.; Rieu, P.; Schroeder, J.I. Abscisic acid-induced degradation of Arabidopsis guanine nucleotide exchange factor requires calcium-dependent protein kinases. Proc. Natl. Acad. Sci. USA 2018, 115, E4522–E4531. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.N.; Schroeder, D.F.; Stasolla, C. Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci. 2012, 188–189, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated Analysis of the Effects of Cold and Dehydration on Rice Metabolites, Phytohormones, and Gene Transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.; Liu, H.; Cai, Z.; Lu, C.; Chen, Y. A novel ABA-insensitive mutant in Arabidopsis reveals molecular network of ABA-induced anthocyanin accumulation and abiotic stress tolerance. J. Plant Physiol. 2022, 278, 153810. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Forneck, A.; Schoedl, K.; Schuhmacher, R. Correlating physiological parameters with biomarkers for UV-B stress indicators in leaves of grapevine cultivars Pinot noir and Riesling. J. Agric. Sci. 2013, 151, 189–200. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, D.; Son, J.E. Spatial and Temporal Bioactive Compound Contents and Chlorophyll Fluorescence of Kale (Brassica oleracea L.) Under UV-B Exposure Near Harvest Time in Controlled Environments. Photochem. Photobiol. 2020, 96, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhang, B.; Hu, J.; Li, K.; Guo, X. Modulation of carotenoid biosynthesis in maize (Zea mays L.) seedlings by exogenous abscisic acid and salicylic acid under low temperature. Plant Cell Rep. 2023, 43, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, N.; Gao, X.; Ma, Q. Integrated metabolite profiling and transcriptome analysis reveals tissue-specific regulation of terpenoid biosynthesis in Artemisia argyi. Genomics 2022, 114, 110388. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, Y.; Li, J.; Zhou, Z.; Deng, X.; Wang, Z.; Wu, S.; Lin, L.; Huang, Y.; Zeng, W.; et al. High Light Intensity Triggered Abscisic Acid Biosynthesis Mediates Anthocyanin Accumulation in Young Leaves of Tea Plant (Camellia sinensis). Antioxidants 2023, 12, 392. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, C.; Liang, D.Y.; Liu, L.; Pandey, L.K.; Xu, H.W.; Zhou, X.F. Sensitivity of wild and domesticated Rhododendron chrysanthum to different light regime (UVA, UVB, and PAR). Photosynthetica 2019, 57, 841–849. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Plyusnina, T.Y.; Klruschev, S.S.; Riznichenko, G.Y.; Rubin, A.B. Analysis of Chlorophyll Fluorescence Transient by Spectral Multi-exponential Approximation. Biofizika 2015, 60, 487–495. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Erratum to: Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Strasser, R.J. Phenotyping of dark and light adapted barley plants by the fast chlorophyll a fluorescence rise OJIP. S. Afr. J. Bot. 2004, 70, 277–283. [Google Scholar] [CrossRef]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant 2012, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Silva Dalberto, D.; Garbin Martinazzo, E.; Antonio Bacarin, M. Chlorophyll a fluorescence reveals adaptation strategies in drought stress in Ricinus communis. Braz. J. Bot. 2017, 40, 861–870. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 2021, 347, 129005. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]







| Gene ID | Q-Value (M-vs.-N) | log2 (N/M) | Q-Value (N-vs.-Q) | log2 (Q/N) |
|---|---|---|---|---|
| TRINITY_DN10705_c0_g2_i1-A1 | 0.035302 | 1.195234 | 0.998232 | −0.007671 |
| TRINITY_DN12335_c0_g4_i1-A1 | 0.025145 | 2.002006 | 0.997827 | 0.684958 |
| TRINITY_DN154_c0_g5_i1-A1 | 0.023931 | −1.070521 | 0.997827 | −0.686958 |
| TRINITY_DN17958_c0_g2_i1-A1 | 0.037824 | −1.997859 | 0.997827 | −1.220038 |
| TRINITY_DN20110_c0_g1_i1-A1 | 0.024333 | 0.685827 | 0.997827 | −0.274792 |
| TRINITY_DN20522_c0_g1_i1-A1 | 0.000908 | −3.206042 | 0.997827 | −0.404955 |
| TRINITY_DN21509_c0_g1_i1-A1 | 0.009498 | −1.769117 | 0.127622 | −2.776231 |
| TRINITY_DN2213_c0_g1_i4-A1 | 0.010107 | −0.844769 | 0.997827 | −0.216735 |
| TRINITY_DN236_c0_g1_i2-A1 | 0.046394 | 0.944447 | 0.997827 | −0.442235 |
| TRINITY_DN236_c0_g1_i3-A1 | 0.001978 | 1.461774 | 0.997827 | −0.601384 |
| TRINITY_DN236_c0_g1_i6-A1 | 0.002181 | 1.409949 | 0.997827 | −0.364035 |
| TRINITY_DN2428_c0_g1_i1-A1 | 0.006753 | −0.980128 | 0.997827 | −0.313276 |
| TRINITY_DN249_c0_g1_i5-A1 | 0.000000 | −1.777728 | 0.997827 | 0.321110 |
| TRINITY_DN27405_c0_g1_i1-A1 | 0.000006 | −2.997685 | 0.997827 | 0.766613 |
| TRINITY_DN2967_c1_g1_i1-A1 | 0.004706 | 0.979329 | 0.413874 | 1.099604 |
| Parameters | Explanation |
|---|---|
| Parameters derived from OJIP transient data | |
| Fo = F20μs | Minimum fluorescence |
| Fk = F300μs | 300 μs instantaneous fluorescence |
| FJ = F2ms | 2 ms instantaneous fluorescence |
| Fi = F30ms | 30 ms instantaneous fluorescence |
| Fp = Fm | Maximum fluorescence |
| Fv/Fm = (Ft − Fo)/(Fm − Fo) | The PSII’s maximum photochemical efficiency |
| VJ = (FJ − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at the J-step |
| VI = (FI − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at the I-step |
| VK = (FK − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at the K-step |
| WK = (FK − Fo)/(FJ − Fo) | Ratio of variable fluorescence Fk to the amplitude FJ − Fo |
| Sm = (Area)/(Fm − Fo) | Normalized total complementary area |
| Flux ratios of PSII | |
| φEo = ETo/ABS = [1 − (Fo/Fm)] ψo | Quantum yield for electron transport (at t = Fo) |
| φDo = 1 − φpo = (Fo/Fm) | Quantum yield at t = Fo for energy dissipation |
| ψo = ETo/TRo = (1 − VJ) | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA-(at t = Fo) |
| Activities per reaction center (RC) | |
| DIo/RC = ABS/RC − TRo/RC | Dissipated energy flux per RC (at t = Fo) |
| ETo/RC = Mo(1/VJ)/(1/VJ) | Electron transport flux per RC (at t = Fo) |
| Vitality indexes | |
| PIABS = RC/ABS[φpo/1 − φpo)][ψo/(1 − ψo)] | Performance index on absorption basis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, F.; Zhou, X.; Yu, W.; Xu, H.; Zhou, X. Carotenoid Accumulation in the Rhododendron chrysanthum Is Mediated by Abscisic Acid Production Driven by UV-B Stress. Plants 2024, 13, 1062. https://doi.org/10.3390/plants13081062
Gong F, Zhou X, Yu W, Xu H, Zhou X. Carotenoid Accumulation in the Rhododendron chrysanthum Is Mediated by Abscisic Acid Production Driven by UV-B Stress. Plants. 2024; 13(8):1062. https://doi.org/10.3390/plants13081062
Chicago/Turabian StyleGong, Fushuai, Xiangru Zhou, Wang Yu, Hongwei Xu, and Xiaofu Zhou. 2024. "Carotenoid Accumulation in the Rhododendron chrysanthum Is Mediated by Abscisic Acid Production Driven by UV-B Stress" Plants 13, no. 8: 1062. https://doi.org/10.3390/plants13081062
APA StyleGong, F., Zhou, X., Yu, W., Xu, H., & Zhou, X. (2024). Carotenoid Accumulation in the Rhododendron chrysanthum Is Mediated by Abscisic Acid Production Driven by UV-B Stress. Plants, 13(8), 1062. https://doi.org/10.3390/plants13081062






