Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix chinensis
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties in Phyllosphere and Rhizosphere of T. chinensis under Salt Stress
2.2. Microbial Diversity in Phyllosphere and Rhizosphere of T. chinensis under Salt Stress
2.3. Microbial Community Structure in Phyllosphere and Rhizosphere of T. chinensis under Salt Stress
2.4. Microbial Co-Occurrence Network in Phyllosphere and Rhizosphere of T. chinensis under Salt Stress
2.5. Microbial Functional Groups in Phyllosphere and Rhizosphere of T. chinensis under Salt Stress
3. Discussion
3.1. Rhizosphere Microorganisms Respond to Salt Stress through Niche Competition and Adaptation Strategies
3.2. Salt Secretion Influences Microbial Occupation of the Phyllosphere Niche
3.3. The Functional Groups of Microorganisms in the Phyllosphere and Rhizosphere of T. chinensis Were Closely Related to Environmental Changes
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Leaf Physiological Indexes and Soil Physicochemical Properties
4.3. Microbial DNA Extraction and PCR Amplification
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar]
- Zucca, C.; Middleton, N.; Kang, U.; Liniger, H. Shrinking water bodies as hotspots of sand and dust storms: The role of land degradation and sustainable soil and water management. Catena 2021, 207, 105669. [Google Scholar] [CrossRef]
- Manousaki, E.; Galanaki, K.; Papadimitriou, L.; Kalogerakis, N. Metal phytoremediation by the halophyte Limoniastrum monopetalum (L.) Boiss: Two contrasting ecotypes. Int. J. Phytoremediat. 2014, 16, 755–769. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Li, T.; Liu, J.H.; Sun, J.K.; Zhang, P. Ecological stoichiometry, salt ions and homeostasis characteristics of different types of halophytes and soils. Front. Plant Sci. 2022, 13, 990246. [Google Scholar] [CrossRef] [PubMed]
- McRose, D.L.; Li, J.; Newman, D.K. The chemical ecology of coumarins and phenazines affects iron acquisition by pseudomonads. Proc. Natl. Acad. Sci. USA 2023, 120, e2217951120. [Google Scholar] [CrossRef] [PubMed]
- Marschmann, G.L.; Tang, J.Y.; Zhalnina, K.; Karaoz, U.; Cho, H.; Le, B.; Jennifer, P.R.; Brodie, E.L. Predictions of rhizosphere microbiome dynamics with a genome-informed and trait-based energy budget model. Nat. Microbiol. 2024, 9, 421–433. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Hu, J.P.; Song, N.P. Bacterial community demonstrates stronger network connectivity than fungal community in desert-grassland salt marsh. Sci. Total Environ. 2021, 798, 149118. [Google Scholar] [CrossRef]
- Li, H.; La, S.K.; Zhang, X.; Gao, L.H.; Tian, Y.Q. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.R.; Kratina, P.; Pereira, A.L.; Tamminen, M.; Thomas, M.K.; Narwani, A. The evolution of competitive ability for essential resources. Philos. Trans. R Soc. Lond. B Biol. Sci. 2020, 375, 20190247. [Google Scholar] [CrossRef]
- Zhao, K.F.; Song, J.; Feng, G.; Zhao, M.; Liu, J.P. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.Y.; Wang, B.S. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Guo, H.; Wang, S.M.; Zhao, B.; Zhang, J.L.; Ma, Q.; Yin, H.J.; Bao, A.K. The photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens in response to salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; War, A.F.; Rafiq, I.; Reshi, Z.A.; Rashid, I.; Shouche, Y.S. Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 2022, 254, 126888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Xiong, C.; Wei, Z.W.; Chen, Q.L.; Ma, B.; Zhou, S.Y.D.; Tan, J.Q.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.P.; Schlechter, R.O. Phyllosphere microbiology: At the interface between microbial individuals and the plant host. New Phytol. 2018, 218, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- O’Banion, B.S.; O’Neal, L.; Alexandre, G.; Lebeis, S.L. Bridging the gap between single-strain and community-level plant-microbe chemical interactions. MPMI 2020, 33, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Prasanna, R.; Ranjan, K.; Velmourougane, K.; Ramakrishnan, B. Nutrients and host attributes modulate the abundance and functional traits of phyllosphere microbiome in rice. Microbiol. Res. 2017, 204, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mechan Llontop, M.E.; Tian, L.; Sharma, P.; Heflin, L.; Bernal-Galeano, V.; Haak, D.C.; Clarke, C.R.; Vinatzer, B.A. Experimental evidence pointing to rain as a reservoir of tomato phyllosphere microbiota. Phytobiomes J. 2021, 5, 382–399. [Google Scholar] [CrossRef]
- Stone, B.W.G.; Jackson, C.R. Seasonal patterns contribute more towards phyllosphere bacterial community structure than short-term perturbations. Microb. Ecol. 2021, 81, 146–156. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.P.; Cipriano, M.A.P.; da Silva, M.J.; Patrício, F.R.A.; Freitas, S.D.S.; Carazzolle, M.F.; Mondego, J.M.C. Functional genomics analysis of a phyllospheric Pseudomonas spp with potential for biological control against coffee rust. BMC Microbiol. 2022, 22, 222. [Google Scholar] [CrossRef] [PubMed]
- Maignien, L.; DeForce, E.A.; Chafee, M.E.; Eren, A.M.; Simmons, S.L. Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. mBio 2014, 5, e00682-13. [Google Scholar] [CrossRef] [PubMed]
- Firrincieli, A.; Khorasani, M.; Frank, A.C.; Doty, S.L. Influences of climate on phyllosphere endophytic bacterial communities of wild poplar. Front. Plant Sci. 2020, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Burch, A.Y.; Elad, T.; Huse, S.M.; Lindow, S.E.; Post, A.F.; Belkin, S. Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamarix trees across the Sonoran Desert. Appl. Environ. Microb. 2012, 78, 6187–6193. [Google Scholar] [CrossRef]
- Qvit-Raz, N.; Finkel, O.M.; Al-Deeb, T.M.; Malkawi, H.I.; Hindiyeh, M.Y.; Jurkevitch, E.; Belkin, S. Biogeographical diversity of leaf-associated microbial communities from salt-secreting Tamarix trees of the Dead Sea region. Res. Microbiol. 2012, 163, 142–150. [Google Scholar] [CrossRef]
- Li, W.J.; Jiang, L.M.; Zhang, Y.; Teng, D.X.; Wang, H.F.; Wang, J.L.; Lv, G.H. Structure and driving factors of the soil microbial community associated with Alhagi sparsifolia in an arid desert. PLoS ONE 2021, 16, e0254065. [Google Scholar] [CrossRef]
- Zhang, W.; Bahadur, A.; Sajjad, W.; Wu, X.K.; Zhang, G.S.; Liu, G.X.; Chen, T. Seasonal variation in fungal community composition associated with Tamarix chinensis roots in the coastal saline soil of Bohai bay, China. Microb. Ecol. 2021, 82, 652–665. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.Y.; Liu, W.Y.; Ke, M.J.; Qu, Q.; Zhou, Z.G.; Lu, T.; Qian, H.F. Phyllosphere bacterial assemblage is affected by plant genotypes and growth stages. Microbiol. Res. 2021, 248, 126743. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Zhao, Y.L.; Wang, Y.C.; Liu, Z.Y.; Gao, C.Q. Comprehensive analysis of MYB gene family and their expressions under abiotic stresses and hormone treatments in Tamarix hispida. Front. Plant Sci. 2018, 9, 1303. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xie, Q.J.; Tang, F.F.; Wu, J.; Dong, W.F.; Wang, C.; Gao, C.Q. The ThSOS3 gene improves the salt tolerance of transgenic Tamarix hispida and Arabidopsis thaliana. Front. Plant Sci. 2021, 11, 597480. [Google Scholar] [CrossRef]
- Feng, Z.T.; Deng, Y.Q.; Zhang, S.C.; Liang, X.; Yuan, F.; Hao, J.L.; Zhang, J.C.; Sun, S.F.; Wang, B.S. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015, 238, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Q.; Kang, P.; Tan, M.; Hu, J.P.; Zhang, Y.Q.; Zhang, J.L.; Song, N.P.; Li, X.R. Root exudates and rhizosphere soil bacterial relationships of Nitraria tangutorum are linked to k-strategists bacterial community under salt stress. Front. Plant Sci. 2022, 13, 997292. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Williams, M.A. Osmolyte dynamics and microbial communities vary in response to osmotic more than matric water deficit gradients in two soils. Soil Biol. Biochem. 2014, 79, 14–24. [Google Scholar] [CrossRef]
- Shi, X.L.; Zhao, X.H.; Ren, J.Y.; Dong, J.L.; Zhang, H.; Dong, Q.Q.; Jiang, C.J.; Zhong, C.; Zhou, Y.F.; Yu, H.Q. Influence of peanut, sorghum, and soil salinity on microbial community composition in interspecific interaction zone. Front. Microbiol. 2021, 12, 678250. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Bao, A.K.; Chai, W.W.; Wang, W.Y.; Zhang, J.L.; Li, Y.X.; Wang, S.M. Transcriptomic analysis of the succulent xerophyte Zygophyllum xanthoxylum in response to salt treatment and osmotic stress. Plant Soil 2016, 402, 343–361. [Google Scholar] [CrossRef]
- Chen, L.N.; Zhao, Z.L.; Li, J.; Wang, H.M.; Guo, G.M.; Wu, W.B. Effects of muddy water irrigation with different sediment particle sizes and sediment concentrations on soil microbial communities in the Yellow River Basin of China. Agric. Water Manag. 2022, 270, 107750. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, Y.; Li, M.M.; Xu, Z.X.; Li, L. Ecological niche selection shapes the assembly and diversity of microbial communities in Casuarina equisetifolia L. Front. Plant Sci. 2022, 13, 988485. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef]
- Finkel, O.M.; Delmont, T.O.; Post, A.F.; Belkin, S. Metagenomic signatures of bacterial adaptation to life in the phyllosphere of a salt-secreting desert tree. Appl. Environ. Microb. 2016, 82, 2854–2861. [Google Scholar] [CrossRef]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Sophie, Z.B.; Richter, A. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol. Biochem. 2013, 60, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Hernández, M.; Dumont, M.G.; Rui, J.P.; Fernández Scavino, A.; Conrad, R. Soil bacterial community mediates the effect of plant material on methanogenic decomposition of soil organic matter. Soil Biol. Biochem. 2018, 116, 99–109. [Google Scholar] [CrossRef]
- Shan, S.P.; Wei, Z.W.; Cheng, W.; Du, D.X.; Zheng, D.F.; Ma, G.H. Biofertilizer based on halotolerant microorganisms promotes the growth of rice plants and alleviates the effects of saline stress. Front. Microbiol. 2023, 14, 1165631. [Google Scholar] [CrossRef]
- Hou, Y.L.; Wei, C.C.; Zeng, W.Z.; Hou, M.L.; Wang, Z.; Xu, G.Y.; Huang, J.S.; Ao, C. Application of rhizobacteria to improve microbial community structure and maize (Zea mays L.) growth in saline soil. Environ. Sci. Pollut. Res. 2024, 31, 2481–2494. [Google Scholar] [CrossRef]
- Brown, R.W.; Rhymes, J.M.; Jones, D.L. Saltwater intrusion induces shifts in soil microbial diversity and carbon use efficiency in a coastal grassland ecosystem. Soil Biol. Biochem. 2022, 170, 108700. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.C.; Wang, Z.W.; Zhu, K.F.; Wu, W.M. Comparative metagenomic analysis reveals rhizosphere microbial community composition and functions help protect grapevines against salt stress. Front. Microbiol. 2023, 14, 1102547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Xu, Z.C.; Liu, H.D.; Liu, Y.; Zhou, Y.N.; Meng, C.; Ma, S.Q.; Xie, Z.H.; Li, Y.Q.; Zhang, C.S. Patterns in the microbial community of salt-tolerant plants and the functional genes associated with salt stress alleviation. Microbiol. Spectr. 2021, 9, e0076721. [Google Scholar] [CrossRef]
- Abadi, V.; Sepehri, M.; Rahmani, H.A.; Dolatabad, H.K.; Shamshiripour, M.; Khatabi, B. Diversity and abundance of culturable nitrogen-fixing bacteria in the phyllosphere of maize. J. Appl. Microbiol. 2021, 131, 898–912. [Google Scholar] [CrossRef]
- Stanton, D.E.; Batterman, S.A.; Von Fischer, J.C.; Hedin, L.O. Rapid nitrogen fixation by canopy microbiome in tropical forest determined by both phosphorus and molybdenum. Ecology 2019, 100, e02795. [Google Scholar] [CrossRef]
- Chalk, P.M.; Lam, S.K.; Chen, D. The significance of endophytic and phyllospheric N2 fixation in forest trees: Evidence from stable (15N) and radioactive (13N) tracer studies. Trees 2022, 36, 1179–1184. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lu, L.; Huang, F.Y.; Zhang, Y.; Li, X.M.; Yang, L.H.; Usman, M.; Kappler, A.; Li, S. Hydrological perturbations facilitated phyllosphere denitrification of an urban greening tree. ACS Earth Space Chem. 2022, 6, 1460–1467. [Google Scholar] [CrossRef]
- Atamna-Ismaeel, N.; Finkel, O.; Glaser, F.; von Mering, C.; Vorholt, J.A.; Koblížek, M.; Belkin, S.; Béjà, O. Bacterial anoxygenic photosynthesis on plant leaf surfaces. Environ. Microbiol. Rep. 2012, 4, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kohzu, A.; Suda, W.; Yamamura, S.; Takamatsu, T.; Takenaka, A.; Koshikawa, M.K.; Hayashi, S.; Watanabe, M. Microbial nitrification in throughfall of a Japanese cedar associated with archaea from the tree canopy. Springerplus 2016, 5, 1596. [Google Scholar] [CrossRef] [PubMed]
- Mwajita, M.R.; Murage, H.; Tani, A.; Kahangi, E.M. Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in Kenya for plant growth promoters. Springerplus 2013, 2, 606. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, X.; He, C.; Maitra, P.; Li, X.C.; Guo, L.D. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Shi, Y.; Geng, L.L.; Chu, H.Y.; Zhang, J.; Song, F.P.; Duan, J.Y.; Shu, C.L. Template preparation affects 16S rRNA high-throughput sequencing analysis of phyllosphere microbial communities. Front. Plant Sci. 2017, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press, Co.: Beijing, China, 2000; pp. 178–200. [Google Scholar]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; de Vos, W.M.; Ross, R.P.; O’Toole, P.W. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Pan, Y.Q.; Yang, P.; Hu, J.P.; Zhao, T.L.; Zhang, Y.Q.; Ding, X.D.; Yan, X.F. A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches. Front. Microbiol. 2022, 13, 1018077. [Google Scholar] [CrossRef]
- Ma, B.; Wang, H.Z.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.M.; Brookes, P.C.; Xu, J.M.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Zhang, Y.Q.; Li, X.R. Kalidium cuspidatum colonization changes the structure and function of salt crust microbial communities. Environ. Sci. Pollut. Res. 2024, 31, 19764–19778. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.P.; Zhang, Q.; Zhu, H.S.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.X.; Shao, M.G.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y.H.; Wei, G.H. Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Change Biol. 2022, 28, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal. Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Ocean plankton. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Qu, X.; Ran, Y.C.; Li, X.R. Effects of long-term fencing on soil microbial community structure and function in the desert steppe, China. J. Arid. Land 2024, 16, 431–446. [Google Scholar] [CrossRef]
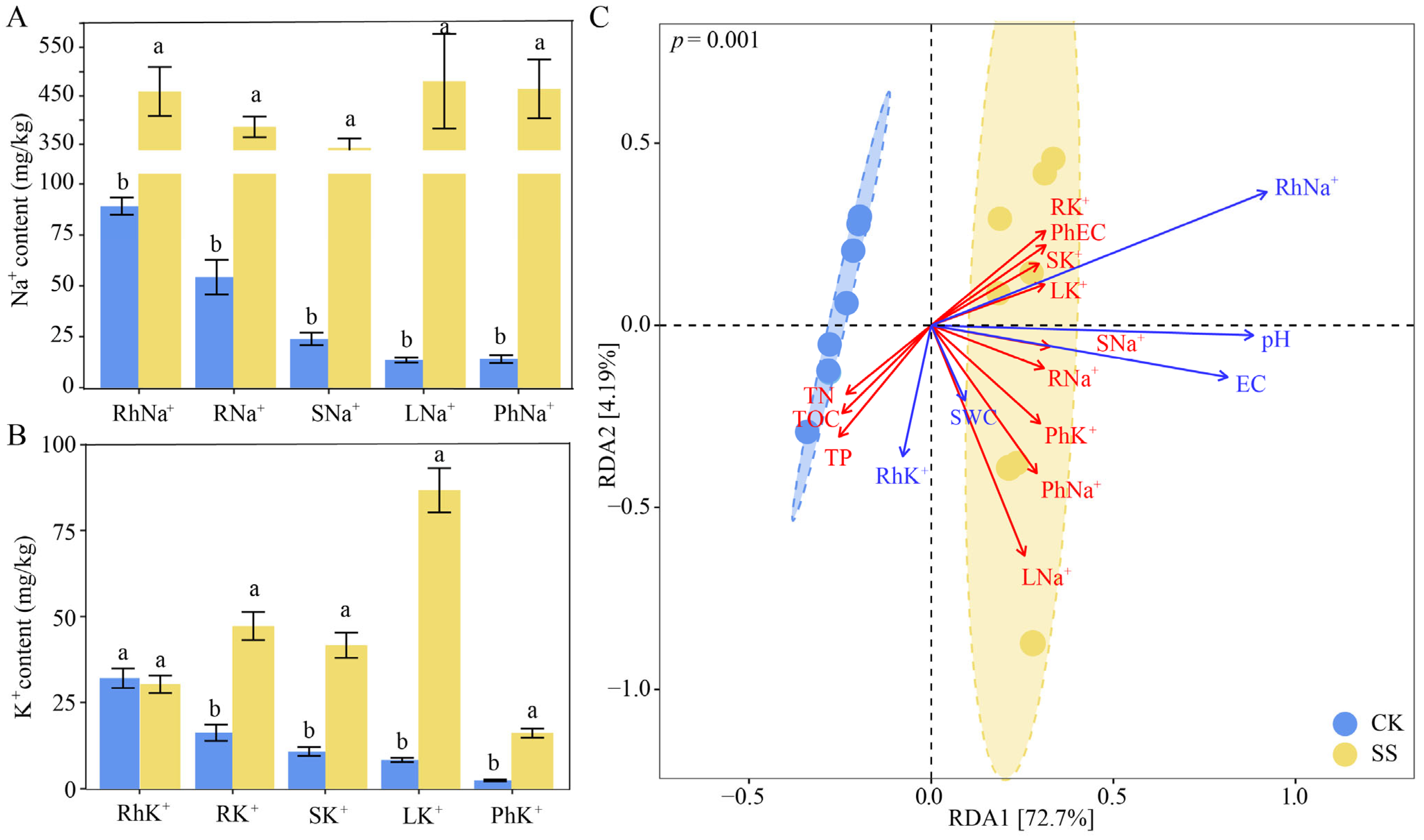
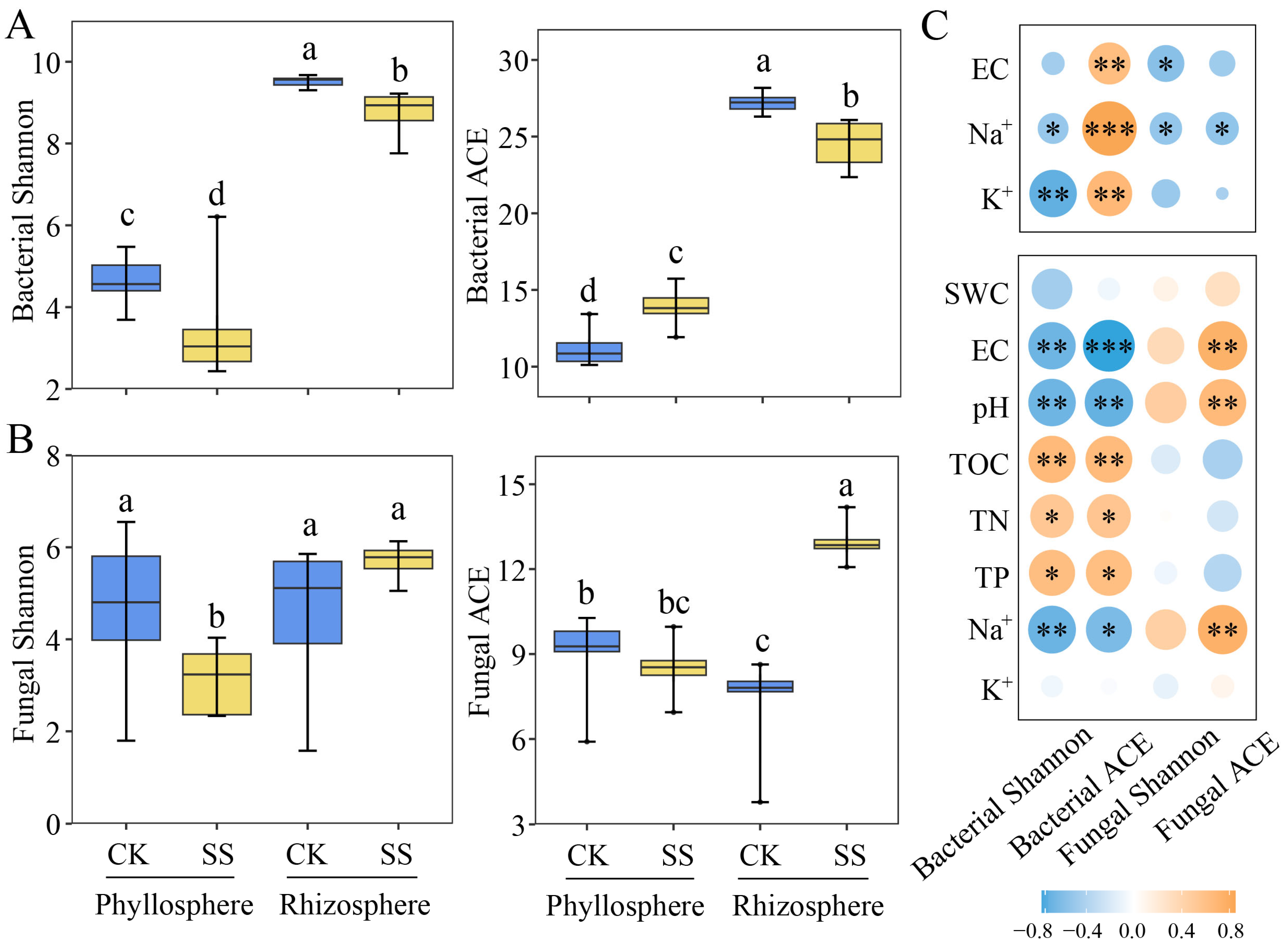

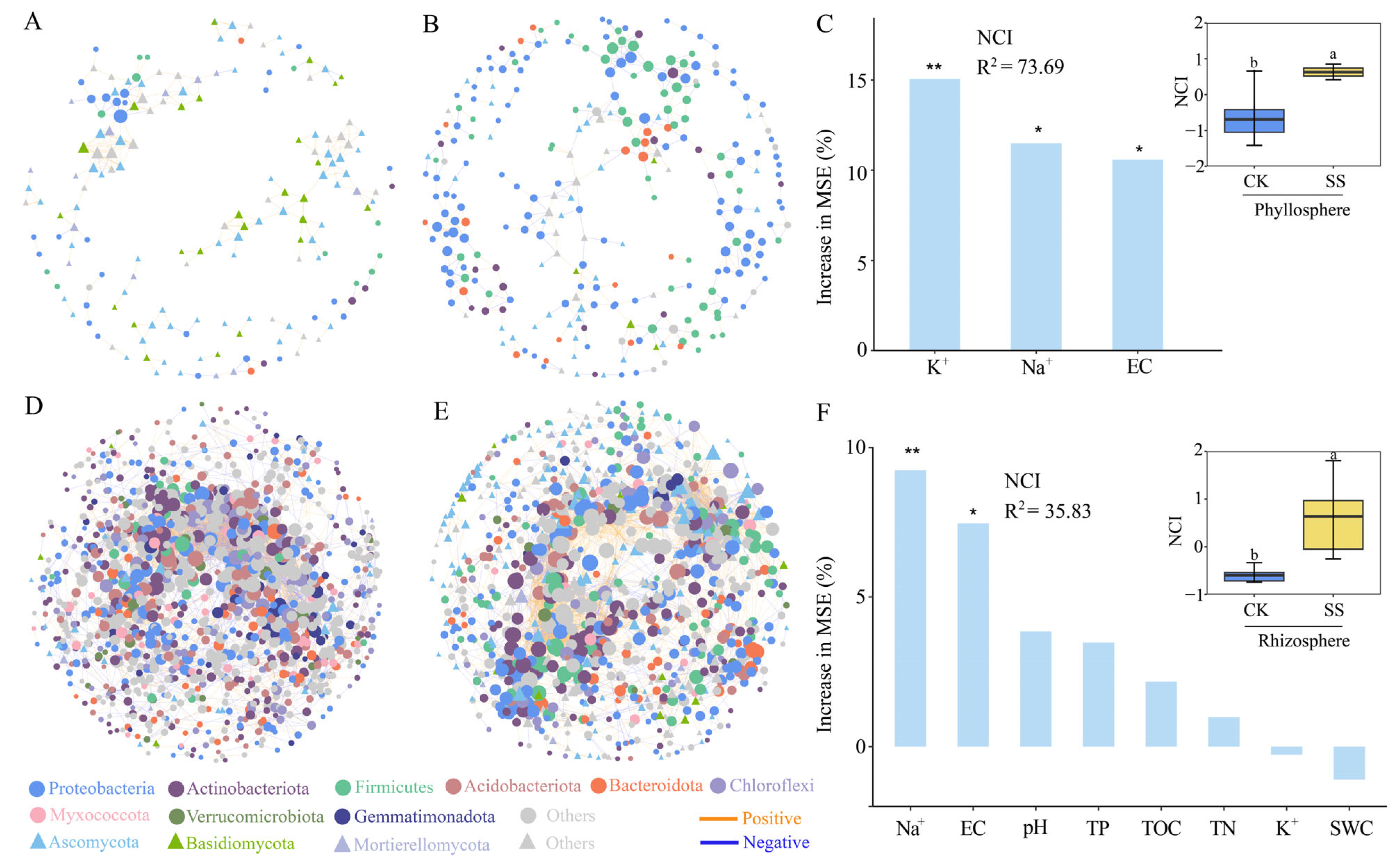
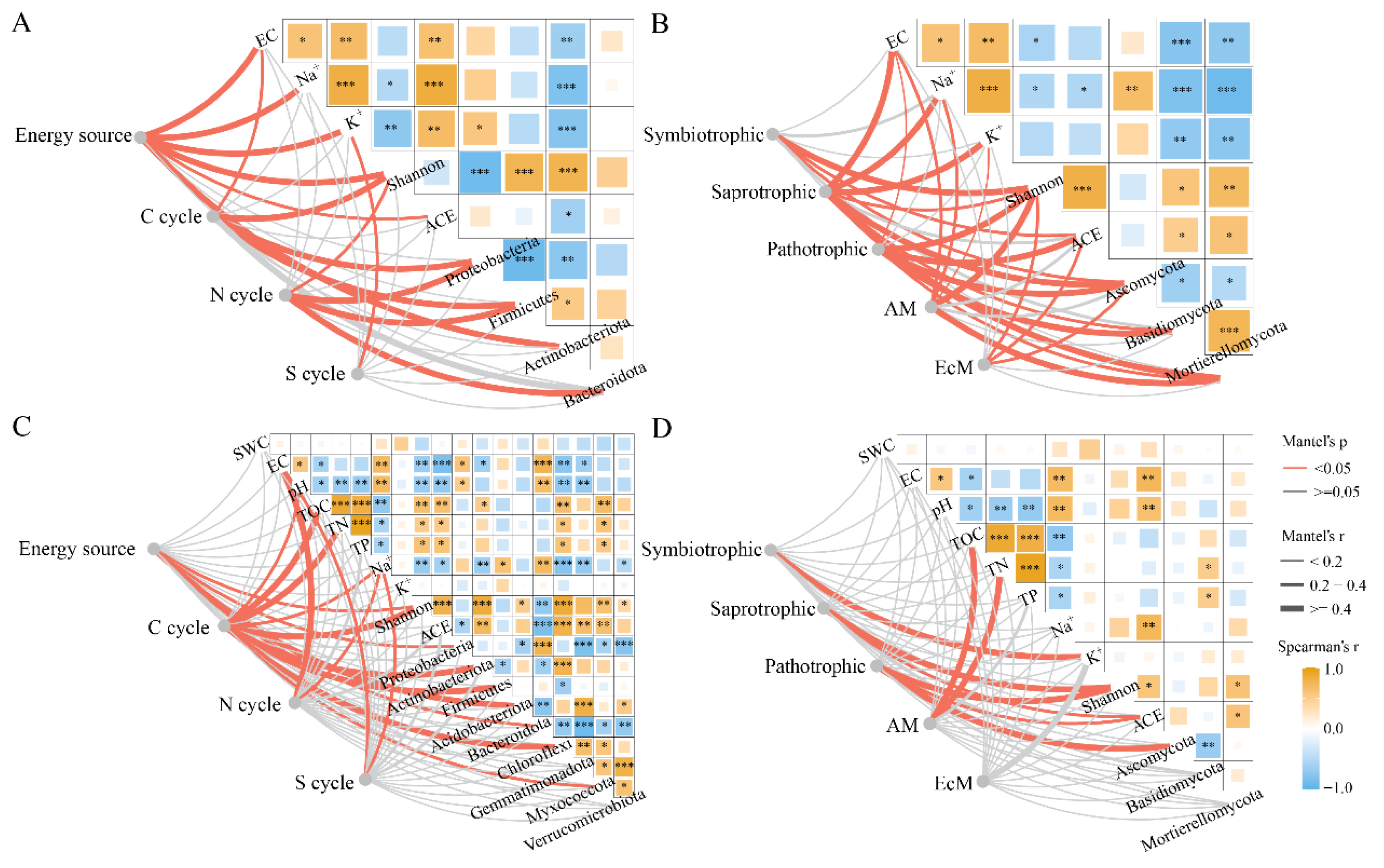
| Physicochemical Properties | CK | SS |
|---|---|---|
| Ph. EC (µs/cm) | 214.13 ± 15.37 b | 811.38 ± 33.15 a |
| Ph-Na+ (mg/kg) | 14.06 ± 1.97 b | 464.38 ± 60.86 a |
| Ph-K+ (mg/kg) | 2.45 ± 0.26 b | 16.20 ± 1.32 a |
| Rh. pH | 8.62 ± 0.03 b | 9.10 ± 0.08 a |
| Rh. SWC | 22.26 ± 1.95 a | 22.91 ± 1.27 a |
| Rh. EC (µs/cm) | 144.04 ± 6.99 b | 2411.25 ± 422.03 a |
| Rh. TOC (g/kg) | 26.68 ± 1.11 a | 21.81 ± 0.73 b |
| Rh. TN (g/kg) | 1.31 ± 0.09 a | 0.98 ± 0.05 a |
| Rh. TP (g/kg) | 0.55 ± 0.02 a | 0.46 ± 0.02 a |
| Rh. Na+ (mg/kg) | 89.04 ± 4.23 b | 459.02 ± 50.56 a |
| Rh. K+ (mg/kg) | 32.06 ± 2.84 a | 30.32 ± 2.53 a |
| Root. Na+ (mg/kg) | 54.26 ± 8.47 b | 385.67 ± 21.25 a |
| Root. K+ (mg/kg) | 16.32 ± 2.35 b | 47.27 ± 4.11 a |
| Stem. Na+ (mg/kg) | 23.92 ± 3.01 b | 342.37 ± 19.33 a |
| Stem. K+ (mg/kg) | 10.82 ± 1.29 b | 41.62 ± 3.66 a |
| Leaf. Na+ (mg/kg) | 13.60 ± 1.25 b | 480.28 ± 97.56 a |
| Leaf. K+ (mg/kg) | 8.38 ± 0.61 b | 86.71 ± 6.42 a |
| Topological Features | Ph. CK | Ph. SS | Rh. CK | Rh. SS |
|---|---|---|---|---|
| Nodes | 175 | 280 | 1384 | 1150 |
| Total edges | 247 | 399 | 7531 | 6394 |
| Positive edges | 217 | 238 | 4196 | 5208 |
| Negative edges | 30 | 161 | 3335 | 1186 |
| Modularity | 0.846113 | 0.859674 | 0.574697 | 0.645207 |
| Average path length | 4.163033 | 8.326226 | 5.49876 | 5.181075 |
| Graph diameter | 12.89363 | 22.15457 | 16.57193 | 13.75342 |
| Graph density | 0.009889 | 0.00657 | 0.007581 | 0.008993 |
| Clustering coefficient | 0.490975 | 0.399516 | 0.436952 | 0.428124 |
| Betweenness centralization | 0.026054 | 0.102263 | 0.018319 | 0.023204 |
| Degree centralization | 0.043922 | 0.019292 | 0.042099 | 0.031276 |
| Functional Groups | Ph. CK | Ph. SS | Rh. CK | Rh. SS |
|---|---|---|---|---|
| Energy source | 20.44 ± 2.88 b | 37.17 ± 1.91 a | 23.34 ± 1.18 a | 25.46 ± 1.95 a |
| C cycle | 5.03 ± 0.49 a | 3.25 ± 1.81 a | 5.22 ± 0.69 b | 11.39 ± 1.33 a |
| N cycle | 44.70 ± 3.52 a | 45.07 ± 4.41 a | 4.50 ± 0.38 a | 6.36 ± 1.12 a |
| P cycle | 0.05 ± 0.02 a | 0.22 ± 0.15 a | 0.14 ± 0.04 b | 1.27 ± 0.52 a |
| Functional Groups | Ph. CK | Ph. SS | Rh. CK | Rh. SS |
|---|---|---|---|---|
| Symbiotrophic | 2.14 ± 0.94 a | 0.23 ± 0.05 a | 2.41 ± 0.86 a | 1.34 ± 0.29 a |
| Saprotrophic | 33.21 ± 8.36 a | 3.34 ± 0.65 b | 17.39 ± 3.85 a | 21.58 ± 2.46 a |
| Pathotrophic | 30.84 ± 8.83 b | 70.68 ± 3.74 a | 41.19 ± 8.33 a | 44.61 ± 4.70 a |
| AM | 0.71 ± 0.32 a | 0.07 ± 0.01 a | 0.71 ± 0.24 a | 0.34 ± 0.14 a |
| EcM | 1.28 ± 0.78 a | 0.13 ± 0.04 a | 0.97 ± 0.77 a | 0.30 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Pan, Y.; Wang, P.; Ran, L.; Qin, G.; Li, Q.; Kang, P. Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix chinensis. Plants 2024, 13, 1091. https://doi.org/10.3390/plants13081091
Qu X, Pan Y, Wang P, Ran L, Qin G, Li Q, Kang P. Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix chinensis. Plants. 2024; 13(8):1091. https://doi.org/10.3390/plants13081091
Chicago/Turabian StyleQu, Xuan, Yaqing Pan, Peiqin Wang, Lele Ran, Guifei Qin, Qunfang Li, and Peng Kang. 2024. "Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix chinensis" Plants 13, no. 8: 1091. https://doi.org/10.3390/plants13081091
APA StyleQu, X., Pan, Y., Wang, P., Ran, L., Qin, G., Li, Q., & Kang, P. (2024). Response of Phyllosphere and Rhizosphere Microbial Communities to Salt Stress of Tamarix chinensis. Plants, 13(8), 1091. https://doi.org/10.3390/plants13081091





