Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping
Abstract
:1. Common Bean Characteristics
2. Seed Heterogeneity Phenomenon in Common Beans
3. Background Effects and Mechanisms Reflecting Heterogeneity of Common Bean Seeds
3.1. Heterogeneity of Seeds as a Result of Outcrossing
3.2. Heterogeneity of Seed as a Consequence of Genetic Interactions
| Gene Name | Role of Gene |
|---|---|
| P gene a,b | Basic gene for synthesis of transcription factors that enable formation of seed coat and flower colour; has several alleles. |
| Complex C locus a,b,c,d,e | Gene for sulphur-white seed coat colour with several closely linked genes and alleles encoding different seed coat patterns; patterns contrast between darker colour and lighter background. |
| R gene a | Dominant gene for red colour of seed coat (oxblood); a part of C locus. |
| Prp gene a | Located within C locus and includes Prp gene and allele Prpi; Prp gene influences purple-red pod colour; Prpi codes enhanced anthocyanin expression (IAE) syndrome that results in purple-red flower buds, corollas, pods, petioles, leaf laminae and stems; Prpi gene in conjunction with other genes also influences colour of seed coat. |
| Gy gene a | Produces a greenish-yellow seed coat colour that is associated with complex C locus. |
| Chr gene a | Chr gene with gy gene ensures a greenish-yellow colouration of hilum ring and corona. |
| R-2 gene a | Secondary gene for red seed coat colour. |
| Prpi-2 gene a | Secondary gene for IAE syndrome, independent of C locus; can form two coloured seed coats with other genes. |
| J gene a,b,f | Dominant gene for development of mature seed coat colour; involved in expression of hilum ring colour, darkening effect, and shine; with t restrict partly coloured patterns; L is a synonym for j and l for J. |
| Z gene (former D) a | Affects colour of hilum ring; requires j gene for loss of expression; restrict partly coloured patterns with t gene; zonal. |
| G gene a | Yellowish-brown factor; has a darkening effect. |
| B gene a,c | Greenish-grey-brown factor. |
| V gene a,b | Purple factor that changes seed coat colour to dark purple or black and flower colour to light pink or white to bishop purple. |
| Ane gene a | With cu express nebulosus mottling; synonym for vein expression on seed coat and is also associated with B gene. |
| Bic gene a | With genotype TPCJBVRk express dark olive-brown seed coat colour and bicolour flowers. |
| Rk gene a,b,e | Produces recessive red seed coat colour and influences flower pattern; independent of C locus and has multiple alleles. |
| Sal gene a,b | With v or Vwf genes produces a red haze on seed coat and salmon red (expressed in a vein pattern) or China rose flowers. |
| Am gene a | Expressed only with the Sal gene as oxblood seed coat colour and amaranth (scarlet) flower colour. |
| T locus a | Basic factor for partially coloured seed coats and flowers in homozygous recessive form (tt). |
| Bip gene a | With t and z genes restrict partly coloured patterns, resulting in bipunctata patterns (t z bip); they have multiple alleles. |
| Cl gene a | With t and v genes express circumlineated zone, a sharp, precipitate-like line that separates each coloured area from white part of seed coat. |
| Fib gene a,b | Restrict partly coloured patterns; fibula arcs. |
| Mic gene a | With c and j genes express micropyle inpunctata pattern. |
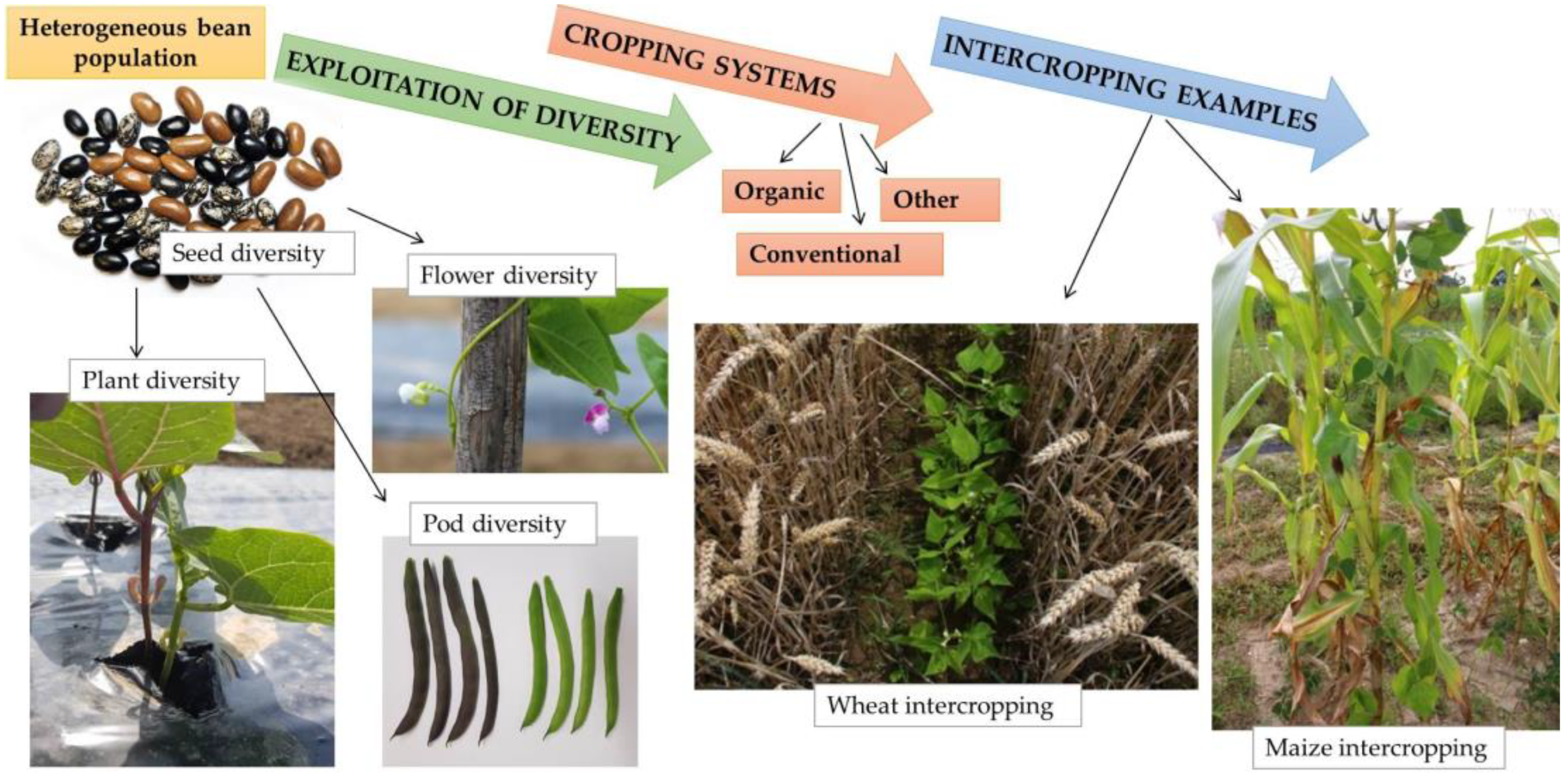
4. Exploiting Heterogeneity and Intercropping Systems in Breeding and Agriculture
4.1. Advantages of Heterogeneous Populations and Breeding
4.2. Intercropping Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meena, R.S.; Lal, R. Legumes and Sustainable Use of Soils. In Legumes for Soil Health and Sustainable Management; Meena, R.S., Das, A., Yadav, G.S., Lal, R., Eds.; Springer: Singapore, 2018; pp. 1–31. ISBN 9789811302527. [Google Scholar]
- Palmero, F.; Fernandez, J.A.; Garcia, F.O.; Haro, R.J.; Prasad, P.V.V.; Salvagiotti, F.; Ciampitti, I.A. A Quantitative Review into the Contributions of Biological Nitrogen Fixation to Agricultural Systems by Grain Legumes. Eur. J. Agron. 2022, 136, 126514. [Google Scholar] [CrossRef]
- Cortinovis, G.; Oppermann, M.; Neumann, K.; Graner, A.; Gioia, T.; Marsella, M.; Alseekh, S.; Fernie, A.R.; Papa, R.; Bellucci, E.; et al. Towards the Development, Maintenance, and Standardized Phenotypic Characterization of Single-Seed-Descent Genetic Resources for Common Bean. Curr. Protoc. 2021, 1, e133. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.A.; Yeken, M.Z.; Shahid, M.Q.; Habyarimana, E.; Yılmaz, H.; Alsaleh, A.; Hatipoğlu, R.; Çilesiz, Y.; Khawar, K.M.; Ludidi, N.; et al. Common Bean as a Potential Crop for Future Food Security: An Overview of Past, Current and Future Contributions in Genomics, Transcriptomics, Transgenics and Proteomics. Biotechnol. Biotechnol. Equip. 2021, 35, 759–787. [Google Scholar] [CrossRef]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus sp.) as a Model for Understanding Crop Evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, C.P.; Santos, I.L.; de Carvalho Paulino, J.F.; Barbosa, C.C.F.; Pereira, C.C.A.; Carvalho, C.R.L.; de Moraes Cunha Gonçalves, G.; Song, Q.; Carbonell, S.A.M.; Chiorato, A.F.; et al. Genome-Wide Association Mapping Reveals New Loci Associated with Light-Colored Seed Coat at Harvest and Slow Darkening in Carioca Beans. BMC Plant Biol. 2021, 21, 343. [Google Scholar] [CrossRef] [PubMed]
- De Ron, A.M.; Papa, R.; Bitocchi, E.; González, A.M.; Debouck, D.G.; Brick, M.A.; Fourie, D.; Marsolais, F.; Beaver, J.; Geffroy, V.; et al. Common Bean. In Grain Legumes; De Ron, A.M., Ed.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2015; Volume 10, pp. 1–36. ISBN 978-1-4939-2796-8. [Google Scholar]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C.; et al. A Reference Genome for Common Bean and Genome-Wide Analysis of Dual Domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Astudillo, C.; Grusak, M.A.; Graham, R.; Beebe, S.E. Inheritance of Seed Iron and Zinc Concentrations in Common Bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Angioi, S.A.; Rau, D.; Attene, G.; Nanni, L.; Bellucci, E.; Logozzo, G.; Negri, V.; Spagnoletti Zeuli, P.L.; Papa, R. Beans in Europe: Origin and Structure of the European Landraces of Phaseolus vulgaris L. Theor. Appl. Genet. 2010, 121, 829–843. [Google Scholar] [CrossRef]
- Pipan, B.; Sinkovič, L. Multi-Elemental Composition, Nutrients and Total Phenolics in Seeds of Phaseolus vulgaris L. Breeding Material. J. Elem. 2021, 26, 613–623. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.; Barros, A.I. Nutrients, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and Their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Spagnoletti Zeuli, P.; Gioia, T.; Logozzo, G.; et al. Molecular Analysis of the Parallel Domestication of the Common Bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; Valdés-López, O. Common Bean: A Legume Model on the Rise for Unraveling Responses and Adaptations to Iron, Zinc, and Phosphate Deficiencies. Front. Plant Sci. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, G.; Frascarelli, G.; Di Vittori, V.; Papa, R. Current State and Perspectives in Population Genomics of the Common Bean. Plants 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Pipan, B.; Meglič, V. Diversification and Genetic Structure of the Western-to-Eastern Progression of European Phaseolus vulgaris L. Germplasm. BMC Plant Biol. 2019, 19, 442. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Rau, D.; Bitocchi, E.; Bellucci, E.; Biagetti, E.; Carboni, A.; Gepts, P.; Nanni, L.; Papa, R.; Attene, G. Landscape Genetics, Adaptive Diversity and Population Structure in Phaseolus vulgaris. New Phytol. 2016, 209, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Sedlar, A.; Zupin, M.; Maras, M.; Razinger, J.; Šuštar-Vozlič, J.; Pipan, B.; Meglič, V. QTL Mapping for Drought-Responsive Agronomic Traits Associated with Physiology, Phenology, and Yield in an Andean Intra-Gene Pool Common Bean Population. Agronomy 2020, 10, 225. [Google Scholar] [CrossRef]
- Campa, A.; Murube, E.; Ferreira, J.J. Genetic Diversity, Population Structure, and Linkage Disequilibrium in a Spanish Common Bean Diversity Panel Revealed through Genotyping-by-Sequencing. Genes 2018, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.H.; Ranalli, P. Common Bean (Phaseolus vulgaris L.). Field Crops Res. 1997, 53, 131–146. [Google Scholar] [CrossRef]
- Fiore, M.C.; Raimondo, F.M.; Mercati, F.; Digangi, I.; Sunseri, F.; Scialabba, A. Preserving Biodiversity in Marginal Rural Areas: Assessment of Morphological and Genetic Variability of a Sicilian Common Bean Germplasm Collection. Plants 2020, 9, 989. [Google Scholar] [CrossRef]
- Savić, A.; Pipan, B.; Vasić, M.; Meglič, V. Genetic Diversity of Common Bean (Phaseolus vulgaris L.) Germplasm from Serbia, as Revealed by Single Sequence Repeats (SSR). Sci. Hortic. 2021, 288, 110405. [Google Scholar] [CrossRef]
- Almeida, C.P.D.; Paulino, J.F.D.C.; Morais Carbonell, S.A.; Chiorato, A.F.; Song, Q.; Di Vittori, V.; Rodriguez, M.; Papa, R.; Benchimol-Reis, L.L. Genetic Diversity, Population Structure, and Andean Introgression in Brazilian Common Bean Cultivars after Half a Century of Genetic Breeding. Genes 2020, 11, 1298. [Google Scholar] [CrossRef]
- García-Fernández, C.; Campa, A.; Ferreira, J.J. Dissecting the Genetic Control of Seed Coat Color in a RIL Population of Common Bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2021, 134, 3687–3698. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santos, M.E.; Peña-Valdivia, C.B.; García-Esteva, A.; Aguilar-Benítez, G.; Kohashi-Shibata, J. Seed and Seedlings Physical Characteristics and Seeds Germination of Wild and Domesticated Common Bean (Phaseolus vulgaris L.) and Their Progeny. Agrociencia 2017, 51, 43–62. [Google Scholar]
- Dawson, J.C.; Goldringer, I. Breeding for Genetically Diverse Populations: Variety Mixtures and Evolutionary Populations. In Organic Crop Breeding; Lammerts van Bueren, E.T., Myers, J.R., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 77–98. ISBN 978-0-470-95858-2. [Google Scholar]
- Bassett, M.J. Genetics of Seed Coat Color and Pattern in Common Bean. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 239–315. ISBN 978-0-470-16802-8. [Google Scholar]
- Konzen, E.R.; Tsai, S.M. Seed Coat Shininess in Phaseolus Vulgaris: Rescuing a Neglected Trait by Its Screening on Commercial Lines and Landraces. J. Agric. Sci. 2014, 6, 113. [Google Scholar] [CrossRef]
- Sadohara, R.; Long, Y.; Izquierdo, P.; Urrea, C.A.; Morris, D.; Cichy, K. Seed Coat Color Genetics and Genotype × Environment Effects in Yellow Beans via Machine-Learning and Genome-Wide Association. Plant Genome 2022, 15, e20173. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Russi, L.; Romani, M.; Notario, T.; Pecetti, L. Value of Heterogeneous Material and Bulk Breeding for Inbred Crops: A Pea Case Study. Field Crops Res. 2023, 293, 108831. [Google Scholar] [CrossRef]
- Cooper, H.D.; Spillane, C.; Hodgkin, T.; Food and Agriculture Organization of the United Nations, International Plant Genetic Resources Institute (Eds.) Broadening the Genetic Bases of Crop Production; CABI Pub. in association with Food and Agriculture Organization of the United Nations and International Plant Genetic Resources Institute: Wallingford, UK, 2001; ISBN 978-0-85199-411-6. [Google Scholar]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological Principles Underlying the Increase of Productivity Achieved by Cereal-Grain Legume Intercrops in Organic Farming. A Review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J.; et al. Crop Genetic Erosion: Understanding and Responding to Loss of Crop Diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef]
- Mariyono, J.; Kuntariningsih, A.; Suswati, E.; Kompas, T. Quantity and Monetary Value of Agrochemical Pollution from Intensive Farming in Indonesia. Manag. Environ. Qual. Int. J. 2018, 29, 759–779. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed Vigour and Crop Establishment: Extending Performance beyond Adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J. Exploring Breakthroughs in Three Traits Belonging to Seed Life. Plants 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Acosta, Y.; Pérez, L.; Escalante, D.; Pérez, A.; Martínez-Montero, M.E.; Fontes, D.; Ahmed, L.Q.; Sershen; Lorenzo, J.C. Heteromorphic Seed Germination and Seedling Emergence in the Legume Teramnus labialis (L.f.) Spreng (Fabacaeae). Botany 2020, 98, 371–379. [Google Scholar] [CrossRef]
- Georgieva, N. Seed Heterogeneity in Dependence of Their Position on the Mother Plant in Lupinus albus L. Banats J. Biotechnol. 2020, XI, 76–82. [Google Scholar] [CrossRef]
- Gianella, M.; Bradford, K.J.; Guzzon, F. Ecological, (Epi)Genetic and Physiological Aspects of Bet-Hedging in Angiosperms. Plant Reprod. 2021, 34, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, L.; Baskin, C.C.; Tian, C.; Huang, Z. Maternal Effects on Seed Heteromorphism: A Dual Dynamic Bet Hedging Strategy. Seed Sci. Res. 2019, 29, 149–153. [Google Scholar] [CrossRef]
- Mitchell, J.; Johnston, I.G.; Bassel, G.W. Variability in Seeds: Biological, Ecological, and Agricultural Implications. J. Exp. Bot. 2016, 68, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Clavijo Michelangeli, J.A.; Gezan, S.A.; Lee, H.; Vallejos, C.E. Maternal Effects on Seed and Seedling Phenotypes in Reciprocal F1 Hybrids of the Common Bean (Phaseolus vulgaris L.). Front. Plant Sci. 2017, 8, 42. [Google Scholar] [CrossRef]
- Scholl, J.P.; Calle, L.; Miller, N.; Venable, D.L. Offspring Polymorphism and Bet Hedging: A Large-scale, Phylogenetic Analysis. Ecol. Lett. 2020, 23, 1223–1231. [Google Scholar] [CrossRef]
- Dello Jacovo, E.; Valentine, T.A.; Maluk, M.; Toorop, P.; Lopez del Egido, L.; Frachon, N.; Kenicer, G.; Park, L.; Goff, M.; Ferro, V.A.; et al. Towards a Characterisation of the Wild Legume Bitter Vetch (Lathyrus linifolius L. (Reichard) Bässler): Heteromorphic Seed Germination, Root Nodule Structure and N-fixing Rhizobial Symbionts. Plant Biol. 2019, 21, 523–532. [Google Scholar] [CrossRef]
- Imbert, E. Ecological Consequences and Ontogeny of Seed Heteromorphism. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 13–36. [Google Scholar] [CrossRef]
- Sethi, R.; Kaur, N.; Singh, M. Morphological and Physiological Characterization of Seed Heteromorphism in Medicago Denticulata Willd. Plant Physiol. Rep. 2020, 25, 107–119. [Google Scholar] [CrossRef]
- Reyes, J.L.M.; Mesa, H.G.A.; Bolanos, E.N.A.; Meza, S.H.; Ramirez, N.C.; Servia, J.L.C. Classification of Bean (Phaseolus vulgaris L.) Landraces with Heterogeneous Seed Color Using a Probabilistic Representation. In Proceedings of the 2021 IEEE International Autumn Meeting on Power, Electronics and Computing (ROPEC), Ixtapa, Mexico, 10 November 2021; IEEE: Ixtapa, Mexico; pp. 1–7. [Google Scholar]
- Vidak, M.; Šatović, Z.; Liber, Z.; Grdiša, M.; Gunjača, J.; Kilian, A.; Carović-Stanko, K. Assessment of the Origin and Diversity of Croatian Common Bean Germplasm Using Phaseolin Type, SSR and SNP Markers and Morphological Traits. Plants 2021, 10, 665. [Google Scholar] [CrossRef]
- Assefa, T.; Assibi Mahama, A.; Brown, A.V.; Cannon, E.K.S.; Rubyogo, J.C.; Rao, I.M.; Blair, M.W.; Cannon, S.B. A Review of Breeding Objectives, Genomic Resources, and Marker-Assisted Methods in Common Bean (Phaseolus vulgaris L.). Mol. Breed. 2019, 39, 20. [Google Scholar] [CrossRef]
- Yang, C.J.; Russell, J.; Ramsay, L.; Thomas, W.; Powell, W.; Mackay, I. Overcoming Barriers to the Registration of New Plant Varieties under the DUS System. Commun. Biol. 2021, 4, 302. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union Council Directive 2002/53/EC of 13 June 2002 on the Common Catalogue of Varieties of Agricultural Plant Species. 2002, p. 11. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32002L0053 (accessed on 1 March 2024).
- Council of the European Union Council Regulation (EC) No 2100/94 of 27 July 1994 on Community Plant Variety Rights. 1994, p. 30. Available online: https://eur-lex.europa.eu/eli/reg/1994/2100/oj (accessed on 20 February 2024).
- UPOV General Introduction to the Examination of Distinctness, Uniformity and Stability and the Development of Harmonized Descriptions of New Varieties of Plants. 2002. Available online: https://www.upov.int/tgp/en/introduction_dus.html (accessed on 15 December 2023).
- Šajgalík, M.; Ondreičková, K.; Hauptvogel, P.; Mihálik, D.; Glasa, M.; Kraic, J. Higher Effectiveness of New Common Bean (Phaseolus vulgaris L.) Germplasm Acquisition by Collecting Expeditions Associated with Molecular Analyses. Sustainability 2019, 11, 5270. [Google Scholar] [CrossRef]
- Council of the European Union Regulation (EU) 2018/848 of the European Parlament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. 2018. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj (accessed on 16 January 2024).
- Klaedtke, S.M.; Caproni, L.; Klauck, J.; de la Grandville, P.; Dutartre, M.; Stassart, P.M.; Chable, V.; Negri, V.; Raggi, L. Short-Term Local Adaptation of Historical Common Bean (Phaseolus vulgaris L.) Varieties and Implications for In Situ Management of Bean Diversity. Int. J. Mol. Sci. 2017, 18, 493. [Google Scholar] [CrossRef]
- Tiranti, B.; Negri, V. Selective Microenvironmental Effects Play a Role in Shaping Genetic Diversity and Structure in a Phaseolus vulgaris L. Landrace: Implications for on-Farm Conservation. Mol. Ecol. 2007, 16, 4942–4955. [Google Scholar] [CrossRef]
- Musango, R.; Kudzai, K.; Mhungu, S.; Tibugar, H. Phenotypic Characterization of Common Bean (Phaseolus vulgaris L.) Accessions Conserved at the Genetic Resources and Biotechnology Institute. J. Biodivers. Environ. Sci. 2016, 8, 26–36. [Google Scholar]
- Chacón-Sánchez, M.I.; Martínez-Castillo, J.; Duitama, J.; Debouck, D.G. Gene Flow in Phaseolus Beans and Its Role as a Plausible Driver of Ecological Fitness and Expansion of Cultigens. Front. Ecol. Evol. 2021, 9, 618709. [Google Scholar] [CrossRef]
- Ferreira, J.L.; De Souza Carneiro, J.E.; Teixeira, A.L.; De Lanes, F.F.; Cecon, P.R.; Borém, A. Gene Flow in Common Bean (Phaseolus vulgaris L.). Euphytica 2007, 153, 165–170. [Google Scholar] [CrossRef]
- Ibarra-Perez, F.J.; Barnhart, D.; Ehdaie, B.; Knio, K.M.; Waines, J.G. Effects of Insect Tripping on Seed Yield of Common Bean. Crop Sci. 1999, 39, 428–433. [Google Scholar] [CrossRef]
- Gross, Y.; Kigel, J. Differential Sensitivity to High Temperature of Stages in the Reproductive Development of Common Bean (Phaseolus vulgaris L.). Field Crops Res. 1994, 36, 201–212. [Google Scholar] [CrossRef]
- Van Ginkel, M.; Flipphi, R.C.H. Why Self-Fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination. Agronomy 2020, 10, 349. [Google Scholar] [CrossRef]
- Royer, M.R.; Gonçalves-Vidigal, M.C.; Scapim, C.A.; Vidigal Filho, P.S.; Terada, Y. Outcrossing in Common Bean. Cropp Breed. Appl. Biotechnol. 2002, 2, 49–54. [Google Scholar] [CrossRef]
- Anisimova, I.N. Structural and Functional Organization of Genes That Induce and Suppress Cytoplasmic Male Sterility in Plants. Russ. J. Genet. 2020, 56, 1288–1297. [Google Scholar] [CrossRef]
- Gautam, R.; Shukla, P.; Kirti, P.B. Male Sterility in Plants: An Overview of Advancements from Natural CMS to Genetically Manipulated Systems for Hybrid Seed Production. Theor. Appl. Genet. 2023, 136, 195. [Google Scholar] [CrossRef] [PubMed]
- Hervieu, F.; Charbonnier, L.; Bannerot, H.; Pelletier, G. The Cytoplasmic Male-Sterility (CMS) Determinant of Common Bean Is Widespread in Phaseolus coccineus L. and Phaseolus vulgaris L. Curr. Genet. 1993, 24, 149–155. [Google Scholar] [CrossRef]
- Mackenzie, S. Male Sterility and Hybrid Seed Production. In Plant Biotechnology and Agriculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 185–194. ISBN 978-0-12-381466-1. [Google Scholar]
- Schnable, P.S.; Wise, R.P. The Molecular Basis of Cytoplasmic Male Sterility and Fertility Restoration. Trends Plant Sci. 1998, 3, 175–180. [Google Scholar] [CrossRef]
- Wells, W.C.; Isom, W.H.; Waines, J.G. Outcrossing Rates of Six Common Bean Lines. Crop Sci. 1988, 28, 177–178. [Google Scholar] [CrossRef]
- Brunner, B.R.; Beaver, J.S. Estimation of outcrossing of the common bean in Puerto Rico. HortScience 1989, 24, 669–671. [Google Scholar] [CrossRef]
- Gepts, P.; González, A.; Papa, R.; Acosta, J.; Wong, A.; Delgado Salinas, A. Outcrossing in Mexican Wild and Domesticated Populations of Common Bean. Ann. Rep. Bean Improv. Coop. 2000, 43, 25–26. [Google Scholar]
- García-Díaz, Y.D.; Aquino-Bolaños, E.N.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Bioactive Compounds and Antioxidant Activity in the Common Bean Are Influenced by Cropping Season and Genotype. Chil. J. Agric. Res. 2018, 78, 255–265. [Google Scholar] [CrossRef]
- McClean, P.E.; Bett, K.E.; Stonehouse, R.; Lee, R.; Pflieger, S.; Moghaddam, S.M.; Geffroy, V.; Miklas, P.; Mamidi, S. White Seed Color in Common Bean (Phaseolus vulgaris) Results from Convergent Evolution in the P (Pigment) Gene. New Phytol. 2018, 219, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Bassett, M.J. A Complex C Region Genotype [? R] That with GB vlae Produces Dark Seal-Brown Seedcoat Color in Common Bean. J. Am. Soc. Hortic. Sci. 1996, 121, 594–598. [Google Scholar] [CrossRef]
- Prakken, R. Inheritance of Colour in Phaseolus vulgaris L. II.: A Critical Review; Mededelingen van de Landbouwhogeschool te Wageningen: Wageningen, The Netherlands, 1970; Volume 70, pp. 73–94. [Google Scholar] [CrossRef]
- McClean, P.E.; Lee, R.K.; Otto, C.; Gepts, P.; Bassett, M.J. Molecular and Phenotypic Mapping of Genes Controlling Seed Coat Pattern and Color in Common Bean (Phaseolus vulgaris L.). J. Hered. 2002, 93, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and Antioxidant Capacity of Seed Coat and Cotyledon from Brazilian and Peruvian Bean Cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Suárez Valles, B.; Ferreira Fernández, J.J. Phenolic Content and Antioxidant Activity in Seeds of Common Bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef]
- Leakey, C.L.A. Genotypic and Phenotypic Markers in Common Bean. In Genetic Resources of Phaseolus Beans; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1988; Volume 6, pp. 245–327. ISBN 978-94-009-2786-5. [Google Scholar]
- Bassett, M.J.; Miklas, P.N.; Caldas, G.V.; Blair, M.W. A Dominant Gene for Garnet Brown Seed Coats at the Rk Locus in ‘Dorado’ Common Bean and Mapping Rk to Linkage Group 1. Euphytica 2010, 176, 281–290. [Google Scholar] [CrossRef]
- Chen, P.X.; Bozzo, G.G.; Freixas-Coutin, J.A.; Marcone, M.F.; Pauls, P.K.; Tang, Y.; Zhang, B.; Liu, R.; Tsao, R. Free and Conjugated Phenolic Compounds and Their Antioxidant Activities in Regular and Non-Darkening Cranberry Bean (Phaseolus vlgaris L.) Seed Coats. J. Funct. Foods 2015, 18, 1047–1056. [Google Scholar] [CrossRef]
- Islam, N.S.; Bett, K.E.; Pauls, K.P.; Marsolais, F.; Dhaubhadel, S. Postharvest Seed Coat Darkening in Pinto Bean (Phaseolus vulgaris) Is Regulated by Psd, an Allele of the Basic Helix-Loophelix Transcription Factor P. Plants People Planet 2020, 2, 663–677. [Google Scholar] [CrossRef]
- Felicetti, E.; Song, Q.; Jia, G.; Cregan, P.; Bett, K.E.; Miklas, P.N. Simple Sequence Repeats Linked with Slow Darkening Trait in Pinto Bean Discovered by Single Nucleotide Polymorphism Assay and Whole Genome Sequencing. Crop Sci. 2012, 52, 1600–1608. [Google Scholar] [CrossRef]
- List of Genes—Phaseolus vulgaris L. 2022. Available online: http://www.bic.uprm.edu/wp-content/uploads/2022/09/Bean-Genes-List-2022.pdf (accessed on 3 November 2023).
- Tian, Z.; Wang, J.; Li, J.; Han, B. Designing Future Crops: Challenges and Strategies for Sustainable Agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, L.; Munir, S.; Bashir, N.H.; Wang, Y.; Yang, J.; Li, C. Crop Diversity and Pest Management in Sustainable Agriculture. J. Integr. Agric. 2019, 18, 1945–1952. [Google Scholar] [CrossRef]
- Tooker, J.F.; Frank, S.D. Genotypically Diverse Cultivar Mixtures for Insect Pest Management and Increased Crop Yields. J. Appl. Ecol. 2012, 49, 974–985. [Google Scholar] [CrossRef]
- Bocci, R.; Bussi, B.; Petitti, M.; Franciolini, R.; Altavilla, V.; Galluzzi, G.; Di Luzio, P.; Migliorini, P.; Spagnolo, S.; Floriddia, R.; et al. Yield, Yield Stability and Farmers’ Preferences of Evolutionary Populations of Bread Wheat: A Dynamic Solution to Climate Change. Eur. J. Agron. 2020, 121, 126156. [Google Scholar] [CrossRef]
- Bybee-Finley, K.; Ryan, M. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Organic Agriculture and Evolutionary Populations to Merge Mitigation and Adaptation Strategies to Fight Climate Change. South Sustain. 2020, 1, e013. [Google Scholar] [CrossRef]
- Wuest, S.E.; Peter, R.; Niklaus, P.A. Ecological and Evolutionary Approaches to Improving Crop Variety Mixtures. Nat. Ecol. Evol. 2021, 5, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.K.; Vista, S.P.; Gurung, S.B.; Ghimire, K.H.; Gurung, R.; Pant, S.; Gautam, S.; Paneru, P.B. Cultivar Mixture for Minimizing Risk in Farming and Conserving Agrobiodiversity. In Traditional Crop Biodiversity for Mountain Food and Nutrition Security in Nepal; Tools and Research Results of the UNEP GEF Local Crop Project; NAGRC, LI-BIRD and the Alliance of Bioversity International and CIAT: Kathmandu, Nepal, 2020; pp. 14–25. ISBN 978-92-9255-181-0. [Google Scholar]
- Reinprecht, Y.; Schram, L.; Smith, T.H.; Pauls, K.P. Enhancing In-Crop Diversity in Common Bean by Planting Cultivar Mixtures and Its Effect on Productivity. Front. Sustain. Food Syst. 2020, 4, 126. [Google Scholar] [CrossRef]
- Alemayehu, A.; Tamado, T.; Nigussie, D.; Yigzaw, D.; Kinde, T.; Wortmann, C.S. Maize–Common Bean Intercropping to Optimize Maize-Based Crop Production. J. Agric. Sci. 2017, 155, 1124–1136. [Google Scholar] [CrossRef]
- Singh, A.; Lehner, I.; Schöb, C. Effect of Drought on Bean Yield Is Mediated by Intraspecific Variation in Crop Mixtures. Front. Plant Sci. 2022, 13, 813417. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.W.; Bennett, A.E.; Cong, W.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- De Ron, A.M.; Leonardo, J.; Hinojoza, T. A Model for Sustainable Agriculture: The Intercropping System Common Bean-Maize; Science Society of Galicia (SCG): Pontevedra, Spain, 2018. [Google Scholar]
- Lizarazo, C.I.; Tuulos, A.; Jokela, V.; Mäkelä, P.S.A. Sustainable Mixed Cropping Systems for the Boreal-Nemoral Region. Front. Sustain. Food Syst. 2020, 4, 103. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Keshavarz Afshar, R.; Liu, X.; Herbert, S.J. Understanding Intercropping to Improve Agricultural Resiliency and Environmental Sustainability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 162, pp. 199–256. ISBN 978-0-12-820767-3. [Google Scholar]
- Bitew, Y.; Derebe, B.; Worku, A.; Chakelie, G. Response of Maize and Common Bean to Spatial and Temporal Differentiation in Maize-Common Bean Intercropping. PLoS ONE 2021, 16, e0257203. [Google Scholar] [CrossRef] [PubMed]
- Mahallati, M.N.; Koocheki, A.; Mondani, F.; Feizi, H.; Amirmoradi, S. Determination of Optimal Strip Width in Strip Intercropping of Maize (Zea mays L.) and Bean (Phaseolus vulgaris L.) in Northeast Iran. J. Clean. Prod. 2015, 106, 343–350. [Google Scholar] [CrossRef]
- Hussen, A. Important of Applying Intercropping for Sustainable Crop Production: A Review. Int. J. Res. Agron. 2021, 4, 37–40. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Zhang, F.; Clairotte, M.; Drevon, J.J.; Le Cadre, E.; Hinsinger, P. Dynamics of Phosphorus Fractions in the Rhizosphere of Common Bean (Phaseolus vulgaris L.) and Durum Wheat (Triticum turgidum durum L.) Grown in Monocropping and Intercropping Systems. Plant Soil 2008, 312, 139–150. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.-Y.; Wu, H.-M.; Zhang, F.-F.; Li, C.-J.; Li, X.-X.; Lambers, H.; Li, L. Root Exudates Drive Interspecific Facilitation by Enhancing Nodulation and N2 Fixation. Proc. Natl. Acad. Sci. USA 2016, 113, 6496–6501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, X.; Xiao, J.; Tang, L.; Zheng, Y. Interactive Influences of Intercropping by Nitrogen on Flavonoid Exudation and Nodulation in Faba Bean. Sci. Rep. 2019, 9, 4818. [Google Scholar] [CrossRef] [PubMed]
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. Origin of Agriculture and Plant Domestication in West Mesoamerica. Genet. Resour. Crop Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Bilodeau, P.; Arcand, Y.; Charles, M.T.; Diederichsen, A.; Germain, I.; Liu, Q.; MacKinnon, S.; Messiga, A.J.; Mondor, M.; et al. Historical Indigenous Food Preparation Using Produce of the Three Sisters Intercropping System. Foods 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Weih, M.; Karley, A.J.; Newton, A.C.; Kiær, L.P.; Scherber, C.; Rubiales, D.; Adam, E.; Ajal, J.; Brandmeier, J.; Pappagallo, S.; et al. Grain Yield Stability of Cereal-Legume Intercrops Is Greater Than Sole Crops in More Productive Conditions. Agriculture 2021, 11, 255. [Google Scholar] [CrossRef]
- Dawo, M.I.; Wilkinson, J.M.; Pilbeam, D.J. Interactions between Plants in Intercropped Maize and Common Bean. J. Sci. Food Agric. 2008, 89, 41–48. [Google Scholar] [CrossRef]
- Nurk, L.; Graß, R.; Pekrun, C.; Wachendorf, M. Effect of Sowing Method and Weed Control on the Performance of Maize (Zea mays L.) Intercropped with Climbing Beans (Phaseolus vulgaris L.). Agriculture 2017, 7, 51. [Google Scholar] [CrossRef]
- Latati, M.; Bargaz, A.; Belarbi, B.; Lazali, M.; Benlahrech, S.; Tellah, S.; Kaci, G.; Drevon, J.J.; Ounane, S.M. The Intercropping Common Bean with Maize Improves the Rhizobial Efficiency, Resource Use and Grain Yield under Low Phosphorus Availability. Eur. J. Agron. 2015, 72, 80–90. [Google Scholar] [CrossRef]
- Zhanbota, A.; Noor, R.S.; Khan, A.I.; Wang, G.; Waqas, M.M.; Shah, A.N.; Ullah, S. A Two-Year Study on Yield and Yield Components of Maize-White Bean Intercropping Systems under Different Sowing Techniques. Agronomy 2022, 12, 240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plestenjak, E.; Meglič, V.; Sinkovič, L.; Pipan, B. Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping. Plants 2024, 13, 1112. https://doi.org/10.3390/plants13081112
Plestenjak E, Meglič V, Sinkovič L, Pipan B. Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping. Plants. 2024; 13(8):1112. https://doi.org/10.3390/plants13081112
Chicago/Turabian StylePlestenjak, Eva, Vladimir Meglič, Lovro Sinkovič, and Barbara Pipan. 2024. "Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping" Plants 13, no. 8: 1112. https://doi.org/10.3390/plants13081112
APA StylePlestenjak, E., Meglič, V., Sinkovič, L., & Pipan, B. (2024). Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping. Plants, 13(8), 1112. https://doi.org/10.3390/plants13081112








