Abstract
The proteinogenic amino acid proline plays crucial roles in both plant development and stress responses, far exceeding its role in protein synthesis. However, the molecular mechanisms and the relative importance of these additional functions of proline remain under study. It is well documented that both stress responses and developmental processes are associated with proline accumulation. Under stress conditions, proline is believed to confer stress tolerance, while under physiological conditions, it assists in developmental processes, particularly during the reproductive phase. Due to proline’s properties as a compatible osmolyte and potential reactive oxygen species (ROS) scavenger, most of its beneficial effects have historically been attributed to the physicochemical consequences of its accumulation in plants. However, emerging evidence points to proline metabolism as the primary driver of these beneficial effects. Recent reports have shown that proline metabolism, in addition to supporting reproductive development, can modulate root meristem size by controlling ROS accumulation and distribution in the root meristem. The dynamic interplay between proline and ROS highlights a sophisticated regulatory network essential for plant resilience and survival. This fine-tuning mechanism, enabled by the pro-oxidant and antioxidant properties of compartmentalized proline metabolism, can modulate redox balance and ROS homeostasis, potentially explaining many of the multiple roles attributed to proline. This review uniquely integrates recent findings on the dual role of proline in both ROS scavenging and signaling, provides an updated overview of the most recent research published to date, and proposes a unified mechanism that could account for many of the multiple roles assigned to proline in plant development and stress defense. By focusing on the interplay between proline and ROS, we aim to provide a comprehensive understanding of this proposed mechanism and highlight the potential applications in improving crop resilience to environmental stress. Additionally, we address current gaps in understanding and suggest future research directions to further elucidate the complex roles of proline in plant biology.
1. Introduction
Plants are sessile organisms and therefore have to coordinate the completion of their life cycle with whatever environmental conditions they face. Both developmental decisions and adaptive or defense responses are therefore regulated by a multitude of environmental and internal cues. Accumulation of free proline in excess of the need for protein synthesis has traditionally been associated with stress tolerance but is now emerging additionally as a crucial factor in plant development. The multifunctional nature of proline makes this amino acid a key mediator in stress-related responses, including osmoprotection, protection of proteins and membranes, antioxidant defense, protein folding, and signal transduction [1]. In addition, proline has been involved in several developmental processes such as floral transition [2,3,4,5], pollen fertility [6,7], embryo development [8,9], cell wall synthesis [10], and root elongation [11,12,13].
Proline metabolism or its accumulation is believed to counteract dehydration, stabilize proteins and membranes, scavenge free radicals, maintain cellular homeostasis, buffer redox environment, trigger signal transduction, and affect gene expression. The underlying molecular mechanisms and the relative importance of these multiple functions, however, are still not fully understood. In order to use modification of proline metabolism for crop fortification, further investigations of its contributions to the regulatory network of adaptive and developmental processes are needed. Although several excellent papers have separately addressed the importance of ROS in plants [14,15,16,17,18] and their interactions with proline under stress [19,20] and non-stress conditions [13], the possibility that a single, or predominant, mechanism may control the responses to proline under stress and non-stress conditions has never been discussed. This review aims to fill this gap by addressing the importance of proline–ROS interactions, exploring their role in plant development and stress response, and discussing the possibility that proline control of redox balance and ROS signaling may underlie most of the multiple functions attributed to proline.
2. The State of the Art: Multiple Roles of Proline in Stress and Development
Proline in Stress Responses
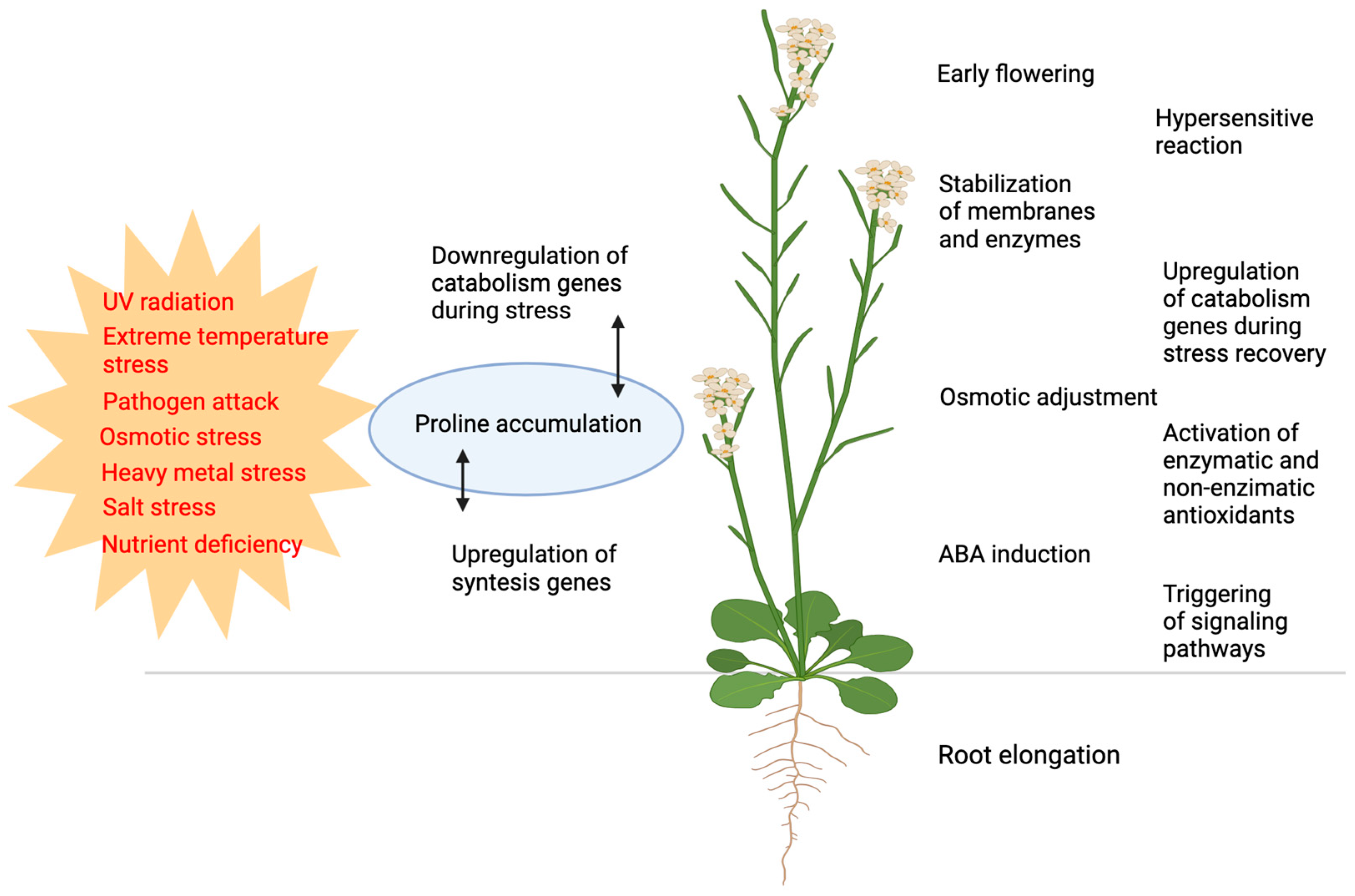
Historically, the primary role of free proline accumulation has been associated, ever since the middle of the last century, with its protective effects against a number of biotic and abiotic stresses, including salinity, drought stress, extreme temperature, water stress, heavy metal stress, UV light stress, nutrient deficiency, and pathogen attacks [1,21,22,23,24,25] (Figure 1). However, compelling evidence for the beneficial effects of proline accumulation was only provided in 1981, specifically in bacterial tolerance to osmotic stress [26].
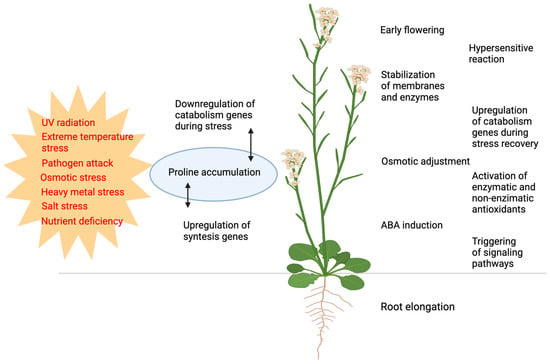
Figure 1.
Functions of proline and proline metabolism in responses to biotic and abiotic stresses. Various types of biotic and abiotic stresses (shown in red) induce changes in proline metabolism, most frequently resulting in proline accumulation (blue). The alterations in proline metabolism result in or are correlated to a broad range of defense responses (shown in black). Figure created with BioRender.com.
The main reasons for the popularity of proline as a potential stress protector were the peculiar chemical properties of proline and its rapid and abundant accumulation observed under stress conditions in many plant species [22,23,25,27]. Indeed, although some plant species can respond to stress by accumulating other types of osmolytes, such as glycine betaine, γ-aminobutyric acid (GABA), trehalose, mannitol, sorbitol, and polyamines, among others [28], proline accumulation is the most widespread among plant species, as well as in various organisms such as protozoa, eubacteria, marine invertebrates, and algae [29,30,31,32].
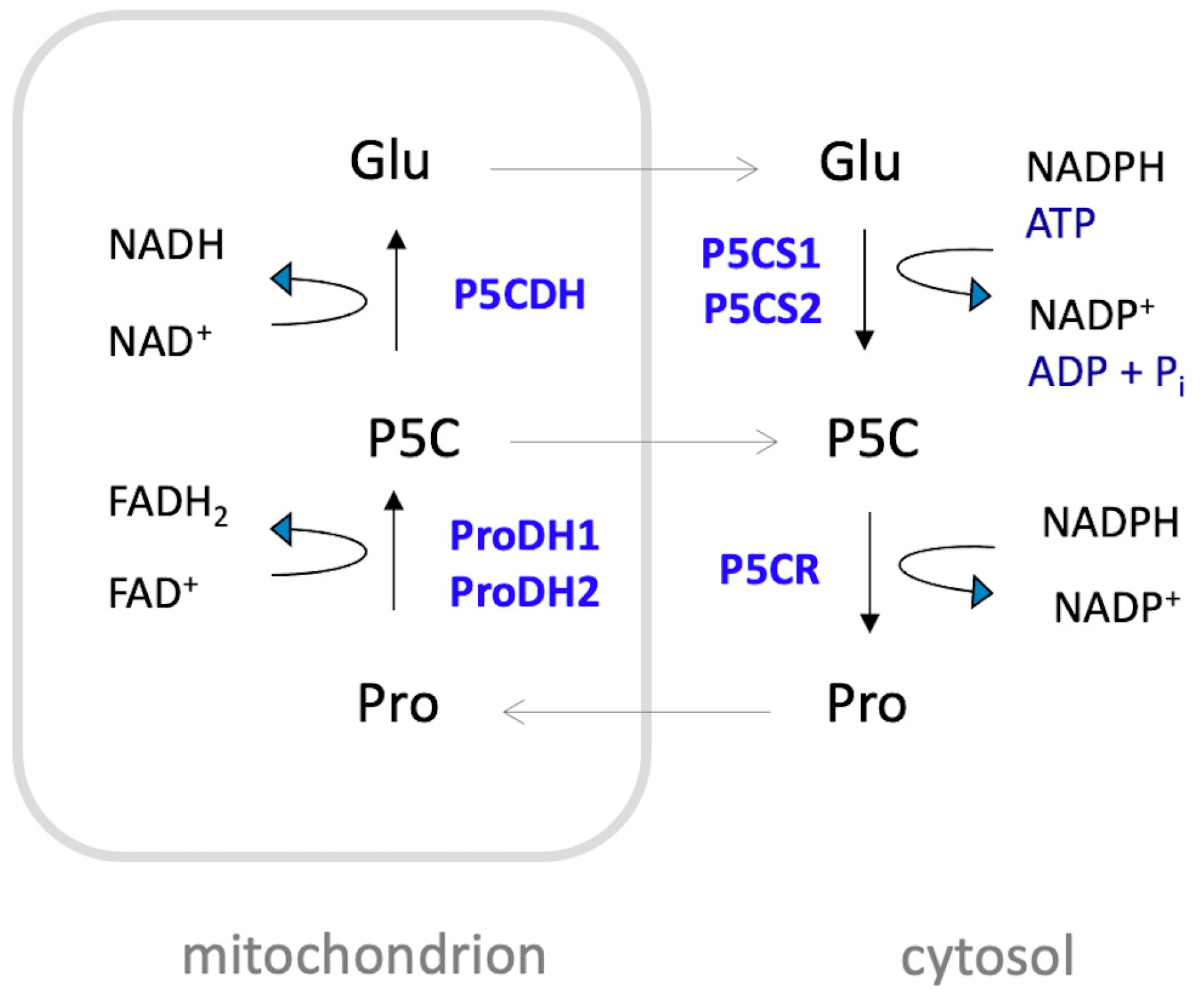
The extent of proline accumulation under adverse conditions depends on the plant species and can increase, in strong accumulators, by several hundred-fold compared to unstressed levels [33]. Proline is primarily synthesized in the cytosol from glutamate through a two-step reduction process (Figure 2). This process is catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS) and Δ1-pyrroline-5-carboxylate reductase (P5CR). During the first step, P5CS catalyzes the conversion of glutamate to glutamate-γ-semialdehyde (GSA), which is in tautomeric equilibrium with Δ1-pyrroline-5-carboxylate (P5C). This step involves the consumption of ATP and is coupled with the oxidation of NADPH to NADP+. In the second step, P5CR catalyzes the reduction of P5C to proline, also coupled with the oxidation of NAD(P)H to NAD(P)+. An alternative route from ornithine is also possible in animals and fungi, but it seems to be prevented in plants due to the localization of ornithine aminotransferase and P5CR in separate cell compartments [34,35,36]. Proline catabolism occurs in the mitochondria, where ProDH catalyzes the oxidation of proline to P5C, reducing FAD to FADH2, and P5CDH catalyzes the conversion of P5C to glutamate, reducing NAD+ to NADH (for a thorough review on proline metabolism see Trovato et al., 2019 [37]).
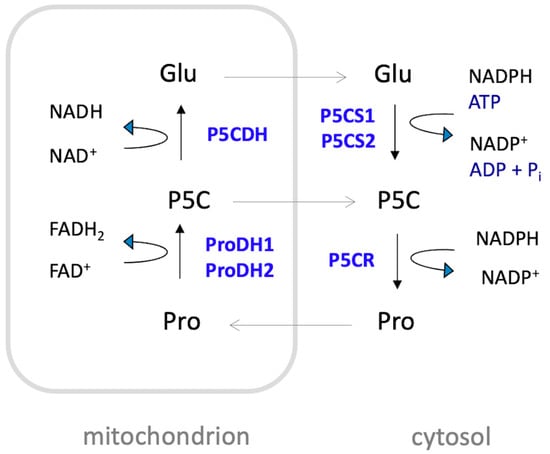
Figure 2.
Schematic representation of proline metabolism in Arabidopsis. Proline (Pro) is mainly synthesized in the cytosol from glutamate (Glu) by a two-step reduction catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS) and Δ1-pyrroline-5-carboxylate reductase (P5CR). The process involves the consumption of one molecule of ATP and the oxidation of two molecules of NADPH to NADP+. Proline catabolism takes place in the mitochondrion, where proline is oxidized to Δ1-pyrroline-5-carboxylate (P5C) by the action of ProDH1 and ProDH2, with concomitant reduction of FAD to FADH2. Subsequently, P5C is oxidized to glutamate via P5C dehydrogenase (P5CDH), coupled with the reduction of NAD+ to NADH. In Arabidopsis, P5CS and ProDH are encoded by two pairs of paralogous genes: P5CS1 and P5CS2, and ProDH1 and ProDH2, respectively. These genes encode the isozymes P5CS1, P5CS2, ProDH1, and ProDH2.
The electrons from FADH2 and NADH are transferred via the electron transport chain to molecular oxygen, generating a proton gradient across the inner mitochondrial membrane, which drives ATP synthesis via ATP synthase. However, as a by-product of mitochondrial respiration, superoxide anions (O2•–) are continuously generated and can cause oxidative damage to cellular components unless they are quenched by the antioxidant defense system [38]. In the mitochondrion, O2•– is rapidly converted to hydrogen peroxide (H2O2) by the action of superoxide dismutases (SOD). H2O2 can then pass through the mitochondrial membrane and diffuse into the cytosol or even neighboring cells, where it can act as either a signaling molecule activating the cellular antioxidant defense and further adaptive responses or a toxic agent leading to oxidative damage and death [38]. Depending on the amount of H2O2 produced, proline catabolism can therefore induce antioxidant or pro-oxidant responses.
At the molecular level, stress triggers the rapid transcriptional upregulation of P5CS, the gene encoding the rate-limiting enzyme in proline biosynthesis, and possibly P5CR, which catalyzes the second committed step in this pathway. In Arabidopsis, P5CS is encoded by the paralogous genes P5CS1 and P5CS2, and only P5CS1 is rapidly induced by stress through ABA-dependent and ABA-independent mechanisms, while P5CS2 has housekeeping and developmental functions [8,9,39,40,41]. Duplication of P5CS genes has occurred independently in several plant lineages and the functional specialization appears similar, although not identical, in different plant species [42,43,44]. While proline accumulation in the cytosol is largely driven by enhanced biosynthesis, it is further promoted by the downregulation of proline dehydrogenase (ProDH), the gene coding for the rate-limiting enzyme in proline catabolism, and possibly Δ1-pyrroline-5-carboxylate dehydrogenase (P5CDH), which codes for the second enzyme of proline degradation. In Arabidopsis, ProDH is encoded by two paralogous genes, ProDH1 and ProDH2. The downregulation of the expression of these genes reduces proline degradation during stress [33].
Chemically, proline has unique properties that make it important in protein folding and effective as a compatible osmolyte. Structurally, it is the only amino acid in which the α-amino group is reconnected to the side chain to form a secondary amine in a heterocyclic structure. This conformation confers exceptional structural rigidity to the protein structure and makes it important in protein folding [45,46]. It is often found in polyproline- or hydroxyproline stretches in cell wall proteins, such as extensins, and arabinogalactan proteins, which are thought to play key roles in cell elongation and signal transduction [47]. In addition, free proline is a zwitterionic molecule with a pKa in the neutral pH range, high compatibility with cellular metabolism, and high solubility in water, where it can reach concentrations of up to 6.5 M [48], making this amino acid a perfect candidate as a compatible osmolyte.
While clear evidence of proline conferring stress tolerance in plants is still debated, numerous transgenic plants have been engineered over the years to alter proline metabolism, by overexpressing proline biosynthesis genes, downregulating proline catabolism genes, or increasing transport from one tissue to another. The studies have shown increased stress tolerance, at least under laboratory conditions, supporting the idea that proline accumulation helps plants withstand adverse conditions [49]. Additionally, treatments with proline have been reported to effectively protect crops from various environmental stresses, further supporting its positive role in stress responses [25,50]. However, despite general agreement on proline as a reliable stress marker, consensus on its positive effects under stress conditions is not unanimous. Some researchers have reported neutral or negative effects of proline accumulation under stress [51,52,53,54,55,56], suggesting that in some cases proline might be a mere symptom of stress rather than a cure to relieve it [55].
3. Mechanisms of Proline’s Protective Role Under Abiotic Stress
3.1. Osmoprotection
Assuming that proline accumulation improves plant tolerance to stress damage, it remains to be understood how and by which molecular mechanisms. Given the peculiar chemical properties of proline as a compatible osmolyte, it is difficult not to believe that these properties are at the basis of proline-induced stress tolerance.
Indeed, the osmoprotective function of proline is one of the earliest and most widely accepted mechanisms to explain the role of proline in stress tolerance [22,57]. Under osmotic stress conditions, such as drought or salinity, the accumulation of osmolytes in the cytoplasm can help reduce the cellular water potential below the external water potential without adversely affecting cellular metabolism. In this way cells limit or stop water loss and preserve turgor pressure. This osmotic adjustment is thought to be critical for protecting cellular structures and maintaining the integrity of membranes and proteins, thus preventing cellular damage and ensuring continued metabolic activity. Since its first formulation, this hypothesis has been confirmed by numerous publications (See Forlani et al., 2019 [33] for a review) and has become broadly accepted as a mechanism to explain proline-induced tolerance to osmotic stress.
However, despite its popularity, this putative mechanism of action has never been conclusively proven and has been questioned by some authors [33,54,58,59,60]. The first doubts were raised in a seminal paper by Hare and Cress (1997) [51], who noted that in most cases the cytoplasmic pool of free proline appeared to be too small to account for a relevant contribution of proline to the overall osmolarity, even during a stressful episode. Later, subsequent studies made on cell suspension cultures have supported this early hypothesis by demonstrating that even under severe osmotic stress, the concentration of proline rarely increases beyond a few micromoles per gram of fresh weight. For instance, some studies that quantified the cellular sap concentration along with its chemical components found that proline’s contribution to the necessary increase in osmolarity was no more than 3 to 15%, even though its concentration had increased by a factor of one hundred compared to the controls [61,62,63]. Along the same line, only modest or no contribution to osmotic adjustment was found in flour wheat (Triticum aestivum) [64], barley (Hordeum vulgare) [65], and durum wheat (Triticum turgidum durum) [66]. Overall, apart from a few exceptions, such as in maize (Zea mays) roots grown at low water potentials, where proline accumulation can account for 45% of the total osmotic adjustment [67], or in the halophytes salt cress (Thellungiella halophila) and thick-leaved pepperwort (Lepidium crassifolium), which accumulate large amounts of proline [68,69], in most glycophytes the level of proline accumulation appears to be insufficient for meaningful osmotic adjustment compared to other osmolytes such as potassium (K+) and sodium (Na+), which contribute much more to the osmotic pressure of the cell [33]. Moreover, a recent meta-analysis found that the transgenic expression of proline metabolism genes has overall positive effects on drought and salinity tolerance, but only marginally through osmotic regulation [49].
3.2. Protection of Proteins and Membranes
Another property of proline thought to be responsible for or contribute to stress tolerance is its capability to protect and stabilize enzymes and membranes from environmental stresses [51,70,71]. It is not clear which mechanism or mechanisms can explain these effects on protein and membrane stability, but obviously, the compatible osmolyte’s properties of proline have the potential to perform this role. With the limitations discussed in the previous paragraph, the effect of proline on overall osmolarity may help cells maintain their volume and water content during periods of environmental stress, preserving proteins and membranes from dehydration. However, considering the controversial contribution of proline in osmotic adjustment, other mechanisms have been proposed to act in substitution to or synergistically with osmotic adjustment, the most convincing of which stems from the kosmotropic properties of proline.
When cells experience osmotic stress, the withdrawal of water can lead to the accumulation of chaotropic substances that disrupt protein structure and function. Proline, similarly to other compatible solutes, exerts a kosmotropic (anti-chaotropic) effect by promoting the ordering of water molecules around macromolecules or by replacing them, which is critical for maintaining proper three-dimensional protein folding and enzymatic activity. This ordered hydration shell reduces entropy, helping to stabilize proteins and prevent unfolding. Furthermore, according to Schobert and Tschesche (1978) [48], proline could increase the hydrophilic surface area of proteins and enhance their water-binding capacity by stepwise stacking, and hydrophobic interactions involving its pyrrolidine ring. This increased hydration capacity has been shown to alleviate the negative effects of dehydration on protein stability and activity [71]. Additionally, proline is believed to facilitate protein renaturation and prevent aggregation by trapping folding intermediates in a supramolecular assembly [72].
In terms of membrane stabilization, proline interacts with phospholipid headgroups, modulating membrane fluidity and permeability [73]. This interaction helps to maintain the structural integrity of cellular membranes during stress, preventing leakage and preserving cellular compartmentalization. Additionally, proline’s ability to form hydrogen bonds with water molecules contributes to the formation of a protective hydration layer around membranes, further enhancing their stability under adverse conditions [74].
3.3. ROS Scavenging
Among the functions proposed for proline to explain its beneficial effects on environmental stress, the ROS scavenger has been one of the most accredited but, at the same time, most controversial. In plant cells, ROS are constantly produced as by-products of photosynthetic and mitochondrial electron transport, as well as by peroxisomal metabolism, and the production rates are typically increased by stress [38]. Additionally, NADPH oxidases and peroxidases mediate extracellular production of ROS, which can react in the cell wall or diffuse back into the cells [75,76].
The ROS scavenging function of proline was first reported in the seminal work of Smirnoff and Cumbes [77], who suggested, as early as 1989, that proline could effectively scavenge hydroxyl radicals (•OH) and proposed that proline could react with various ROS, laying the foundation for further research into the antioxidant capabilities of proline. Later, proline was shown to reduce lipid peroxidation as shown by reduced malondialdehyde (MDA) formation, first in brown mustard (Brassica juncea) cotyledons under various stress conditions [78,79,80], and subsequently in a number of different species [49,81], confirming the antioxidant effects of proline.
While the protective effects of proline against oxidative damage are generally accepted by the scientific community, the ability of proline to be a direct ROS scavenger is based more on logical considerations—considering that one of the main detrimental effects of stress is the generation of ROS—than on solid evidence, which is mainly based on theoretical and computational studies or in vitro studies. Overall, there is some correlative or theoretical evidence to support the hypothesis that proline can directly scavenge the hydroxyl radical and, perhaps, singlet oxygen (1O2), but there is no sound evidence for a direct effect on the superoxide anion (O2•–) and hydrogen peroxide (H2O2).
For •OH, the conclusions of Smirnoff and Cumbes were confirmed and extended by Signorelli et al. [82], who found by computational chemistry that hydrogen abstraction from proline is the most favored reaction with •OH and yields less reactive intermediates. However, for a true protection mechanism, proline would have to be far more abundant than the compounds or proteins damaged by •OH.
With respect to 1O2, the original claim that proline is a quencher of singlet oxygen in isolated thylkoids because of its ability to reduce singlet oxygen-mediated 2,2,6,6-tetramethylpiperidin (TEMP) oxidation [83] was refuted by Signorelli et al. [84], who reanalyzed the putative mechanisms of quenching by real-time measurement of singlet oxygen fluorescence and concluded that proline was not able to quench singlet oxygen.
More recently, Rehman et al. [85] employed paramagnetic resonance spin trapping with TEMPD fluorescence probing using singlet oxygen sensor green and oxygen uptake measurements in isolated thylakoids, demonstrating that proline quenches both singlet oxygen and superoxide radicals (O2•−) in vitro through an electron transfer mechanism. However, these claims are questionable because they have only been demonstrated in in vitro systems and because they are in contrast with the elegant conclusions of Hamilton and Heckathorn [86], who demonstrated that the primary cause of mitochondrial electron transport disruption due to saline stress is oxidative damage in complex I and Na+ toxicity in complex II, and that proline was unable to protect complex I. As superoxide dismutase (SOD) provided the highest protection of complex I, the authors concluded that O2•− was causing most of the oxidative damage of complex I. Altogether, these results challenge the notion that proline has a prominent function as an antioxidant and suggest that it does not provide protection against superoxide.
Contrary to the direct scavenging hypothesis, the possibility that proline may indirectly eliminate ROS, particularly by protecting antioxidant enzymes and increasing their activity, seems more likely and convincing. Indeed, a number of studies have reported that proline induces the activity of antioxidant enzymes such as catalase (CAT), peroxidase, polyphenol oxidase, and ascorbate peroxidase (APX) [13,49,87], suggesting that some of the observations of proline amelioration of oxidative damage may be related to an enhancement of the enzymatic antioxidant machinery. The stimulating effect of proline on the antioxidant defense could either be mediated by the kosmotropic or osmoprotective properties of proline or by direct or indirect induction of the expression of the genes of antioxidant enzymes.
3.4. Proline as Signaling Molecule
Under non-stressed conditions, homeostatic levels of proline are maintained by feedback regulation of P5CS activity, as well as reciprocal regulation of gene expression of P5CS and ProDH, indicating that plants sense and respond to intracellular proline concentration [37,88]. External proline application induced 85 out of 7000 analyzed Arabidopsis transcripts and a rehydration- and proline-responsive CIS-element (ACTCAT) was identified in the promoter of ProDH and other proline-responsive genes [89,90]. Members of the bZIP family of transcription factors have been identified as mediators of hypo-osmolarity-induced induction of ACTCAT-containing promoters [91,92]. Transcriptome analysis of chromium stressed Arabidopsis seedlings revealed that the beneficial effect of additional proline treatment was correlated with the differential expression of genes encoding proteins involved in cell wall biosynthesis and DNA repair [93,94]. Together with the data on the induction of the antioxidant defense system, these observations show that a significant part of the functions of proline could be mediated by specific signal transduction. However, the molecular mechanisms of proline sensing and signaling and the full range of target genes remain to be discovered.
3.5. Proline Metabolism
Despite the clear correlation between proline accumulation and its beneficial effects under stress, growing evidence suggests that proline metabolism, rather than proline itself, plays a crucial role in the impact of proline on stress responses and development [49,51,95,96]. As originally proposed by Hare and Cress [51], and described above, in most plant species the pool of free proline that accumulates in the cytoplasm during and after stress appears to be of insufficient size to account for the beneficial effects of proline on stress tolerance (see also Forlani et al. [33]). This observation implies that proline accumulation per se may not be the cause of proline-induced stress resistance to environmental stress. Second, an increasing number of papers report a lack of correlation between proline levels and stress tolerance or interpret the positive effects of proline against stress in terms of proline metabolism [13,49,53,58,96,97,98,99,100]. This controversial finding suggests that the simple accumulation of proline may not be sufficient to fully explain the adaptive response of proline under stress, prompting the search for other mechanisms.
In contrast, the unique characteristics of proline metabolism and its central role in intermediary metabolism may better explain its beneficial effects under stressful conditions. An important feature of proline metabolism is that its synthesis occurs in the cytosol, where it is either used for protein synthesis or transported to other cell compartments or tissues. According to some authors [8], proline synthesis could also occur in the chloroplast, especially under stress conditions, but this statement is not consistent with the absence of a chloroplast transit peptide in P5CS and is not confirmed by more recent work (see Trovato et al. [37] for more details). For degradation, proline is imported by unknown carriers or transporters into mitochondria. Both proline synthesis from glutamate and proline catabolism to glutamate share the same intermediate, P5C, which is in tautomeric equilibrium with GSA. As noted by Phang [101,102], proline and P5C are not interconverted by a single reversible enzyme but by two different enzymes with distinct mechanisms. A cycle between cytosolic P5CR and mitochondrial ProDH has been proposed to act as a redox shuttle in animal cells and promote ROS formation in mitochondria without net proline degradation [103,104]. Miller et al. [105] proposed the activity of such a cycle also in plants, but it is not yet clear whether P5C is exported in substantial amounts from mitochondria or whether it is primarily converted to glutamate by P5CDH. Several carriers were identified that mediate transport of glutamate across the mitochondrial membrane, thus cycling between proline and glutamate is definitely feasible if the enzymes of proline biosynthesis and catabolism are active at the same time [106,107]. The metabolic cycling between proline and P5C via ProDH and P5CR may provide a mechanism for transferring reducing equivalents from NAD(P)H into mitochondria, helping to maintain the balance of NAD(P)+ and NAD(P)H in the cytosol, especially when excess proline saturates the capacity of P5CDH to oxidize P5C to glutamate.
As for proline synthesis, the biosynthetic pathway from glutamate consumes two molecules of NADPH and one molecule of ATP per molecule of proline (Figure 1), with important implications in the reduction of stress-induced acidosis caused by NADPH accumulation. Even more important, the oxidation of NADPH to NADP+ stimulates the activity of the oxidative pentose phosphate pathway, which is essential for the synthesis of nucleotides and nucleic acids, the synthesis of aromatic acids and polyphenols, and the regeneration and sustenance of the ascorbate/glutathione pathway for ROS scavenging [51,108]. Additionally, if shuttled into chloroplasts, the NADP+ could be used as a terminal electron acceptor in the photosynthetic electron transport chain, reducing the risk of photoinhibition and of damaging the photosystem II (PSII) complex.
As for proline catabolism, the oxidation of proline to P5C and glutamate yields reducing equivalents in the form of FADH2 and NADH, which can be fed into the mitochondrial electron transport chain to produce ATP, thereby providing energy for the cell. This process is extremely efficient, as the oxidation of one molecule of proline to glutamate yields approximately 5 ATP equivalents, and complete oxidation of proline via glutamate and the tricarboxylic acid cycle yields approximately 30 ATP equivalents [109]. Proline catabolism is crucial for energy-consuming processes such as recovery from stress [110], root elongation [12,67], and the initial flight phase in some insects [111,112]. Due to the presence of glutamate dehydrogenase (GDH) in the mitochondrial matrix, glutamate may be converted to α-ketoglutarate (AKG) and fed into the mitochondrial TCA cycle.
Overall, the importance of anabolic and catabolic pathways in plant cell metabolism and the ability to control the balance between NAD+/NADH and NADP+/NADPH as a redox buffer through the proline-P5C cycle may well explain the beneficial effects of proline under and after stress.
4. Proline in Plant Development
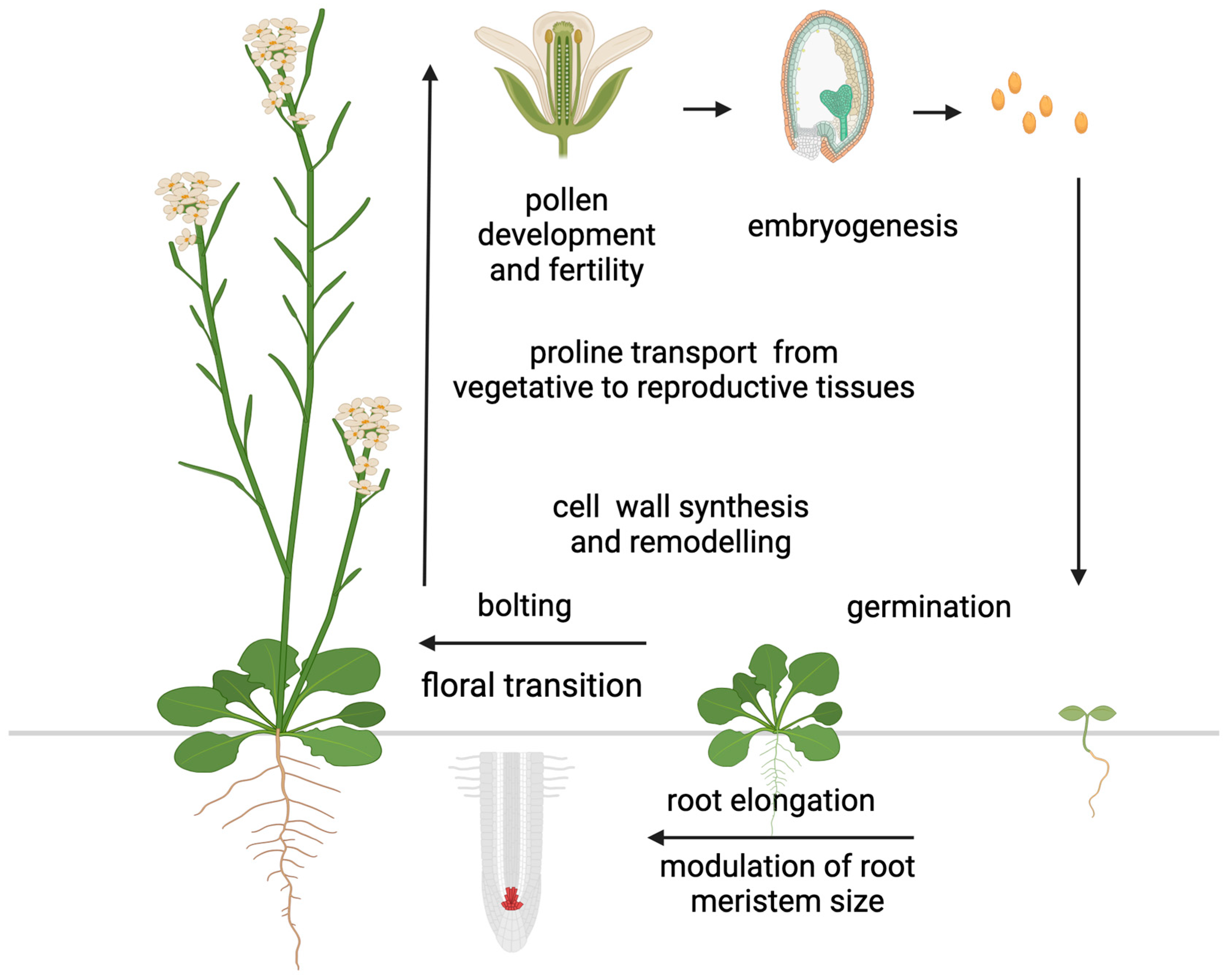
In addition to accumulating in response to biotic and abiotic stress, large amounts of proline have been detected in the reproductive organs of some plant species under non-stressed conditions. Specifically, high proline concentrations have been detected in pollen grains of petunia (Petunia hybrida), cowpea (Vigna unguiculata), and tomato (Lycopersicum esculentum) [113,114,115,116], ovules of broad bean (Vicia faba) [117], inflorescences and siliques of rapeseed (Brassica napus) [118], flower buds and young pods in barrel medic (Medicago truncatula) [119], and seeds in thale cress (Arabidopsis thaliana) [120], suggesting a role for proline in plant development (Figure 3).
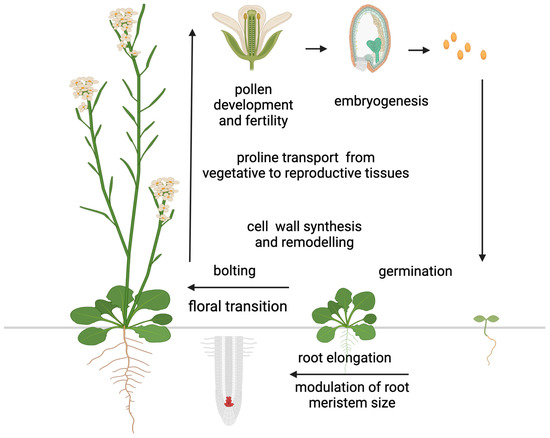
Figure 3.
Proline functions in plant development. The diagram illustrates the main functions and developmental processes proline is involved in. Most of these functions are related to the reproductive stage, with the exception of germination and root elongation. Figure created with BioRender.com.
The most convincing example of this evidence came from the excellent work of Chiang and Dandekar [121], who found that the percentage of proline relative to the total amino acid pool increased from 1–3% in the vegetative rosette of Arabidopsis prior to floral transition to about 26% in the reproductive tissue after floral transition. Schwacke et al. [116] reported a similar finding in Lycopersicum esculentum, where the proline content in flowers was 60 times higher than in any other organ analyzed. Although the striking difference in proline concentrations between vegetative and floral tissues found in tomato (Lycopersicum esculentum) is exceptional, a remarkable remobilization of proline from vegetative to floral tissues after floral transition is widespread among plant species, suggesting a special role for proline in floral transition and flower development. A complex developmental regulation underlies the redistribution of proline after floral transition involving long-distance transport [122], active transport between different cell compartments [116,120,123,124], direct synthesis within target tissues [121,125], selective catabolism [126,127], as well as the rate of protein synthesis and protein degradation [128]. However, despite the number of papers reporting accumulation of proline under normal conditions to much higher concentration than other proteinogenic amino acids, this phenomenon has long been overlooked. A major step in understanding the role of proline in plant development came from the genetic manipulation of the short proline pathway, particularly P5CS1 and P5CS2, the paralog genes encoding the enzyme that controls the rate-limiting steps of proline synthesis in Arabidopsis. The study of plants transgenic for proline metabolism genes and Arabidopsis mutants defective in proline synthesis or degradation has helped to uncover a role for proline in germination [129,130,131], floral transition [2,3,4,5,47], embryo development [8,9], pollen fertility [6,7,125], and root development [12,13,132]. Moreover, in the cell wall matrix, proline and hydroxyproline are major constituents of proteins and glycoproteins, such as extensins, arabinogalactans, and proline-rich proteins [133,134,135]. These proteins are essential for cell wall synthesis and remodeling and play crucial roles in cell elongation, division, and differentiation [10,136], highlighting the importance of proline in plant development (Figure 3).
The first evidence supporting the role of proline in normal plant development was likely provided by the work of Kavi Kishor et al. [47], who described increased root biomass and flower development in transgenic tobacco overexpressing P5CS. Later, Nanjo et al. [2] generated transgenic Arabidopsis for a P5CS gene in antisense orientation, which showed morphologically altered leaves and stunted inflorescences. In addition, Samach et al. [3] identified P5CS2 as an early target of CONSTANS (CO), a transcription factor that promotes flowering in Arabidopsis in response to day length. Collectively, these early findings suggested a role for proline in bolting and flower development. Consistent with these observations, Mattioli et al. [4] reported that CaMV35S-driven overexpression of P5CS1 led to early flowering and bolting during the initial stages of plant development in Arabidopsis, although, in the later stages, reduced proline levels and a bushy appearance were observed, likely due to homology-driven gene silencing of both P5CS1 and P5CS2 [6]. Supporting these findings, a partial double mutant homozygous for P5CS1 and heterozygous for P5CS2 (hereafter referred to as p5cs sesquimutant) contained less proline than the wild type and exhibited late flowering, probably due to the upregulation of FLOWERING LOCUS C (FLC), a master repressor of flowering in Arabidopsis [5,9]. In the p5cs sesquimutant the effects of the two parental mutants on flowering time are additive, since the p5cs sesquimutant flowers later than the two single mutants. However, the effects of the mutations on Arabidopsis development are very different, indicating largely non-redundant functions of P5CS1 and P5CS2 [8,40] with P5CS1 mediating stress-induced proline accumulation and P5CS2 more involved in plant development, particularly embryo development and pollen fertility [6,7,8,9,125]. More recently, by physiologic and metabolic analyses, Park et al. [137] found that the Ca++-induced flowering delay in peach (Prunus persica) is mediated by proline metabolism.
Regarding embryo development, two independent studies [8,9] have shown that embryos homozygous for a p5cs2 mutation exhibit developmental arrest and a number of aberrations at different stages of embryo development, failing to develop into viable plants and suggesting a specific role for P5CS2 in embryogenesis. Confirming the specific effect of proline in embryo development, proline supplementation was shown to complement this lethal defect, although different authors report different levels of complementation. Mattioli et al. [9] reported only partial complementation, while Székely et al. [8] were able to rescue whole but abnormal and infertile plants. Funck et al. [6] reported that in vitro culturing of immature homozygous p5cs2 embryos yielded fertile plants that produced viable seeds without further proline supplementation under short-day conditions. In addition to the critical role of P5CS2, P5CR is also required for embryo development, as homozygous p5cr mutants exhibit embryo lethality, with embryos aborting after only a few cell divisions [6]. Interestingly, while P5CR expression is essential for embryo development, it is not required for pollen or egg cell fertility, unlike P5CS2 (see next paragraph), likely due to the extreme stability of the P5CR protein [6].
In addition, Dourmap et al. [138] have recently reported a novel role for P5CDH in reallocating carbon and nitrogen from the stem to the seed, which is critical for accumulating the reserves required for seed development. They found that mutants defective in P5CDH activity produce small seeds with low carbon and nitrogen content and exhibit low germination rates, particularly under high nitrate availability. It is currently unknown whether the small size and wrinkled appearance of p5cdh mutant seeds are caused by small embryo size or a lack of reserve material. As P5CDH is involved in the degradation of both proline and arginine, the defects in p5cdh mutants may derive from the block in either one or both of these pathways.
Regarding pollen fertility, Funck et al. [6] and Mattioli et al. [7] independently found a novel role for proline in pollen development by crossing p5cs sesquimutant plants with the wild type. When the p5cs sesquimutant was used as the female, approximately 50% of the offspring contained a p5cs2 allele, as expected. However, less than 1% of the progeny received a p5cs2 allele when the p5cs sesquimutant was used as the pollen donor. Morphological and functional analysis of the mutant pollen revealed a group of small, degenerated, and non-viable pollen grains mixed with normal-looking pollen grains, confirming that the p5cs sesquimutant is defective in pollen development and suggesting that proline biosynthesis plays a vital role in male gametophyte development.
Proline accumulation has also been positively correlated with seed germination, although the evidence remains limited, and a definitive demonstration of proline’s role in germination is still awaited. Several studies have reported improved germination rates under stress in transgenic tobacco plants overproducing proline by expressing ornithine-δ-aminotransferase [129] or P5CS [139]. Hare et al. [130] demonstrated that plants expressing an antisense version of P5CS exhibited low proline content and impaired germination, which could be partially rescued by exogenous proline supplementation. Furthermore, they observed a four-fold increase in proline levels in most Arabidopsis seeds prior to radicle emergence. This increase was correlated with an activation of rate-limiting enzymes in the OPPP, leading to the hypothesis that the OPPP plays a significant role in seed germination by providing the necessary reducing power and metabolic intermediates required for biosynthetic processes during early seedling growth [130,140]. Furthermore, as described for embryo development, the inability of p5cdh mutants to oxidize P5C to glutamate negatively affects germination, supporting a correlation between proline (or arginine) metabolism and germination [138].
In addition to reproductive development, recent reports [13,132] have revealed the importance of proline in root development. This novel function is not completely unexpected, as the elongation of hairy roots induced by transformation with Agrobacterium rhizogenes was initially attributed to the action of rolD contained on the T-DNA [141,142]. rolD was later identified as ornithine cyclodeaminase (OCD), a gene of bacterial origin coding for an enzyme that directly converts ornithine to proline [12]. In addition, a specific requirement for proline metabolism has been reported in the elongation of primary roots of maize and Arabidopsis under low water potential conditions [11,95]. Later, proline was found to modulate root meristem size in Arabidopsis by affecting cell division and controlling the ratio between cell division and cell differentiation [9,132].
Although most of the known effects of proline on plant development have been found in Arabidopsis, the conservation of its biochemical pathway and role in stress defense across plant species suggests that these effects are likely to be generalizable to most plants. However, further research and experimental confirmations are needed to fully validate this hypothesis across a broader range of species.
5. Proline–ROS Interactions
Intriguingly, the effects of proline on root meristem size were found parallel to and independent from auxin, cytokinin, and gibberellic acid (GA) pathways and not involving genes controlling cell differentiation at the transition zone, such as ARR1, ARR12, and SHY2 [132]. Subsequent work has shown that proline metabolism affects the levels and distribution of superoxide anion (O2•–) and hydrogen peroxide (H2O2), which in turn modulate root meristem size and root elongation [13]. This finding was in agreement with Foreman et al. [143], who had previously shown that ROS produced by NADPH oxidase regulate root elongation through the activation of Ca++ channels, as well as with Dunand et al. [144] and Tsukagoshi et al. [145], who disclosed the relative role of O2•– and H2O2 in root elongation. Bauduin et al. [13] found that exogenous proline concentrations at low µM levels induced root elongation and correlated with high levels of O2•– and low levels of H2O2 in the root meristem, whereas high proline concentrations or proline-deficient mutants inhibited root elongation and correlated with H2O2 accumulation in the transition zone. Importantly, H2O2 scavenger treatment fully rescued the short root phenotype of the proline-deficient p5cs sesquimutant, as well as wild-type roots treated with high concentrations of proline. Furthermore, using mutants lacking ProDH activity, the same study revealed the critical role of ProDH in regulating root meristem size, probably as the main player in proline-induced O2•– production in mitochondria. It is at present not fully clear how different concentrations of proline or different rates of proline degradation can have inverse effects on the ratio between O2•– and H2O2 in different regions of the root meristem, especially since dismutation of O2•– by SOD is presumably the predominant source of H2O2 [146]. In addition, crosstalk between ROS and auxin signaling in the regulation of root growth has been repeatedly observed [147,148], raising the unresolved question how the effects of proline can be mediated by ROS and at the same time be independent of auxin signaling. Interestingly, Bauduin et al. (2022) found that while proline itself may not directly control H2O2 levels, it appears to modulate the activity of catalase (CAT) and ascorbate peroxidase (APX), the two most potent H2O2 enzymatic scavengers in plant cells.
Although Bauduin et al. were unable to localize precisely the site of O2•– in the root tip and limited their main conclusions to the effects of H2O2, their findings on the proline-mediated effects of O2•– and H2O2 on root meristem size are largely consistent with the work of Dunand et al. [144] and Tsukagoshi et al. [145]. The latter authors identified the transcription factor UPBEAT1, which encodes a repressor of root peroxidases, and characterized mutant and overexpressor lines. Mutants deficient in UPBEAT showed low H2O2 and long roots, whereas UPBEAT overexpressors had high H2O2 levels and short roots. The authors could phenocopy the mutant phenotypes with exogenous H2O2 and complement the short root phenotype with scavengers of H2O2. Overall, they concluded that meristem proliferation in the root is determined by the balance between O2•– and H2O2. Specifically, higher levels of O2•– relative to H2O2 promote root elongation and meristem proliferation, while higher levels of H2O2 relative to O2•– inhibit root elongation and reduce meristem size. Furthermore, they provided evidence that O2•– is mainly located in the apoplast of the meristematic and elongation zones, whereas H2O2 is found in the transition and differentiation zones, and that the ratio between O2•– and H2O2 determines root meristem growth in a hormone-independent manner.
To confirm the interactions between proline and H2O2 at the genetic level, Bauduin et al. [13] crossed the p5cs sesquimutant with upb1 [145]—a mutant allele of the transcription factor UPBEAT—whose inactivation leads to increased peroxidase activity and low levels of H2O2, resulting in roots longer than wild type, and analyzed the meristem size of the resulting p5cs upb1 quasi-triple mutant. The root meristem size of p5cs upb1 was intermediate between p5cs and upb1 root meristems, suggesting that the effects of H2O2 on root length are dose-dependent and that the balance of H2O2 levels is crucial for determining root meristem size. This finding corroborates the link between proline and ROS and supports the idea that root meristem size and root elongation are regulated by a delicate balance between O2•– and H2O2 levels, and that both proline synthesis and peroxidase activity play significant, although independent, roles in modulating this balance.
Since ROS are highly reactive and potentially toxic molecules that are constantly produced by the cell during normal metabolism and in response to environmental stresses, they are tightly controlled by a dynamic and complex array of enzymatic and non-enzymatic antioxidants, presumably including proline. Indeed, some non-enzymatic antioxidants such as glutathione [149], catechol [150], melatonin [151], and gallic acid [152] have been shown to modulate root meristem growth by controlling ROS levels. The correlation between H2O2 accumulation and the short root phenotype of the p5cs sesquimutant, as well as the inhibition of root growth induced by high concentrations of H2O2 (or proline), is easily understood given the known cellular toxicity of hydrogen peroxide [153,154]. It is well known that high concentrations of hydrogen peroxide, as well as other ROS and reactive nitrogen species (RNS), can be toxic and even lethal to plant cells. They can cause lipid peroxidation leading to membrane damage and loss of cell integrity, protein oxidation with serious consequences for enzyme activity and metabolic processes, and irreversible DNA damage and modification affecting gene expression and signaling pathways [154].
It is less intuitive to understand the stimulatory effects on root growth induced by low concentrations of proline (or H2O2). However, although earlier research focused mainly on ROS toxicity, current research has well established that ROS have a dual role in plants: toxic at high concentrations and beneficial at low concentrations, where they act as important signaling molecules that regulate normal plant development and responses to stress [17,155,156,157]. These latter processes involve interactions between NADPH oxidases, calcium (Ca2+) channels, and oxidative stress-induced Ca2+ fluxes. Intriguingly, proline-mediated Ca2+-dependent signaling was recently reported in the regulation of carbon/nitrogen metabolism of rice (Oryza sativa) subjected to Cr(VI) stress [158]. Similarly, by physiologic and metabolic analyses, Park et al. [137] showed that the Ca2+-induced late flowering in peach (Prunus persica) is dependent on proline metabolism. Overall, these results suggest a potential crosstalk between proline and ROS signaling pathways.
Proline–ROS interactions have also been described in response to biotic [159] and abiotic stress [160,161]. However, in contrast to unstressed conditions, where it seems to act mainly as a pro-oxidant, stress-induced proline accumulation or suppression of proline degradation seems to promote antioxidant activities. In a study investigating the basis of the hypersensitive response elicited by Arabidopsis in response to infection by an avirulent race of Pseudomonas syringae, Fabro et al. [159] found a positive correlation between ROS and proline. The authors observed a local increase in reporter activity in transgenic P5CS2:GUS or P5CS2:LUC plants infiltrated with a superoxide generator, suggesting that ROS can trigger the activation of P5CS2 and lead to proline accumulation during biotic stress. Additionally, under abiotic stress, several papers reported correlative evidence of ROS-induced proline accumulation, as reported by Uchida et al. [160], who found an upregulation of P5CS in rice seedling leaves in response to H2O2 treatment. Similarly, Yang et al. [162] described a significant accumulation of proline in coleoptiles and radicles of maize seedlings treated with H2O2, caused by the simultaneous increase in P5CS activity and decrease in ProDH activity. In later studies, Ben Rejeb et al. [161] investigated the relationship between ROS and proline and convincingly demonstrated that H2O2 generated from NADPH oxidase-produced O2•– leads to proline accumulation in Arabidopsis thaliana plants treated with 200 mM NaCl or 400 mM mannitol. Similarly, Liu et al. [163] reported that NADPH oxidase-mediated H2O2 reduced salt stress in wheat seedlings by enhancing proline accumulation.
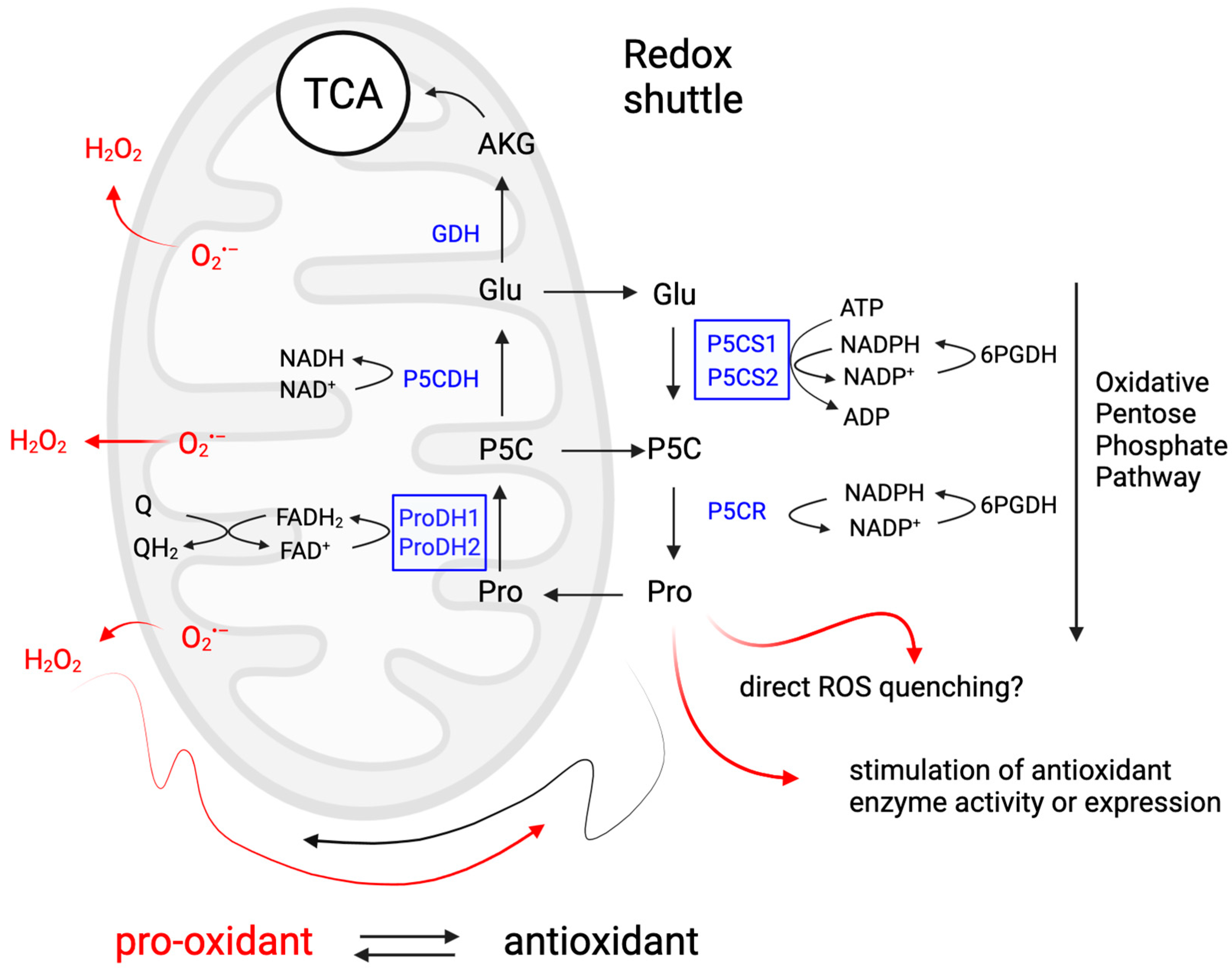
Overall, these studies highlight the importance of ROS in plant development and stress responses, emphasizing the need to control their levels. Thanks to its ability to (re)generate ROS and ATP in the mitochondrion via the proline–P5C or proline–glutamate cycle between the mitochondrion and cytosol, and the feedback loop between antioxidant activity during proline synthesis and pro-oxidant activity during catabolism, proline can control root development by regulating ROS levels and distribution in the root meristem (Figure 4).
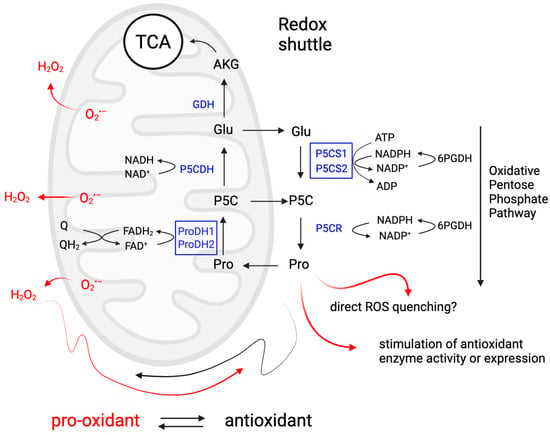
Figure 4.
Proline metabolism as a redox hub in plant response to stress and developmental stimuli. This diagram illustrates how proline metabolism can act as a redox shuttle between cytosol and mitochondria and modulate ROS homeostasis. Proline catabolism generates ROS in mitochondria and acts as a pro-oxidant, while proline synthesis lowers the cytosolic NADPH/NADP+ ratio, which stimulates the oxidative pentose phosphate pathway. Proline accumulation acts as an antioxidant by scavenging ROS, protecting membranes and organelles from ROS through its kosmotropic properties and by activating antioxidant enzymes. Through these mechanisms, proline metabolism can alter both the levels and species composition of ROS, which allows plants to integrate and respond to different stimuli and has the potential to explain most of the functions attributed to proline. Figure created in BioRender.com.
6. Can Interactions with ROS Explain Other Functions of Proline in Stress and Development?
It is well known that all stresses, both biotic and abiotic, generate different types of ROS in various locations [38,164,165]. In parallel, proline accumulates in most plants in response to stress, likely participating in and stimulating an antioxidant response. Accordingly, the interactions between proline metabolism and ROS, along with the consequent control of cellular redox balance and activation of signaling pathways to trigger antioxidant responses, may be considered a general or widespread mechanism of stress response. Other possible mechanisms, on the contrary, seem to be linked to specific situations. The accumulation of proline as a possible osmoregulation factor, for example, could be justified in response to salt or drought stress but seems doubtful in response to stresses without an osmotic component, such as nutritional stress, heavy metal stress, mechanical stress, and biotic stress from pathogen attack.
It is therefore likely that most, if not all, early responses to biotic or abiotic stress involve an interaction between proline and reactive oxygen species (ROS). However, does this also apply to all developmental processes modulated by proline, or only to the fine tuning of root meristem size?
6.1. Germination
As to germination, it is well documented that ROS play a pivotal role in regulating seed dormancy, germination, and viability [166,167]. In dry seeds, ROS are generated through non-enzymatic reactions, such as lipid auto-oxidation [168]. However, as soon as seed imbibition starts, enzymatic reactions begin to prevail [169,170]. Low levels of ROS act as signaling molecules that promote the release of physiological dormancy and trigger seed germination [171,172], by promoting endosperm weakening [173], stimulating gibberellic acid (GA) synthesis, and reducing abscisic acid (ABA) levels [174,175]. In contrast, high levels of ROS typically lead to seed deterioration by affecting lipid peroxidation, membrane permeability, protein integrity, and mitochondrial function [166]. Another important role of H2O2 in seed germination is the activation of α-amylase and the induction of programmed cell death (PCD) in the aleurone layer, in cooperation with the GA signaling repressor DELLA proteins [174,176]. Additionally, lower viability in long-term stored common bean (Phaseolus vulgaris) seeds has been shown to correlate with higher contents of O2•–, H2O2, and •OH [177]. In agreement with this finding, Trovato et al. [178] observed low viability associated with high contents of O2•– and H2O2 in four-year stored seeds of p5cs sesquimutants compared to wild-type seeds of the same age. In addition, the low germination rate of p5cdh mutants, as a consequence of impaired carbon and nitrogen remobilization during seed filling [138], could potentially be triggered by ROS signaling. This is suggested by the increase in ROS production exhibited by p5cdh plants infected with avirulent strains of Pseudomonas syringae [159]. Without P5CDH to convert P5C into glutamate, P5C may damage mitochondria and stimulate ROS formation [179]. Alternatively, P5C may be exported from mitochondria and be converted back to proline by P5C reductase (P5CR), return to the mitochondrion, and be oxidized again to P5C [20]. In this proposed Pro/P5C cycle, O2•– could be continuously produced as a by-product without net proline consumption (Figure 4).
6.2. Pollen Development and Pollination
Although at present there are no reports showing a direct link between proline and ROS in pollen development and fertility, indirect evidence strongly suggests that this might indeed be the case. In fact, ROS are essential in various stages of pollen development and fertilization, including tapetum degeneration, pollen tube growth, and pollen–stigma interactions [180,181,182], but because of their potential toxicity, they must be tightly regulated by enzymatic and non-enzymatic antioxidants. Since proline has been found in large amounts in pollens of several plant species at different stages of development [183], it is a strong candidate to play a major role as a ROS antioxidant in the pollen grain, as well as an important energy source.
Consistently, insufficient amounts of proline in pollen caused by mutations in proline synthesis genes lead to pollen degeneration and male sterility, indicating that proline accumulation in pollen grains is essential for their viability and fertility [6,7,125].
In addition, proline accumulation in response to environmental stress may play a critical role in protecting pollen grains by modulating ROS levels that are inevitably associated with environmental stress. It is well known that flowering plants are highly sensitive to abiotic stresses that severely impair sexual reproduction and reduce crop yield. This sensitivity is critically dependent on pollen viability, which is dramatically reduced by environmental stresses such as drought, salinity, and extreme temperatures [184]. Most of the yield reduction caused by these stresses is due to ROS accumulation, which leads to impaired anther and pollen development, incomplete anther dehiscence, reduced grain set, and precocious grain abortion [185,186,187,188]. Since both proline and ROS are crucial for pollen development and stress responses, it is plausible that proline may help modulate ROS levels to ensure proper pollen development and function under abiotic stress conditions. Accordingly, in wheat grown in the presence of 120 mM NaCl, pretreatment with proline increased grain yield and grain weight [189]. In maize treated with different concentrations of NaCl, proline solutions sprayed on maize leaves improved growth and grain yield at 25 mM and 50 mM NaCl [190]. Moreover, Mattioli et al. [56] reported that treatment with 150 mM NaCl administered after floral transition led to a significant reduction in the number of seeds per silique of wild-type Arabidopsis plants. However, when p5cs sesquimutants were subjected to the same treatment, the detrimental effect was more pronounced, with a significantly stronger reduction in the number of seeds per silique observed. Importantly, when a p5cs sesquimutant expressing an additional copy of the proline biosynthesis gene P5CS2 under the control of the pollen-specific promoter of the At5g17340 gene [191] was treated with 150 mM NaCl, the number of seeds per silique was significantly higher compared to the parental p5cs sesquimutant and close to wild type, supporting the hypothesis that proline may reduce salinity damage on seed production.
6.3. Female Gametophyte and Embryo Development
Despite the striking effect of proline deficiency on embryo development, leading to a number of morphological aberrations and ultimately embryo abortion [8,9], evidence for ROS involvement in plant embryogenesis is relatively scarce. ROS play a crucial role in the development and function of the female gametophyte in plants. They are involved in processes such as embryo sac patterning and polarity maintenance. ROS are also essential for pollen-pistil interactions, including pollen tube growth and fertilization [192,193]. Accordingly, the known role of proline as an antioxidant suggests that it could help modulate ROS levels and thereby protect the female gametophyte from oxidative damage during stress. In animal systems, however, proline has been shown to improve the development of preimplantation embryos by protecting them from oxidative stress. Studies in mouse embryos indicate that proline and its analogues can reduce mitochondrial activity and ROS levels, leading to improved embryo development and increased blastocyst formation [194], suggesting that the antioxidant properties of proline may play a role in maintaining redox balance during early embryo development. Overall, these findings support the hypothesis that proline–ROS interactions may be important for female gametophyte and embryo development, particularly under abiotic stress conditions, although further research is needed to establish direct links and fully understand the mechanisms involved.
6.4. Flowering Time
A number of studies report correlations between ROS and flowering time in the photoperiodic [195,196,197,198], GA-dependent [199,200], and stress-induced [201,202,203] pathways. Given the ability of proline to control the redox state and distribution of ROS in the plant cell, it is reasonable to speculate that proline may also influence flowering time through its interaction with ROS. Nevertheless, there are not many indications in the literature to support this view. One possibility is that proline-mediated variation in ROS accumulation may affect flowering time by controlling the expression of FLOWERING LOCUS C (FLC), a master repressor of flowering time in Arabidopsis [204], whose expression is influenced by cellular redox status and ROS levels [198]. Indeed, it was recently reported that the p5cs sesquimutant, which is deficient in proline synthesis, shows an upregulation of FLC expression compared to wild-type plants [5]. Vernalization-induced down-regulation of FLC abolishes the flowering delay in these mutants, suggesting that FLC acts downstream of proline biosynthesis genes and is required for proline-modulated flowering. Since all proline synthesis mutants (p5cs1, p5cs2, and p5cs1p5cs2/P5CS2) accumulate H2O2 [8,9,13], it is reasonable to speculate that the altered redox balance due to proline deficiency leads to increased ROS levels, which in turn upregulate FLC expression and delay flowering.
Moreover, a floral pathway convincingly modulated by ROS, through interactions with the circadian clock and the FLAVIN-BINDING KELCH-REPEAT F-BOX1 (FKF1) transcription factor, is the photoperiodic pathway by which Arabidopsis regulates flowering time in response to day length. The CONSTANS (CO) protein acts as a master regulator of this pathway [205], integrating light signals and the circadian clock to regulate the expression of the FLOWERING LOCUS T (FT) gene [3,206]. Since proline has been found to oscillate with light and dark cycles due to the circadian regulation of the enzymes P5CS and ProDH [207], and P5CS2 has been shown to be one of the early targets of CO [3], the involvement of proline in the photosynthetic pathway, through interactions with ROS and the circadian cycle, is conceivable.
7. Conclusions and Future Perspectives
As described above, most of the mechanisms hypothesized to account for the beneficial effects of proline, both in response to biotic or abiotic stresses and in plant development, are related to ROS or redox balance, suggesting a universal role for proline metabolism in controlling plant responses through regulation of ROS homeostasis (Figure 4). Can we therefore consider the redox buffering properties of proline metabolism and its role in maintaining ROS homeostasis as a unifying mechanism underlying all known functions of proline in plant development and stress defense?
Perhaps not, at least not yet. Despite the remarkable physicochemical properties of this amino acid, which could potentially explain all the functions attributed to proline in terms of ROS modulation and redox buffering, we currently lack sufficient evidence to support such a general claim. However, this review suggests that proline metabolism, together with a battery of enzymatic and non-enzymatic antioxidants, participates in the complex regulatory system that serves to regulate and control ROS signaling. Because of its antioxidant and pro-oxidant properties, proline metabolism is involved in modulating redox balance and ROS homeostasis, which could potentially explain many of the multiple roles attributed to proline and deserves further investigation. There are still many gaps in our knowledge regarding the relationships between proline and ROS that warrant further investigation. First, we should study the role of proline metabolism, if any, in all the physiologic processes proline is involved in, and improve our knowledge of those in which the interactions between ROS and proline are already known. For example, we still lack understanding of the downstream genes or the signaling pathways involved that respond directly to proline or to proline metabolism-derived ROS stimuli in the root meristem to adjust meristem size to environmental and developmental variations. Even more complex, and deserving of further investigation, is the synergy between proline metabolism and ROS homeostasis during sexual reproduction, from the development of male and female gametophytes to the formation of a viable embryo, of which we currently have only a fragmented and incomplete picture. Another important question that we may want to address in the future is how the multiple players that contribute to the control and regulation of redox balance and ROS signaling—the enzymatic and non-enzymatic antioxidants—communicate with each other, and likely also with plant hormones, to produce integrated responses. It is still unclear how proline can control synthesis and catabolism to tailor ROS production and removal to specific tasks, nor do we know how much the proline-P5C or proline-glutamate cycles contribute to this mechanism and the details of the signal perception/transduction pathways upstream of proline metabolism.
Furthermore, in a global context characterized by increasing temperatures and droughts, especially in some geographical areas, there is a growing interest in finding biotechnological solutions to accelerate the adaptation of crops to new environmental conditions [208,209,210]. A better understanding of the interactions between proline and ROS and between enzymatic and non-enzymatic antioxidants promises to have important applicative implications.
Author Contributions
M.T. and D.F. wrote the review. M.R. collected the bibliography and prepared the figures. All the authors reviewed the draft and discussed improvements. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Sapienza Departmental Projects 2023 RD12318A998C70B8, 393 and Sapienza Research Project 2021 RM12117A51B2CED7 from Sapienza University of Rome.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, T.; Kobayashi, M.; Yoshiba, Y.; Sanada, Y.; Wada, K.; Tukaya, H.; Kakubari, Y.; Yamaguchi- Shinozaki, K.; Shinozaki, K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.S.; Yanofsky, M.F.; Coupland, G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Trovato, M. Proline Affects Flowering Time in Arabidopsis by Modulating FLC Expression: A Clue of Epigenetic Regulation? Plants 2022, 11, 2348. [Google Scholar] [CrossRef]
- Funck, D.; Winter, G.; Baumgarten, L.; Forlani, G. Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biol. 2012, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Biancucci, M.; Lonoce, C.; Costantino, P.; Trovato, M. Proline is required for male gametophyte development in Arabidopsis. BMC Plant Biol. 2012, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Székely, G.; Ábrahám, E.; Cséplő, A.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Falasca, G.; Sabatini, S.; Altamura, M.M.; Costantino, P.; Trovato, M. The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol. Plant. 2009, 137, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Hima Kumari, P.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Skarp, R.E. Proline accumulation in maize (Zea mays L.) primary roots at low water potentials. II. Metabolic source of increased proline deposition in the elongation zone. Plant Physiol. 1999, 119, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Maras, B.; Linhares, F.; Costantino, P. The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc. Natl. Acad. Sci. USA 2001, 98, 13449–13453. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, S.; Latini, M.; Belleggia, I.; Migliore, M.; Biancucci, M.; Mattioli, R.; Francioso, A.; Mosca, L.; Funck, D.; Trovato, M. Interplay between Proline Metabolism and ROS in the Fine Tuning of Root-Meristem Size in Arabidopsis. Plants 2022, 11, 1512. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Honig, A.; Stein, H.; Suzuki, N.; Mittler, R.; Zilberstein, A. Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 2009, 284, 26482–26492. [Google Scholar] [CrossRef]
- Kemble, A.R.; Macpherson, H.T. Liberation of amino acids in perennial rye grass during wilting. Biochem. J. 1954, 58, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plant. Plant J. 1993, 4, 9. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple Roles of Proline in Plant Stress Tolerance and Development. Rend. Lincei-Sci. Fis. 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Csonka, L.N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. MGG 1981, 182, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Verslues, P.E.; Sharp, R.E. Role of amino acids in abiotic stress resistance. In Plant Amino Acids: Biochemistry and Biotechnology; Singh, B.K., Ed.; Marcel Dekker, NY: New York, NY, USA, 1999; pp. 319–356. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Schobert, B. The influence of water stress on the metabolism of diatoms. II. Proline accumulation under different conditions of stress and light. Z. Pflanzenphysiol. 1977, 85, 11. [Google Scholar]
- Brown, L.M.; Hellebust, J.A. Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can. J. Bot. 1978, 56, 676–679. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.S. Regulation of proline synthesis in osmotic response: Effects of protein synthesis inhibitors. J. Exp. Zool. 1991, 259, 272–277. [Google Scholar] [CrossRef]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P., Eds.; Springer Press: Berlin/Heidelberg, Germany, 2019; pp. 73–97. [Google Scholar]
- Funck, D.; Stadelhofer, B.; Koch, W. Ornithine-δ-aminotransferase is essential for arginine catabolism but not for proline biosynthesis. BMC Plant Biol. 2008, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- De Ingeniis, J.; Ratnikov, B.; Richardson, A.D.; Scott, D.A.; Aza-Blanc, P.; De, S.K.; Kazanov, M.; Pellecchia, M.; Ronai, Z.; Osterman, A.L.; et al. Functional specialization in proline biosynthesis of melanoma. PLoS ONE 2012, 7, e45190. [Google Scholar] [CrossRef] [PubMed]
- Brandriss, M.C.; Magasanik, B. Proline: An essential intermediate in arginine degradation in Saccharomyces cerevisiae. J. Bacteriol. 1980, 143, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Forlani, G.; Signorelli, S.; Funck, D. Proline Metabolism and Its Functions in Development and Stress Tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Savouré, A.; Hua, X.J.; Bertauche, N.; Van Montagu, M.; Verbruggen, N. Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol. Gen. Genet. 1997, 254, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Strizhov, N.; Ábrahám, E.; Ökrész, L.; Blickling, S.; Zilberstein, A.; Schell, J.; Koncz, C.; Szabados, L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book 2010, 8, e0140. [Google Scholar] [CrossRef] [PubMed]
- Turchetto-Zolet, A.C.; Margis-Pinheiro, M.; Margis, R. The evolution of pyrroline-5-carboxylate synthase in plants: A key enzyme in proline synthesis. Mol. Genet. Genom. 2009, 281, 87–97. [Google Scholar] [CrossRef]
- Forlani, G.; Bertazzini, M.; Zarattini, M.; Funck, D. Functional characterization and expression analysis of rice delta(1)-pyrroline-5-carboxylate dehydrogenase provide new insight into the regulation of proline and arginine catabolism. Front. Plant Sci. 2015, 6, 591. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Sabbioni, G.; Barera, S.; Funck, D. A complex array of factors regulate the activity of Arabidopsis thaliana delta(1)-pyrroline-5-carboxylate synthetase isoenzymes to ensure their specific role in plant cell metabolism. Plant Cell Environ. 2024, 47, 1348–1362. [Google Scholar] [CrossRef]
- MacArthur, M.W.; Thornton, J.M. Influence of proline residues on protein conformation. J. Mol. Biol. 1991, 218, 397–412. [Google Scholar] [CrossRef]
- Ge, M.; Pan, X.M. The contribution of proline residues to protein stability is associated with isomerization equilibrium in both unfolded and folded states. Extremophiles 2009, 13, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.; Hong, Z.; Miao, G.H.; Hu, C.; Verma, D. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Schobert, B.; Tschesche, H. Unusual solution properties of proline and its interaction with proteins. Biochim. Biophys. Acta 1978, 541, 270–277. [Google Scholar] [CrossRef]
- Renzetti, M.; Bertolini, E.; Trovato, M. Proline Metabolism Genes in Transgenic Plants: Meta-Analysis under Drought and Salt Stress. Plants 2024, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- de Lacerda, C.F.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A. Changes in growth and in solute concentrations in sorghum leaves and roots during salt stress recovery. Environ. Exp. Bot. 2005, 54, 69–76. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [PubMed]
- Poustini, K.; Siosemardeh, A.; Ranjbar, M. Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Genet. Resour. Crop Evol. 2007, 54, 925–934. [Google Scholar] [CrossRef]
- Signorelli, S. The Fermentation Analogy: A Point of View for Understanding the Intriguing Role of Proline Accumulation in Stressed Plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Palombi, N.; Funck, D.; Trovato, M. Proline Accumulation in Pollen Grains as Potential Target for Improved Yield Stability Under Salt Stress. Front. Plant Sci. 2020, 11, 582877. [Google Scholar] [CrossRef]
- Jones, W.R.G.; Storey, L.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell Membrane Activities in Plants; Marré, E., Ciferri, O., Eds.; North Holland: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Blum, A.; Munns, R.; Passioura, J.B.; Turner, N.C.; Sharp, R.E.; Boyer, J.S.; Nguyen, H.T.; Hsiao, T.C.; Verma, D.; Hong, Z. Genetically Engineered Plants Resistant to Soil Drying and Salt Stress: How to Interpret Osmotic Relations? Plant Physiol. 1996, 110, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Shevyakova, N.I.; Ratikin, V.Y.; Muzycho, L.M.; Kuznetzov, V.V. Stress-induced accumulation of proline in relation to salt tolerance of intact plants and isolated cells. Appl. Biochem. Microbiol. 1998, 34, 5. [Google Scholar]
- Gupta, S.; Srivastava, J. Effect of salt stress on morphophysiological parameters in wheat. Indian J. Plant Physiol. 1990, 32, 10. [Google Scholar]
- Binzel, M.L.; Hasegawa, P.M.; Rhodes, D.; Handa, S.; Handa, A.K.; Bressan, R.A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987, 84, 1408–1415. [Google Scholar] [CrossRef]
- Handa, S.; Bressan, R.A.; Handa, A.K.; Carpita, N.C.; Hasegawa, P.M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983, 73, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Santos-Díaz, M.S.; Ochoa-Alejo, N. Effect of water stress on growth, osmotic potential and solute accumulation in cell cultures from chili pepper (a mesophyte) and creosote bush (a xerophyte). Plant Sci. 1994, 96, 21–29. [Google Scholar] [CrossRef]
- Chu, T.; Aspinall, D.; Paleg, L. Stress Metabolism. VII. Salinity and Proline Accumulation in Barley. Funct. Plant Biol. 1976, 3, 219–228. [Google Scholar] [CrossRef]
- Colmer, T.D.; Epstein, E.; Dvorak, J. Differential Solute Regulation in Leaf Blades of Various Ages in Salt-Sensitive Wheat and a Salt-Tolerant Wheat x Lophopyrum elongatum (Host) A. Love Amphiploid. Plant Physiol. 1995, 108, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Bajji, M.; Lutts, S.; Kinet, J.-M. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 2001, 160, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Voetberg, G.S.; Sharp, R.E. Growth of the Maize Primary Root at Low Water Potentials: III. Role of Increased Proline Deposition in Osmotic Adjustment. Plant Physiol. 1991, 96, 1125–1130. [Google Scholar] [CrossRef]
- Murakeözy, É.P.; Nagy, Z.; Duhazé, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Seki, M.; Satou, M.; Sakurai, T.; Kobayashi, M.; Ishiyama, K.; Narusaka, Y.; Narusaka, M.; Zhu, J.K.; Shinozaki, K. Comparative genomics in salt tolerance between Arabidopsis and aRabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol. 2004, 135, 1697–1709. [Google Scholar] [CrossRef]
- Paleg, L.G.; Douglas, T.J.; van Daal, A.; Keech, D.B. Proline, Betaine and Other Organic Solutes protect Enzymes against Heat Inactivation. Funct. Plant Biol. 1981, 8, 107–114. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. The stabilization of proteins by osmolytes. Biophys. J. 1985, 47, 411–414. [Google Scholar] [CrossRef]
- Samuel, D.; Kumar, T.K.; Ganesh, G.; Jayaraman, G.; Yang, P.W.; Chang, M.M.; Trivedi, V.D.; Wang, S.L.; Hwang, K.C.; Chang, D.K.; et al. Proline inhibits aggregation during protein refolding. Protein Sci. 2000, 9, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Situ, A.J.; Ulmer, T.S. Structural and thermodynamic basis of proline-induced transmembrane complex stabilization. Sci. Rep. 2016, 6, 29809. [Google Scholar] [CrossRef] [PubMed]
- De Maio, A.; Hightower, L. The interaction of heat shock proteins with cellular membranes: A historical perspective. Cell Stress Chaperones 2021, 26, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Alia; Saradhi, P.P.; Mohanty, P. Proline in relation to free radical production in seedlings of Brassica juncea raised under sodium chloride stress. Plant Soil 1993, 155, 497–500. [Google Scholar] [CrossRef]
- Alia; Prasad, K.V.S.K.; Pardha Saradhi, P. Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochemistry 1995, 39, 45–47. [Google Scholar] [CrossRef]
- Saradhi, P.P.; AliaArora, S.; Prasad, K.V.S.K. Proline Accumulates in Plants Exposed to uv Radiation and Protects Them against uv-Induced Peroxidation. Biochem. Biophys. Res. Commun. 1995, 209, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Z.; Lin, Y.-J.; Fan, W.-J.; Lu, M.-R. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr(VI). Int. Biodeterior. Biodegrad. 2017, 123, 106–112. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between •OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Alia; Pardha Saradhi, P.; Mohanty, P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. B Biol. 1997, 38, 253–257. [Google Scholar] [CrossRef]
- Signorelli, S.; Arellano, J.B.; Melø, T.B.; Borsani, O.; Monza, J. Proline does not quench singlet oxygen: Evidence to reconsider its protective role in plants. Plant Physiol. Biochem. PPB 2013, 64, 80–83. [Google Scholar] [CrossRef]
- Rehman, A.U.; Bashir, F.; Ayaydin, F.; Kóta, Z.; Páli, T.; Vass, I. Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiol. Plant. 2021, 172, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.W., 3rd; Heckathorn, S.A. Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol. 2001, 126, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, L.; Demir, Y. In vivo and in vitro protective role of proline. Plant Growth Regul. 2002, 38, 259–264. [Google Scholar] [CrossRef]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef]
- Oono, Y.; Seki, M.; Nanjo, T.; Narusaka, M.; Fujita, M.; Satoh, R.; Satou, M.; Sakurai, T.; Ishida, J.; Akiyama, K.; et al. Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca 7000 full-length cDNA microarray. Plant J. 2003, 34, 868–887. [Google Scholar] [CrossRef]
- Satoh, R.; Nakashima, K.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ACTCAT, a novel cis-acting element for proline- and hypoosmolarity-responsive expression of the ProDH gene encoding proline dehydrogenase in Arabidopsis. Plant Physiol. 2002, 130, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Fujita, Y.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A novel subgroup of bZIP proteins functions as transcriptional activators in hypoosmolarity-responsive expression of the ProDH gene in Arabidopsis. Plant Cell Physiol. 2004, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Weltmeier, F.; Ehlert, A.; Mayer, C.S.; Dietrich, K.; Wang, X.; Schutze, K.; Alonso, R.; Harter, K.; Vicente-Carbajosa, J.; Droge-Laser, W. Combinatorial control of Arabidopsis proline dehydrogenase transcription by specific heterodimerisation of bZIP transcription factors. Embo J. 2006, 25, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Feng, Y.X.; Zhang, Q.; Yu, X.Z. Proline-mediated modulation on DNA repair pathway in rice seedlings under chromium stress by integrating gene chip and co-expression network analysis. Ecotoxicology 2022, 31, 1266–1275. [Google Scholar] [CrossRef]
- Ullah, A.; Lin, Y.J.; Zhang, H.; Yu, X.Z. Identification of the Key Genes Involved in Proline-Mediated Modification of Cell Wall Components in Rice Seedlings under Trivalent Chromium Exposure. Toxics 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.-H.; Verslues, P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.-M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- de Lacerda, C.F.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A.; Prisco, J.T. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ. Exp. Bot. 2003, 49, 107–120. [Google Scholar] [CrossRef]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kowaloff, E.M.; Granger, A.S.; Phang, J.M. Alterations in proline metabolic enzymes with mammalian development. Metabolism 1976, 25, 1087–1094. [Google Scholar] [CrossRef]
- Phang, J.M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Cell Regul. 1985, 25, 91–132. [Google Scholar] [CrossRef]
- Phang, J.M. Perspectives, past, present and future: The proline cycle/proline-collagen regulatory axis. Amino Acids 2021, 53, 1967–1975. [Google Scholar] [CrossRef]
- Hagedorn, C.H.; Phang, J.M. Transfer of reducing equivalents into mitochondria by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch. Biochem. Biophys. 1983, 225, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cabassa-Hourton, C.; Planchais, S.; Lebreton, S.; Savoure, A. The proline cycle as an eukaryotic redox valve. J. Exp. Bot. 2021, 72, 6856–6866. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Wahlqvist, M.L. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical bio-synthesis and mechanism of action for human health and environmental applications. Asia Pac. J. Clin. Nutr. 2004, 13, 1–24. [Google Scholar]
- Atkinson, D.E. Cellular Energy Metabolism and its Regulation; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Hare, P.D.; Cress, W.A.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Micheu, S.; Crailsheim, K.; Leonhard, B. Importance of proline and other amino acids during honeybee flight (Apis mellifera carnica POLLMANN). Amino Acids 2000, 18, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Stec, N.; Saleem, A.; Darveau, C.-A. Proline as a Sparker Metabolite of Oxidative Metabolism during the Flight of the Bumblebee, Bombus impatiens. Metabolites 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Croes, A.F. Proline metabolism in pollen—Degradation of proline during germination and early tube growth. Planta 1983, 159, 46–49. [Google Scholar]
- Fujita, T.; Maggio, A.; Garcia-Rios, M.; Bressan, R.A.; Csonka, L.N. Comparative Analysis of the Regulation of Expression and Structures of Two Evolutionarily Divergent Genes for Δ1-Pyrroline-5-Carboxylate Synthetase from Tomato. Plant Physiol. 1998, 118, 661–674. [Google Scholar] [CrossRef]
- Mutters, R.G.; Ferreira, L.G.R.; Hall, A.E. Proline Content of the Anthers and Pollen of Heat-Tolerant and Heat-Sensitive Cowpea Subjected to Different Temperatures. Crop Sci. 1989, 29, 1497–1500. [Google Scholar] [CrossRef]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a Transporter for Proline, Glycine Betaine, and γ-Amino Butyric Acid in Tomato Pollen. Plant Cell 1999, 11, 377–391. [Google Scholar] [CrossRef]
- Venekamp, J.H.; Koot, J.T.M. The Distribution of Free Amino Acids, Especially of Proline, in the Organs of Field Bean Plants, Vicia faba L., during Development in the Field. J. Plant Physiol. 1984, 116, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Flasiński, S.; Rogozińska, J. Effect of water deficit on proline accumulation, protein and chlorophyll content during flowering and seed formation in winter rape (Brassica napus L. var. oleifera). Acta Agrobot. 1985, 38, 11. [Google Scholar] [CrossRef][Green Version]
- Armengaud, P.; Thiery, L.; Buhot, N.; Grenier-de March, G.; Savouré, A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol. Plant. 2004, 120, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Stransky, H.; Koch, W. The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 2007, 226, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.H.; Dandekar, A.M. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Girousse, C.; Bournoville, R.; Bonnemain, J.L. Water Deficit-Induced Changes in Concentrations in Proline and Some Other Amino Acids in the Phloem Sap of Alfalfa. Plant Physiol. 1996, 111, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Gumy, C.; Blatter, E.; Boeffel, S.; Fricke, W.; Rentsch, D. In planta function of compatible solute transporters of the AtProT family. J. Exp. Bot. 2011, 62, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Hirner, B.; Schmelzer, E.; Frommer, W.B. Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell 1996, 8, 1437–1446. [Google Scholar]
- Mattioli, R.; Biancucci, M.; El Shall, A.; Mosca, L.; Costantino, P.; Funck, D.; Trovato, M. Proline synthesis in developing microspores is required for pollen development and fertility. BMC Plant Biol. 2018, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar] [CrossRef]
- Nanjo, T.; Kobayashi, M.; Yoshiba, Y.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999, 461, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Roosens, N.H.; Al Bitar, F.; Loenders, K.; Angenon, G.; Jacobs, M. Overexpression of ornithine-delta-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol. Breed. 2002, 9, 73–80. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W.; van Staden, J. A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul. 2003, 39, 41–50. [Google Scholar] [CrossRef]
- Ambreen, S.; Athar, H.-u.-R.; Khan, A.; Zafar, Z.U.; Ayyaz, A.; Kalaji, H.M. Seed priming with proline improved photosystem II efficiency and growth of wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 502. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Mattioli, R.; Moubayidin, L.; Sabatini, S.; Costantino, P.; Trovato, M. Proline affects the size of the root meristematic zone in Arabidopsis. BMC Plant Biol. 2015, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar] [CrossRef]
- Henrissat, B.; Coutinho, P.M.; Davies, G.J. A census of carbohydrate-active enzymes in the genome of Arabidopsis thaliana. Plant Mol. Biol. 2001, 47, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Park, Y.; Muthuramalingam, P.; Jeong, J.H.; Kim, S.H.; Shin, H. Physiological and metabolic analyses reveal the proline-mediated flowering delay mechanism in Prunus persica. Front. Plant Sci. 2024, 15, 1302975. [Google Scholar] [CrossRef]
- Dourmap, C.; Marmagne, A.; Lebreton, S.; Clément, G.; Guivarc’h, A.; Savouré, A.; Masclaux-Daubresse, C. Carbon and nitrogen remobilization during seed filling in Arabidopsis is strongly impaired in the pyrroline-5-carboxylate dehydrogenase mutant. J. Exp. Bot. 2022, 74, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P.S. Removal of feedback inhibition of Δ1-Pyrroline-5-Carboxylate Synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- White, F.F.; Taylor, B.H.; Huffman, G.A.; Gordon, M.P.; Nester, E.W. Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J. Bacteriol. 1985, 164, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.L.; Trovato, M.; De Paolis, A.; Gallelli, A.; Costantino, P.; Altamura, M.M. The plant oncogene rolD stimulates flowering in transgenic tobacco plants. Dev. Biol. 1996, 180, 693–700. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Dunand, C.; Crèvecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Liu, H.; Mu, Y.; Xuan, Y.; Wu, X.; Wang, W.; Zhang, H. Hydrogen Peroxide Signaling in the Maintenance of Plant Root Apical Meristem Activity. Antioxidants 2024, 13, 554. [Google Scholar] [CrossRef]
- Parveen, N.; Kandhol, N.; Sharma, S.; Singh, V.P.; Chauhan, D.K.; Ludwig-Muller, J.; Corpas, F.J.; Tripathi, D.K. Auxin Crosstalk with Reactive Oxygen and Nitrogen Species in Plant Development and Abiotic Stress. Plant Cell Physiol. 2023, 63, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; des Francs-Small, C.C.; Calder, G.; Mugford, S.T.; Tanz, S.; Lee, B.-R.; Zechmann, B.; Small, I.; Kopriva, S. Identification of a Pentatricopeptide Repeat Protein Implicated in Splicing of Intron 1 of Mitochondrial nad7 Transcripts. J. Biol. Chem. 2010, 285, 32192–32199. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Schoettner, M.; Xu, S.; Paetz, C.; Wilde, J.; Baldwin, I.T.; Groten, K. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. New Phytol. 2017, 213, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Wang, Y.; Bai, H.; Li, H.; Zhang, C.; Chen, J.; Xu, W. Melatonin-ROS signal module regulates plant lateral root development. Plant Signal. Behav. 2021, 16, 1901447. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, B.; Fan, J.; Yuan, Q.; He, F.; Liang, H.; Chen, F.; Liu, W. Gallic acid regulates primary root elongation via modulating auxin transport and signal transduction. Front. Plant Sci. 2024, 15, 1464053. [Google Scholar] [CrossRef]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. PPB 2023, 203, 108032. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Lin, Y.-J.; Tian, P.; Yu, X.-Z. Proline interacts with Ca2+-dependent signaling to enhance chromium tolerance in rice by manipulating nitrate reductase and sucrose phosphate synthase. Int. J. Biol. Macromol. 2023, 253, 126655. [Google Scholar] [CrossRef]
- Fabro, G.; Kovacs, I.; Pavet, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant Microbe Interact. 2004, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Lefebvre-De Vos, D.; Le Disquet, I.; Leprince, A.-S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savouré, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef]
- Yang, S.-L.; Lan, S.-S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, H.; Ouyang, B.; Shi, C.; Demidchik, V.; Hao, Z.; Yu, M.; Shabala, S. NADPH oxidases and the evolution of plant salinity tolerance. Plant Cell Environ. 2020, 43, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.-H. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: Unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2021, 186, 79–92. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Basbouss-Serhal, I.; Soubigou-Taconnat, L.; Bailly, C.; Leymarie, J. Germination Potential of Dormant and Nondormant Arabidopsis Seeds Is Driven by Distinct Recruitment of Messenger RNAs to Polysomes. Plant Physiol. 2015, 168, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef]
- Muller, K.; Linkies, A.; Vreeburg, R.A.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef]
- Aoki, N.; Ishibashi, Y.; Kai, K.; Tomokiyo, R.; Yuasa, T.; Iwaya-Inoue, M. Programmed cell death in barley aleurone cells is not directly stimulated by reactive oxygen species produced in response to gibberellin. J. Plant Physiol. 2014, 171, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Małecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M. Impact of Reactive Oxygen Species on Seed Viability in p5cs Sesquimutants. Unpublished Work. 2017. [Google Scholar]
- Deuschle, K.; Funck, D.; Forlani, G.; Stransky, H.; Biehl, A.; Leister, D.; van der Graaff, E.; Kunze, R.; Frommer, W.B. The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 2004, 16, 3413–3425. [Google Scholar] [CrossRef]
- Xing, S.; Zachgo, S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008, 53, 790–801. [Google Scholar] [CrossRef]
- Hu, L.; Liang, W.; Yin, C.; Cui, X.; Zong, J.; Wang, X.; Hu, J.; Zhang, D. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 2011, 23, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal Production of Reactive Oxygen Species by NADPH Oxidase Is Critical for Tapetal Programmed Cell Death and Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, B.; Jager, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Namuco, O.S.; O’Toole, J.C. Reproductive Stage Water Stress and Sterility. I. Effect of Stress during Meiosis. Crop Sci. 1986, 26, 317–321. [Google Scholar] [CrossRef]
- Ekanayake, I.J.; Datta, S.K.D.; Steponkus, P.L. Spikelet Sterility and Flowering Response of Rice to Water Stress at Anthesis. Ann. Bot. 1989, 63, 257–264. [Google Scholar] [CrossRef]
- Garrity, D.P.; O’Toole, J.C. Screening rice for drought resistance at the reproductive phase. Field Crop. Res. 1994, 39, 99–110. [Google Scholar] [CrossRef]
- Guo, C.; Yao, L.; You, C.; Wang, S.; Cui, J.; Ge, X.; Ma, H. MID1 plays an important role in response to drought stress during reproductive development. Plant J. 2016, 88, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with Proline or an Organic Bio-stimulant Induces Salt Tolerance in Wheat Plants by Improving Antioxidant Redox State and Enzymatic Activities and Reducing the Oxidative Stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Alam, R.; Das, D.K.; Islam, M.R.; Murata, Y.; Hoque, M.A. Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Progress. Agric. 2017, 27, 409–417. [Google Scholar] [CrossRef]
- Honys, D.; Twell, D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004, 5, R85. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, 177. [Google Scholar] [CrossRef]
- Hardy, M.L.M.; Lakhiani, D.; Morris, M.B.; Day, M.L. Proline and Proline Analogues Improve Development of Mouse Preimplantation Embryos by Protecting Them against Oxidative Stress. Cells 2023, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Jiang, S.; Zhang, R. The Role of GIGANTEA Gene in Mediating the Oxidative Stress Response and in Arabidopsis. Plant Growth Regul. 2006, 48, 261–270. [Google Scholar] [CrossRef]
- Lai, A.G.; Doherty, C.J.; Mueller-Roeber, B.; Kay, S.A.; Schippers, J.H.; Dijkwel, P.P. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. USA 2012, 109, 17129–17134. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Kocsy, G.; Tari, I.; Vanková, R.; Zechmann, B.; Gulyás, Z.; Poór, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. 2013, 211, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Q.; Xie, Z.; Yu, B.; Zeng, R.; Min, Q.; Huang, J. OsFPFL4 is Involved in the Root and Flower Development by Affecting Auxin Levels and ROS Accumulation in Rice (Oryza sativa). Rice 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.C.; Takeno, K. Stress-induced flowering. Plant Signal. Behav. 2010, 5, 944–947. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Sureshkumar, S.; Lempe, J.; Weigel, D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2006, 2, e106. [Google Scholar] [CrossRef]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian Clock and Photoperiodic Flowering in Arabidopsis: CONSTANS Is a Hub for Signal Integration. Plant Physiol. 2016, 173, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Ichino, T.; Osanai, M.; Wada, K. Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol. 2000, 41, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Brini, F.; Mseddi, K.; Rhizopoulou, S.; Jones, M.A. A holistic and sustainable approach linked to drought tolerance of Mediterranean crops. Front. Plant Sci. 2023, 14, 1167376. [Google Scholar] [CrossRef]
- Carraro, E.; Di Iorio, A. Eligible strategies of drought response to improve drought resistance in woody crops: A mini-review. Plant Biotechnol. Rep. 2022, 16, 265–282. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).