Chitosan from Mushroom Improves Drought Stress Tolerance in Tomatoes
Abstract
1. Introduction
2. Results
2.1. Effect of Chitosan on Final Fruit Yield and Weight in Tomato Plants under Drought Stress
2.2. Effect of Chitosan on RWC, Proline, and Soluble Sugar Content of Tomato Leaves under Drought Stress
2.3. Effect of Chitosan on MDA, Total Chlorophyll, and Carotenoids Content in Tomato Leaves under Drought Stress
3. Discussion
3.1. Effect of Chitosan on RWC in Tomato Plants under Drought Stress
3.2. Effect of Chitosan on Proline Content in Tomato Plants under Drought Stress
3.3. Effect of Chitosan on Soluble Sugar Content in Tomato Plants under Drought Stress
3.4. Effect of Chitosan on Photosynthetic Pigment in Tomato Plants under Drought Stress
3.5. Effect of Chitosan on Malondialdehyde (MDA) in Tomato Plants under Drought Stress
3.6. Conclusions
4. Materials and Methods
4.1. Materials
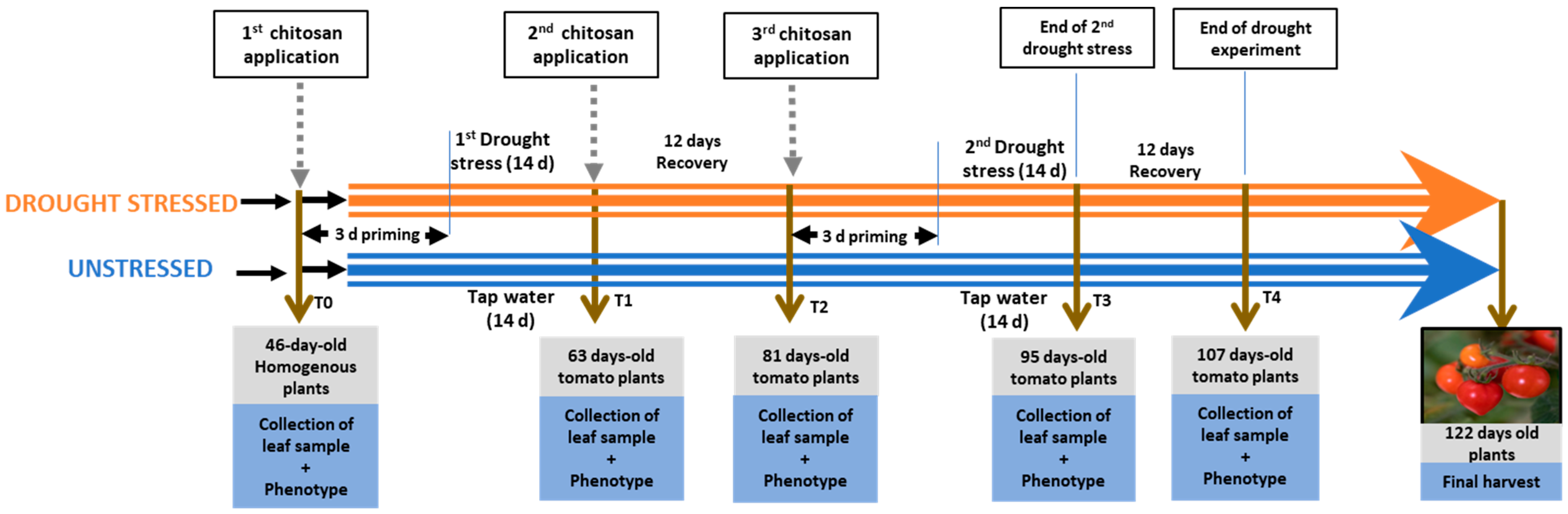
4.2. Experimental Design
4.3. Phenotypic Evaluation of Tomato Plants and Fruit Yield Assessment
4.4. RWC in Leaf Tissue
4.5. Chlorophyll and Carotenoids Content in Leaf Tissue
4.6. Proline Content in Leaf Tissue
4.7. Malondialdehyde Content (MDA) in Leaf Tissue
4.8. Sucrose Content
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Briscoe, J. Genetic Engineering and Water. Science 2001, 292, 2217. [Google Scholar] [CrossRef] [PubMed]
- Wilhite, D.A.; Glantz, M.H. Understanding: The Drought Phenomenon: The Role of Definitions. Water Int. 1985, 10, 111–120. [Google Scholar] [CrossRef]
- Wery, J.; Silim, S.N.; Knights, E.J.; Malhotra, R.S.; Cousin, R. Screening techniques and sources of tolerance to extremes of moisture and air temperature in cool season food legumes. Euphytica 1993, 73, 73–83. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.R.; Link, W.; Sarker, A. Screening Techniques and Sources of Resistance to Abiotic Stresses in Cool-Season Food Legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Siddique, M.; Hamid, A.; Islam, M. Drought stress effects the water relation of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The Jaz Family of Repressors Is the Missing Link in Jasmonate Signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum Nodosum Extract Biostimulants and Their Role in Enhancing Tolerance to Drought Stress in Tomato Plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- EBIC. Promoting the Biostimulant Industry and the Role of Plant Biostimulants in Making Agriculture More Sustainable; European Biostimulants Industry Council: Chatham-Kent, ON, Canada, 2012; Available online: http://www.biostimulants.eu/ (accessed on 30 January 2022).
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Weska, R.; Moura, J.; Batista, L.; Rizzi, J.; Pinto, L. Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Gohel, V.; Singh, A.; Vimal, M.; Ashwini, P.; Chhatpar, H.S. Bioprospecting and antifungal potential of chitinolytic microorganisms. Afr. J. Biotechnol. 2006, 5, 54–72. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of Transpiration through Foliar Application of Chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Interactive Effects of Drought Stress and Chitosan Application on Physiological Characteristics and Essential Oil Yield of Thymus Daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Hassnain, H.; Basit, A.; Alam, M.; Ahmad, I.; Ullah, I.; Alam, N.; Ullah, I.; Khalid, M.A.; Shair, M.; Ain, N.U. Efficacy of Chitosan on Performance of Tomato (Lycopersicon esculentum L.) Plant under Water Stress Condition. Pak. J. Agric. Res. 2020, 33, 1–191. [Google Scholar] [CrossRef]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Reis, C.; Karam, D.; Doriguetto, A.C.; Dos Santos, M.; Filho, P.R.D.S.S.; De Souza, T.C. The Foliar Application of a Mixture of Semisynthetic Chitosan Derivatives Induces Tolerance to Water Deficit in Maize, Improving the Antioxidant System and Increasing Photosynthesis and Grain Yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.; Deshpande, M. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Xue, J.-Y.; Fan, H.-Y.; Zeng, Z.; Zhou, Y.-H.; Hu, S.-Y.; Li, S.-X.; Cheng, Y.-J.; Meng, X.-R.; Chen, F.; Shao, Z.-Q.; et al. Comprehensive regulatory networks for tomato organ development based on the genome and RNAome of MicroTom tomato. Hortic. Res. 2023, 10, uhad14. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S. Biomass Energy and the Environmental Impacts Associated with Its Production and Utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The Effect of Drought Stress on the Leaf RWC and Tuber Yield of a Half-Sib Family of ‘Katahdin’-Derived Potato Cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, C.; Zhou, B. Drought Stress Induces Flowering and Enhances Carbohydrate Accumulation in Averrhoa Carambola. Hortic. Plant J. 2017, 3, 60–66. [Google Scholar] [CrossRef]
- Almeida, L.G.; Magalhães, P.C.; Karam, D.; da Silva, E.M.; Alvarenga, A.A. Chitosan application in the induction of water deficit tolerance in maize plants. Acta Sci. Agron. 2019, 42, e42463. [Google Scholar] [CrossRef]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P.S. Removal of Feedback Inhibition of Δ1-Pyrroline-5-Carboxylate Synthetase Results in Increased Proline Accumulation and Protection of Plants from Osmotic Stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef]
- Mahdavi, B.; Sanavy, S.A.M.M.; Aghaalikhani, M.; Sharifi, M.; Dolatabadian, A. Chitosan Improves Osmotic Potential Tolerance in Safflower (Carthamus tinctorius L.) Seedlings. J. Crop Improv. 2011, 25, 728–741. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic Pathways Regulated by Chitosan Contributing to Drought Resistance in White Clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Massacci, A.; Nabiev, S.; Pietrosanti, L.; Nematov, S.; Chernikova, T.; Thor, K.; Leipner, J. Response of the Photosynthetic Apparatus of Cotton (Gossypium hirsutum) to the Onset of Drought Stress under Field Conditions Studied by Gas-Exchange Analysis and Chlorophyll Fluorescence Imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.P.; Maury, P.; Sarrafi, A.; Grieu, P. QTL Analysis of Chlorophyll Fluorescence Parameters in Sunflower (Helianthus annuus L.) under Well-Watered and Water-Stressed Conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic Carbon Assimilation and Associated Metabolism in Relation to Water Deficits in Higher Plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Nassiri-Mahallati, M.; Azizi, G. Effect of Drought, Salinity, and Defoliation on Growth Characteristics of Some Medicinal Plants of Iran. J. Herbs Spices Med. Plants 2008, 14, 37–53. [Google Scholar] [CrossRef]
- Cornic, G.; Massacci, A. Leaf Photosynthesis under Drought Stress. Photosynth. Environ. 1996, 5, 347–366. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan—Comparision of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 2012, 5–20. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
| Treatments | Number of Fruits | Fruits Weight (g) |
|---|---|---|
| Drought (D) | ||
| Unstressed | 15.38 b | 35.58 b |
| Stressed | 12.38 a | 22.99 a |
| Chitosan (C) | ||
| Control | 11.679 a | 29.57 |
| MC | 14.223 ab | 26.5 |
| CC | 15.732 b | 31.78 |
| D × C | ||
| Unstressed × Ctrl | 12.88 | 36.50 |
| Unstressed × MC | 14.57 | 29.69 |
| Unstressed × CC | 18.71 | 40.55 |
| Stressed × Ctrl | 10.50 | 22.64 |
| Stressed × MC | 13.88 | 23.31 |
| Stressed × CC | 12.75 | 23.01 |
| Statistical significance | ||
| Drought (D) | * | ** |
| Chitosan (C) | * | ns |
| D × C | ns | ns |
| Treatments | T1 | T2 | T3 | T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %RWC | Proline (mg/gDW) | Sucrose (mg/gDW) | %RWC | Proline (mg/gDW) | Sucrose (mg/gDW) | %RWC | Proline (mg/gDW) | Sucrose (mg/gDW) | %RWC | Proline (mg/gDW) | Sucrose (mg/gDW) | |
| Drought (D) | ||||||||||||
| Unstressed | 91.79 b | 2.08 a | 20.38 a | 88.9 | 0.95 a | 19.24 a | 89.65 a | 1.28 a | 17.64 a | 82.89 b | 0.34 a | 16.36 a |
| Stressed | 78.60 a | 10.72 b | 51.14 b | 90.06 | 4.4 b | 24.85 b | 61.45 b | 20.53 b | 41.08 b | 77.55 a | 4.99 b | 23.37 b |
| Chitosan (C) | ||||||||||||
| Control | 83.02 a | 5.56 a | 33.31 a | 88.69 | 3.3 b | 22.22 | 71.83 a | 10.24 a | 28.3 | 80.11 | 2.41 | 19.04 |
| MC | 84.26 a | 8.06 b | 37.53 b | 90.56 | 2.27 a | 20.60 | 79.69 b | 11.64 b | 30.34 | 79.76 | 2.79 | 20.71 |
| CC | 88.31 b | 5.58 a | 36.45 ab | 89.2 | 2.45 a | 23.33 | 75.13 a | 10.84 a | 29.44 | 80.79 | 2.81 | 19.85 |
| D × C | ||||||||||||
| Unstressed × Ctrl | 90.99 | 1.62 a | 18.89 | 88.99 | 0.7 a | 18.51 | 89.62 c | 1.22 | 18.72 | 83.00 | 0.40 a | 15.97 a |
| Unstressed × MC | 91.26 | 2.4 b | 20.52 | 89.83 | 0.7 a | 18.66 | 89.46 c | 1.39 | 16.92 | 82.02 | 0.12 a | 12.94 a |
| Unstressed × CC | 93.12 | 2.22 ab | 21.73 | 87.89 | 1.35 a | 20.55 | 89.86 c | 1.22 | 17.2 | 83.65 | 0.51 a | 20.18 b |
| Stressed × Ctrl | 75.05 a | 9.5 c | 47.73 | 88.39 | 5.90 c | 25.92 | 54.05 a | 19.26 | 37.89 | 77.22 | 4.42 b | 22.10 b |
| Stressed × MC | 77.27 a | 13.72 d | 54.53 | 91.29 | 3.76 b | 22.53 | 69.91 b | 21.89 | 43.76 | 77.49 | 5.46 c | 28.47 c |
| Stressed × CC | 83.50 b | 8.93 c | 51.17 | 90.51 | 3.55 b | 26.12 | 60.40 a | 20.46 | 41.60 | 77.93 | 5.11 bc | 19.52 b |
| Statistical significance | ||||||||||||
| Drought (D) | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | *** |
| Chitosan (C) | *** | *** | * | ns | *** | ns | *** | * | ns | ns | ns | ns |
| D × C | * | *** | ns | ns | *** | ns | *** | ns | ns | ns | ** | *** |
| Treatments | T1 | T2 | T3 | T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA (nmol/mgDW) | Chlorophyll (µg/DW) | Carotenoids (µg/DW) | MDA (nmol/mgDW) | Chlorophyll (µg/DW) | Carotenoids (µg/DW) | MDA (nmol/mgDW) | Chlorophyll (µg/DW) | Carotenoids(µg/DW) | MDA (nmol/mgDW) | Chlorophyll (µg/DW) | Carotenoids (µg/DW) | |
| Drought (D) | ||||||||||||
| Unstressed | 3.37 a | 30.38 b | 2.93 | 1.46 a | 35.86 b | 3.611 | 3.03 a | 28.84 b | 3.05 | 3.78 | 26.41 | 2.66 |
| Stressed | 4.39 b | 28.1 a | 3.06 | 2.09 b | 32.11 a | 3.47 | 3.62 b | 26.57 a | 3.11 | 4.05 | 26.89 | 2.66 |
| Chitosan (C) | ||||||||||||
| Control | 3.79 | 27.86 | 2.8 | 1.72 | 34.03 ab | 3.49 | 3.75 b | 25.61 a | 3.01 | 3.45 | 26.64 | 2.67 |
| MC | 3.96 | 30.24 | 3.12 | 1.8 | 32.13 a | 3.43 | 3.32 ab | 28.43 b | 3.15 | 4.04 | 26.12 | 2.57 |
| CC | 3.89 | 29.62 | 2.99 | 1.8 | 35.79 b | 3.70 | 2.91 a | 29.07 b | 3.08 | 4.25 | 27.19 | 2.74 |
| D × C | ||||||||||||
| Unstressed × Ctrl | 3.45 ab | 27.72 | 2.64 | 1.3 | 34.72 b | 3.38 ab | 3.29 | 28.46 b | 2.97 | 3.89 ab | 26.36 | 2.59 |
| Unstressed × MC | 3.7 ab | 31.73 | 3.06 | 1.53 | 37.2 b | 3.80 b | 2.91 | 29.2 b | 3.15 | 3.61 ab | 25.95 | 2.64 |
| Unstressed × CC | 2.95 a | 31.68 | 3.09 | 1.55 | 35.60 b | 3.65 b | 2.89 | 28.79 a | 3.03 | 3.83 ab | 26.91 | 2.74 |
| Stressed × Ctrl | 4.14 bc | 27.99 | 3.10 | 2.14 | 33.3 b | 3.6 b | 4.1 | 22.75 b | 3.04 | 3.01 a | 26.91 | 2.76 |
| Stressed × MC | 4.21 bc | 28.75 | 3.18 | 2.07 | 27.0 a | 3.0 a | 3.7 | 27.6 b | 3.14 | 4.4 ab | 26.3 | 2.49 |
| Stressed × CC | 4.82 c | 27.56 | 2.91 | 2.05 | 35.99 b | 3.7 b | 2.93 | 29.34 b | 3.13 | 4.67 b | 27.47 | 2.73 |
| Statistical significance | ||||||||||||
| Drought (D) | *** | * | Ns | *** | *** | ns | ** | * | ns | ns | ns | ns |
| Chitosan (C) | Ns | Ns | Ns | ns | * | ns | ** | * | ns | ns | ns | ns |
| D × C | * | Ns | Ns | ns | *** | *** | ns | * | ns | * | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demehin, O.; Attjioui, M.; Goñi, O.; O’Connell, S. Chitosan from Mushroom Improves Drought Stress Tolerance in Tomatoes. Plants 2024, 13, 1038. https://doi.org/10.3390/plants13071038
Demehin O, Attjioui M, Goñi O, O’Connell S. Chitosan from Mushroom Improves Drought Stress Tolerance in Tomatoes. Plants. 2024; 13(7):1038. https://doi.org/10.3390/plants13071038
Chicago/Turabian StyleDemehin, Olusoji, Maha Attjioui, Oscar Goñi, and Shane O’Connell. 2024. "Chitosan from Mushroom Improves Drought Stress Tolerance in Tomatoes" Plants 13, no. 7: 1038. https://doi.org/10.3390/plants13071038
APA StyleDemehin, O., Attjioui, M., Goñi, O., & O’Connell, S. (2024). Chitosan from Mushroom Improves Drought Stress Tolerance in Tomatoes. Plants, 13(7), 1038. https://doi.org/10.3390/plants13071038








