Metabolomic Profile and Functional State of Oat Plants (Avena sativa L.) Sown under Low-Temperature Conditions in the Cryolithozone
Abstract
:1. Introduction
2. Results
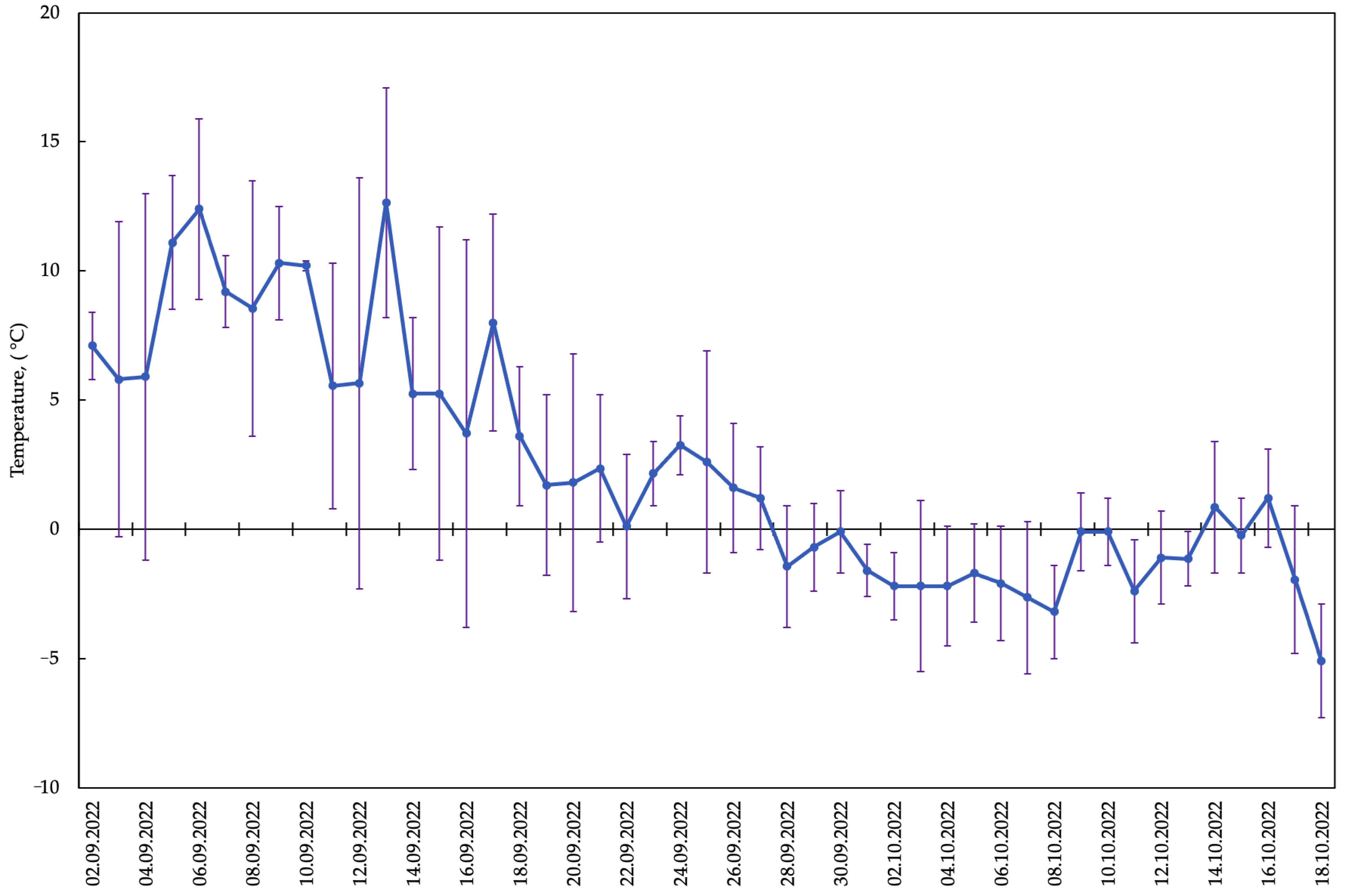
2.1. Periods of Phenological Phases of Development of Late Summer-Sown A. sativa
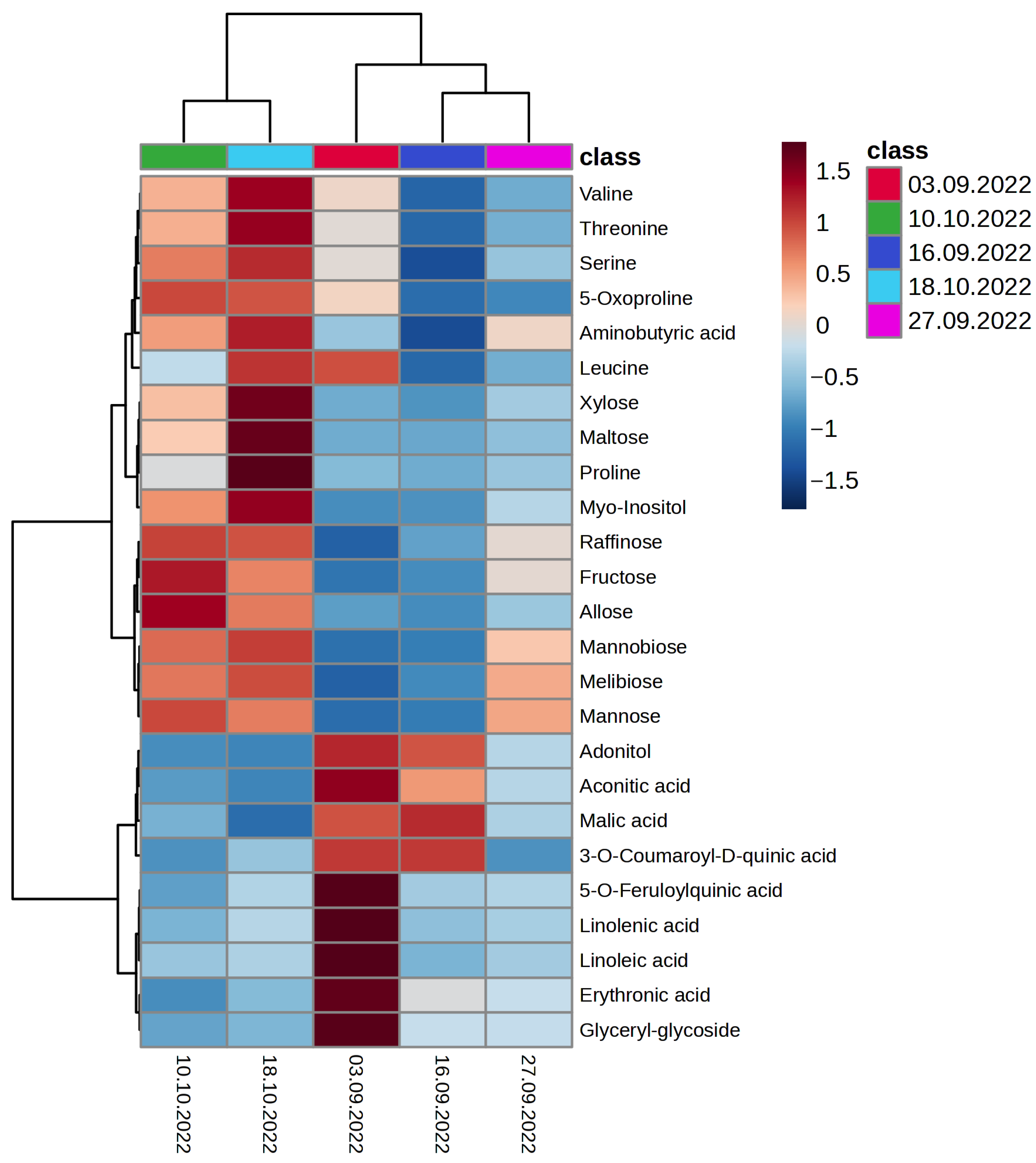
2.2. Metabolomic Changes in Oat Leaves during the Autumn Period with the Onset of Low Quenching Temperatures
2.2.1. Organic and Inorganic Acids
2.2.2. Amino Acids
2.2.3. Fatty Acids and Lipid Residues
2.2.4. Polyols
2.2.5. Monosaccharides
2.2.6. Disaccharides
2.2.7. Phenolic Compounds
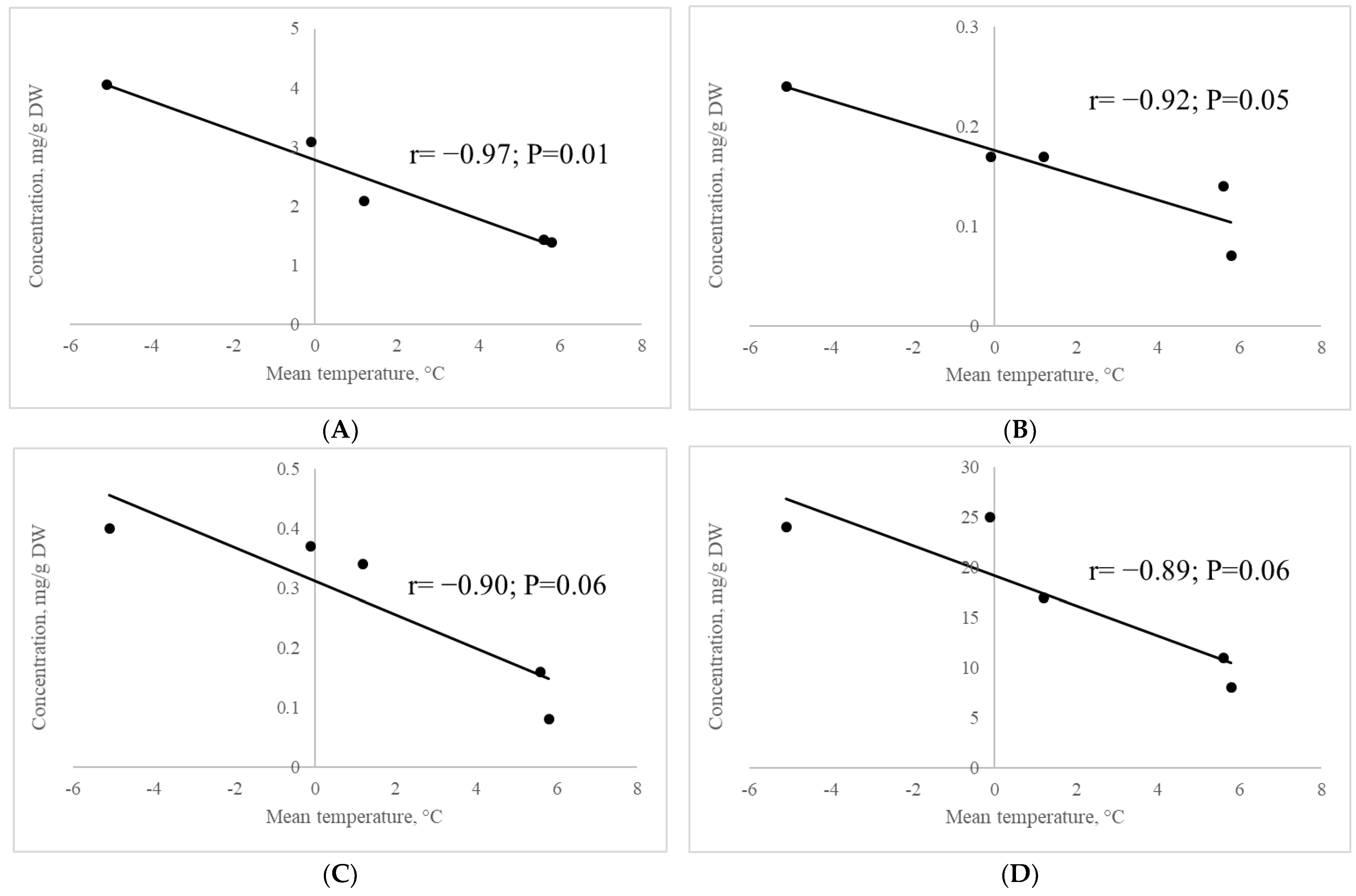
2.2.8. Correlation of the Contents of Metabolites Involved in Raffinose Synthesis with Mean Air Temperature
2.3. Chlorophyll a Fluorescence Parameters (JIP Test)
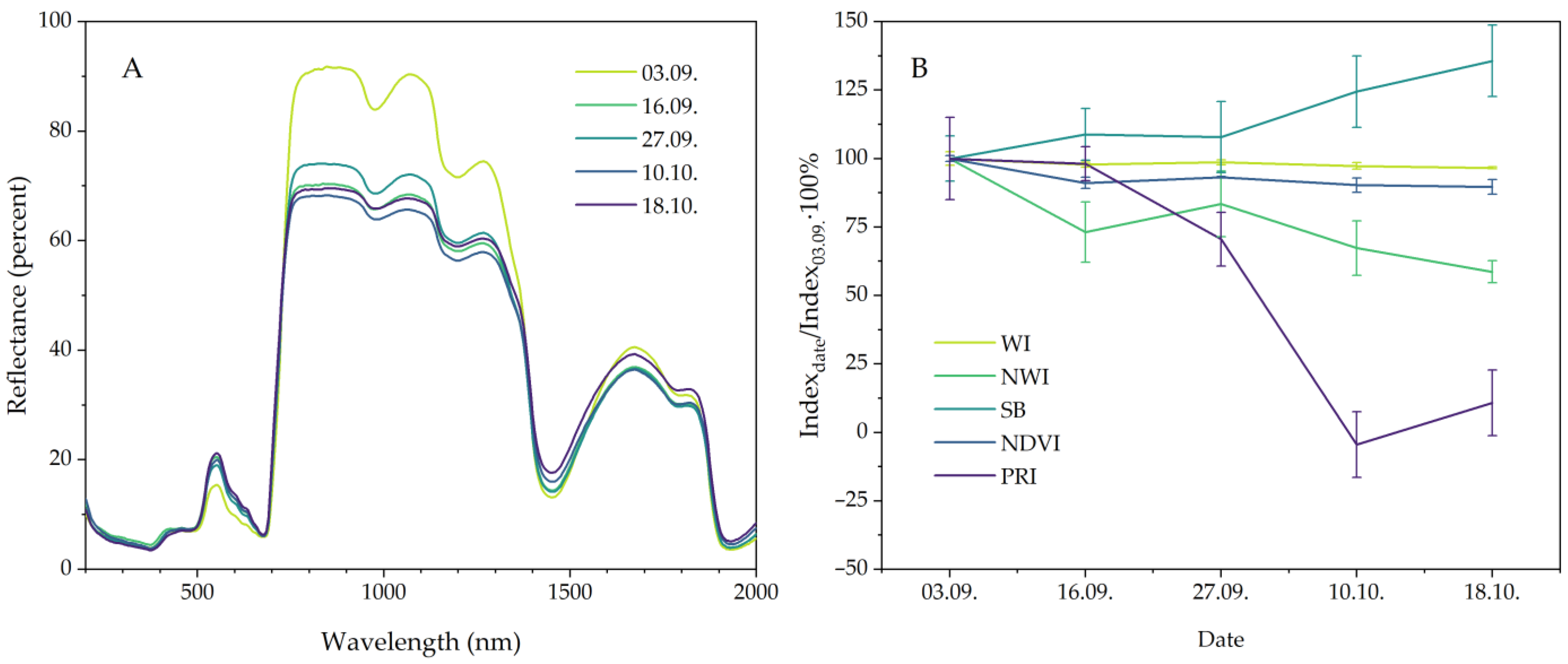
2.4. Leaf Reflectance and Reflectance Indices
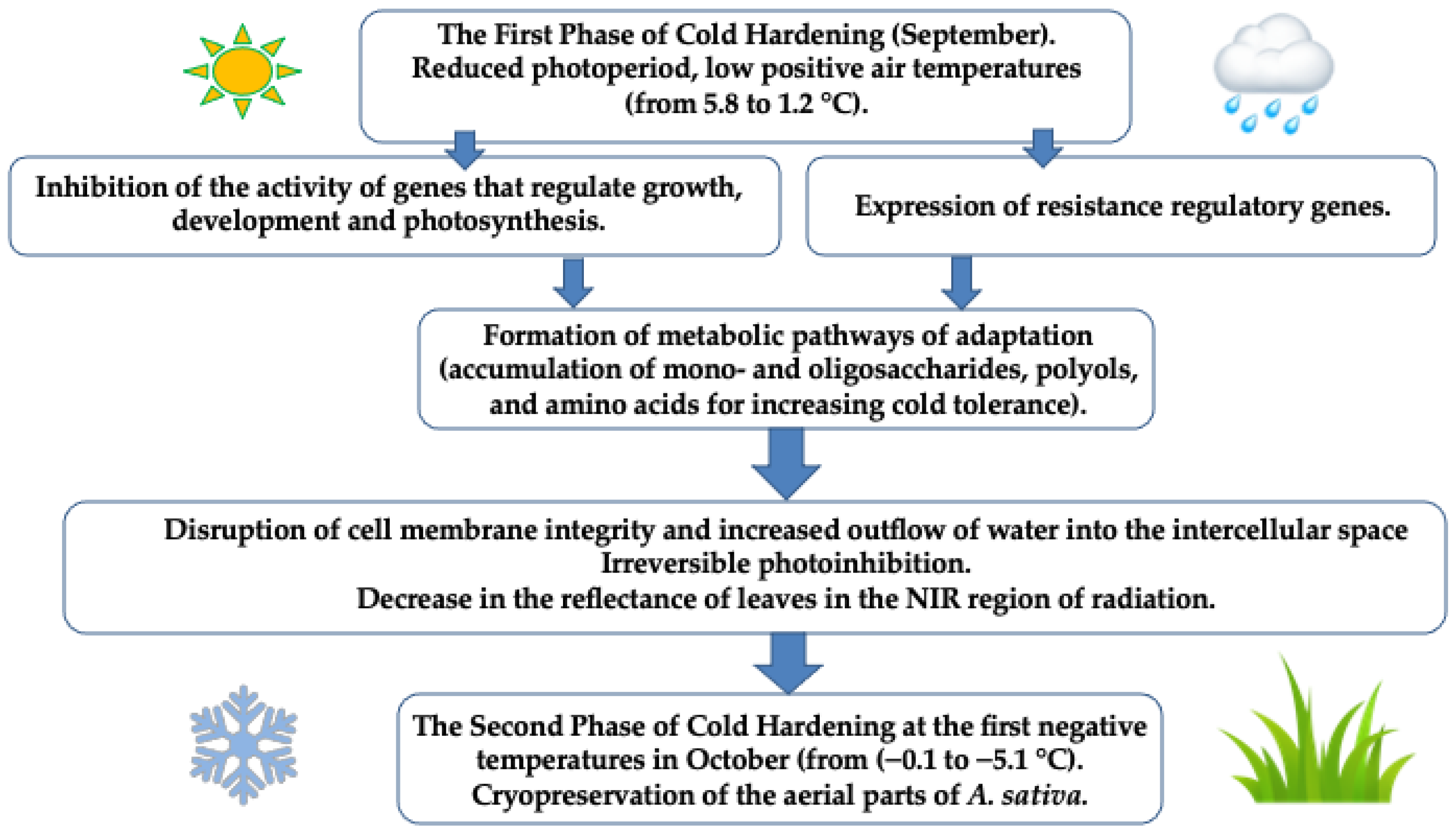
3. Discussion
4. Materials and Methods
4.1. Oat Variety Characteristics
4.2. Cultivation Conditions and Field Experiment
4.3. Sample Collection and Storage Prior to Analysis
4.4. Metabolomic Analysis of Leaves of Late Summer-Sown A. sativa
4.5. Measuring Chlorophyll a Fluorescence Parameters in Leaves
4.6. Measuring Leaf Reflectance and Computing Reflectance Indices
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loskutov, I.G.; Shelenga, T.V.; Konarev, A.V.; Shavarda, A.L.; Blinova, E.V.; Dzubenko, N.I. The metabolomic approach to the comparative analysis of wild and cultivated species of oats (Avena L.). Vavilov J. Genet. Breed. 2016, 20, 636–642. [Google Scholar] [CrossRef]
- Saini, P.; Gani, M.; Saini, P.; Bhat, J.A.; Francies, R.M.; Negi, N.; Chauhan, S.S. Molecular breeding for resistance to economically important diseases of fodder oat. In Disease Resistance in Crop Plants; Wani, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 199–239. [Google Scholar] [CrossRef]
- Pretorius, C.J.; Steenkamp, P.A.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomic Characterization of Discriminatory Metabolites Involved in Halo Blight Disease in Oat Cultivars Caused by Pseudomonas syringae pv. coronafaciens. Metabolites 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in whole grain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef]
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.A.; Sofronova, V.E.; Chepalov, V.A.; Perk, A.A.; Maksimov, T.C. Seasonal changes in the content of photosynthetic pigments in perennial grasses of cryolithic zone. Russ. J. Plant Physiol. 2010, 57, 181–188. [Google Scholar] [CrossRef]
- Selivanov, A.A.; Popov, V.N.; Antipina, O.V.; Pchelkin, V.P.; Tsydendambaev, V.P.; Tsydendambaev, V.D.; Moshkov, I.E. Changes in the content of fatty acid desaturases gene transcripts for Arabidopsis plants under adaptation to hypothermia. Russ. J. Plant Physiol. 2017, 64, 445–451. [Google Scholar] [CrossRef]
- Kidokoro, S.; Yoneda, K.; Takasaki, H.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Different Cold-Signaling Pathways Function in the Responses to Rapid and Gradual Decreases in Temperature. Plant Cell 2017, 29, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, C.; Yang, J.; Tian, Z.; Jiang, R.; Yang, F.; Jiao, K.; Qi, M.; Liu, L.; Zhang, B.; et al. Comprehensive Transcriptome Analysis of Responses during Cold Stress in Wheat (Triticum aestivum L.). Genes 2023, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, C.; Xiang, N.; Li, X.; Yang, S.; Du, J.C.; Yang, Y.; Yang, Y. Activation of secondary cell wall biosynthesis by miR319-targeted TCP4 transcription factor. Plant Biotechnol. J. 2017, 15, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Neuner, G.; Storey, K.B. Cold-loving microbes, plants, and animals-fundamental and applied aspects. Naturwissenschaften 2007, 94, 77–99. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Pieckenstain, F.; Escaray, F.; Erban, A.; Kraemer, U.; Udvardi, M.K.; Kopka, J. Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic preadaptation hypothesis. Plant Cell Environ. 2011, 34, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of chlorophyll a fluorescence transient. In Advances in Photosynthesis and Respiration; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 16, pp. 321–362. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance indices as a potential indirect selection criteria for wheat yield under irrigation. Crop Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Pradhan, S.; Sahoo, R.N.; Singh, R.; Gupta, V.K.; Joshi, D.K.; Sutradhar, A.K. Characterization of water stress and prediction of yield of wheat using spectral indices under varied water and nitrogen management practices. Agric. Water Manag. 2014, 146, 115–123. [Google Scholar] [CrossRef]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crops Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Duarte-Carvajalino, J.M.; Silva-Arero, E.A.; Góez-Vinasco, G.A.; Torres-Delgado, L.M.; Ocampo-Paez, O.D.; Castaño-Marín, A.M. Estimation of Water Stress in Potato Plants Using Hyperspectral Imagery and Machine Learning Algorithms. Horticulturae 2021, 7, 176. [Google Scholar] [CrossRef]
- Amatya, S.; Karkee, M.; Alva, A.K.; Larbi, P.; Adhikari, B. Hyperspectral Imaging for Detecting Water Stress in Potatoes; American Society of Agricultural and Biological Engineers: Dallas, TX, USA, 2012; p. 121345197. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.C.; Jha, S.K. Remote sensing and machine learning for crop water stress determination in various crops: A critical review. Precis. Agric. 2020, 21, 1121–1155. [Google Scholar] [CrossRef]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Kacira, M.; Bartzanas, T.; Kittas, C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Jones, C.L.; Weckler, P.R.; Maness, N.O.; Stone, M.L.; Jayasekara, R. Estimating Water Stress in Plants Using Hyperspectral Sensing; American Society of Agricultural and Biological Engineers: Dallas, TX, USA, 2004, p. 04 3065. [CrossRef]
- Wong, C.Y.; Gamon, J.A. Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytologist 2015, 206, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.A.; Perk, A.A.; Chepalov, V.A.; Sofronova, V.E.; Ilyin, A.N.; Ivanov, R.V. Eco-physiological and biochemical bases of the green cryo-feed forming in Yakutia. Agric. Biol. 2017, 52, 1129–1138. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shino, K. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiologia Plantarum 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Sofronova, V.E.; Chepalov, V.A.; Dymova, O.V.; Golovko, T.K. Functional Condition of Photosystem II in Leaves of Spring Oats during Autumnal Decrease in Temperature. Russ. J. Plant Physiol. 2020, 67, 661–670. [Google Scholar] [CrossRef]
- Dong, X.C.; Qian, T.F.; Chu, J.P.; Zhang, X.; Liu, Y.J.; Dai, X.L.; He, M.R. Late sowing enhances lodging resistance of wheat plant via improving biosynthesis and accumulation of lignin and cellulose. J. Integr. Agricult 2022, 73, 19–24. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Sofronova, V.E.; Petrov, K.A.; Chirikova, N.K. Vybor syr’ya dlya polucheniya lyuteinovogo koncentrata iz list’ev rastenij semejstva zlakovyh, zamorozhennyh estestvennym holodom kriolitozony [Selection of raw materials for obtaining lutein concentrate from leaves of plants of the сereal family frozen by natural cold of the cryolithozone]. Biotekhnologiya Biotechnol. 2022, 4, 96–106. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Z.; Tong, Z.; He, F.; Li, X. Metabolomic analyses reveal substances that contribute to the increased freezing tolerance of alfalfa (Medicago sativa L.) after continuous water deficit. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Thonke, B.; Popp, M. 1d-1-O-methyl-muco-inositol in Viscum album and members of the rhizophoraceae. Phytochemistry 1990, 29, 1785–1786. [Google Scholar] [CrossRef]
- Orthen, B.; Popp, M. Cyclitols as cryoprotectants for spinach and chickpea thylakoids. Environ Exp Bot 2000, 44, 125–132. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Ali, K.; Dahuja, A.; Tyagi, A. Role of phytosterols in drought stress tolerance in rice. Plant. Physiol. Biochem. 2015, 96, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Ivanova, T. Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium. Biology 2022, 11, 507. [Google Scholar] [CrossRef]
- Hu, W.H.; Hu, X.H.; Liu, C.; Wang, B.Q.; Yan, X.H. 24-epibrassinolide enhanced chilled tomato photosynthetic performance by stabilizing electron transport chain and function of photosystem II. Biol. Plant. 2022, 66, 178–187. [Google Scholar] [CrossRef]
- Fischer, C.; Höll, W. Food reserves of Scots pine (Pinus sylvestris L.). Trees 1991, 5, 187–195. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, L.; Chen, C.; Zhou, T.; Wu, Q.; Wen, F.; Chen, J.; Pritchard, H.W.; Peng, C.; Pei, J.; et al. Comparative changes in sugars and lipids show evidence of a critical node for regeneration in safflower seeds during aging. Front. Plant Sci. 2022, 13, 1020478. [Google Scholar] [CrossRef]
- Petrov, K.A.; Dudareva, L.V.; Nokhsorov, V.V.; Stoyanov, K.N.; Makhutova, O.N. Fatty Acid Content and Composition of the Yakutian Horses and Their Main Food Source: Living in Extreme Winter Conditions. Biomolecules 2020, 10, 315. [Google Scholar] [CrossRef]
- Sleptsov, I.V.; Mikhailov, V.V.; Rozhina, S.M.; Kershengolts, B.M. The year-round dynamic of metabolites accumulation in Pinus sylvestris needles in permafrost zone. Trees 2023, 37, 285–296. [Google Scholar] [CrossRef]


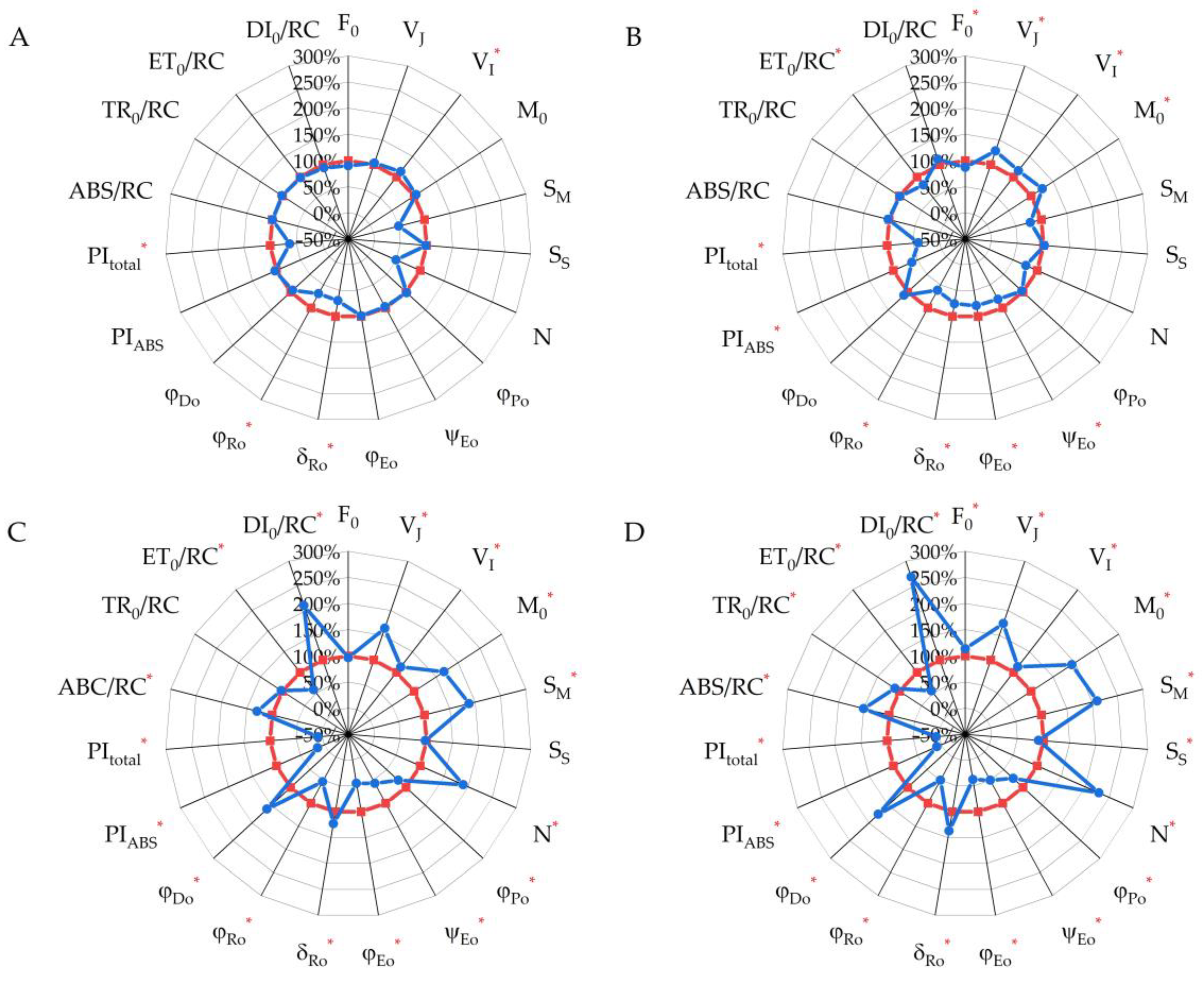
| Parameters/Indices | Formula | Definition |
|---|---|---|
| JIP-test parameters | ||
| F0 | F0 = F50µs | Minimal fluorescence, when all PS II RCs are open (at t = 0) |
| VJ | VJ = (FJ − F0)/(FM −F0) | Relative variable fluorescence at 3 ms, called the J phase |
| VI | VI = (FI − F0)/(FM − F0) | Relative variable fluorescence at 30 ms, called the I phase |
| M0 | M0 ≡ 4(F300µs − F50µs)/(FM − F0) | Initial slope of the fluorescence intensity at 50–300 µs |
| SM | SM = Area/(FM − F0) | Normalized total area above the O-J-I-P fluorescence transient, reflects the capacity of the electron acceptor pool |
| SS | SS = VJ/M0 | Single-turnover QA reduction events |
| N | N = SM/SS = SM M0 (1/VJ) | Turnover number: number of QA reduction events between time 0 and |
| φPo | φPo ≡ TR0/ABS = [1 − (F0/FM)] | Maximum quantum yield shows the efficiency of the primary charge separation processes at PS II RCs |
| ψEo | ψEo ≡ ET0/ABS = [1 − (F0/FM)](1 − VJ) | Efficiency/probability that an electron |
| φEo | φEo = ET0/ABS = [1 − (F0/FM)] ψEo | Quantum yield of electron transfer beyond the acceptor QA of PS II (ET) |
| δRo | δRo ≡ RE0/ET0 = (1 − VI)/(1 − VJ) | Probability of electron transfer to the acceptor side of PS I(RE) |
| φRo | φRo ≡ RE0/ABS = [1 − (F0/FM)] (1 − VI) | Quantum yield of electron transfer to the acceptor side of PS I(RE) |
| φDo | φDo = 1 − φPo | The fraction of dissipated energy of PS II |
| PIABS | Performance index reflects the conversion of energy photons absorbed by PS II to electron flow further from acceptor QA | |
| PItotal | * | Total performance index reflects the conversion of energy photons absorbed by PS II acceptor side of PS I |
| ABS/RC | ABS/RC = M0 (1/VJ) (l/φPo) | Absorption flux per active RC, reflects the relative size of the antenna |
| TR0/RC | TR0/RC = M0 (1/VJ) | Trapped energy flux of photons absorbed by PS II per active RC |
| ET0/RC | ET0/RC = M0 (1/VJ) ψEo | Electron transport flux further of acceptor QA per active RC |
| DI0/RC | DI0/RC = (ABS/RC) − (TR0/RC) | Dissipated energy flux per active RC |
| Reflectance indices | ||
| Water index (WI) | WI = R900/R970 | Index based on the water absorption band at 970 nm, reflecting the relative water content in plant leaves [15,16] |
| Normalized water index (NWI) | NWI = (R970 − R900)/(R970 + R900) | Normalized index based on the water absorption band at 970 nm, reflecting the relative water content in plant leaves [17,18,19] |
| Normalized difference vegetation index (NDVI) | NDVI = (R860 − R640)/(R860 + R640) | The index, which is an indicator of nitrogen content and biomass accumulation, also shows the water content in leaf tissues [20,21] |
| Photo-chemical reflectance index (PRI) | PRI = (R530 − R570)/(R530 + R570) | Index related to the influence of ecophysiological factors on the photosynthetic efficiency of plants [22,23] |
| Single band (SB) | SB = R1450/100 | Single band at 1450 nm related to water absorption [24,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokhsorov, V.V.; Protopopov, F.F.; Sleptsov, I.V.; Petrova, L.V.; Petrov, K.A. Metabolomic Profile and Functional State of Oat Plants (Avena sativa L.) Sown under Low-Temperature Conditions in the Cryolithozone. Plants 2024, 13, 1076. https://doi.org/10.3390/plants13081076
Nokhsorov VV, Protopopov FF, Sleptsov IV, Petrova LV, Petrov KA. Metabolomic Profile and Functional State of Oat Plants (Avena sativa L.) Sown under Low-Temperature Conditions in the Cryolithozone. Plants. 2024; 13(8):1076. https://doi.org/10.3390/plants13081076
Chicago/Turabian StyleNokhsorov, Vasiliy V., Fedor F. Protopopov, Igor V. Sleptsov, Lidia V. Petrova, and Klim A. Petrov. 2024. "Metabolomic Profile and Functional State of Oat Plants (Avena sativa L.) Sown under Low-Temperature Conditions in the Cryolithozone" Plants 13, no. 8: 1076. https://doi.org/10.3390/plants13081076








