Comparative Metabolic Profiling and Biological Evaluation of Essential Oils from Conocarpus Species: Antidiabetic, Antioxidant, and Antimicrobial Potential
Abstract
1. Introduction
2. Results
2.1. GC/MS Analysis of the Essential Oils
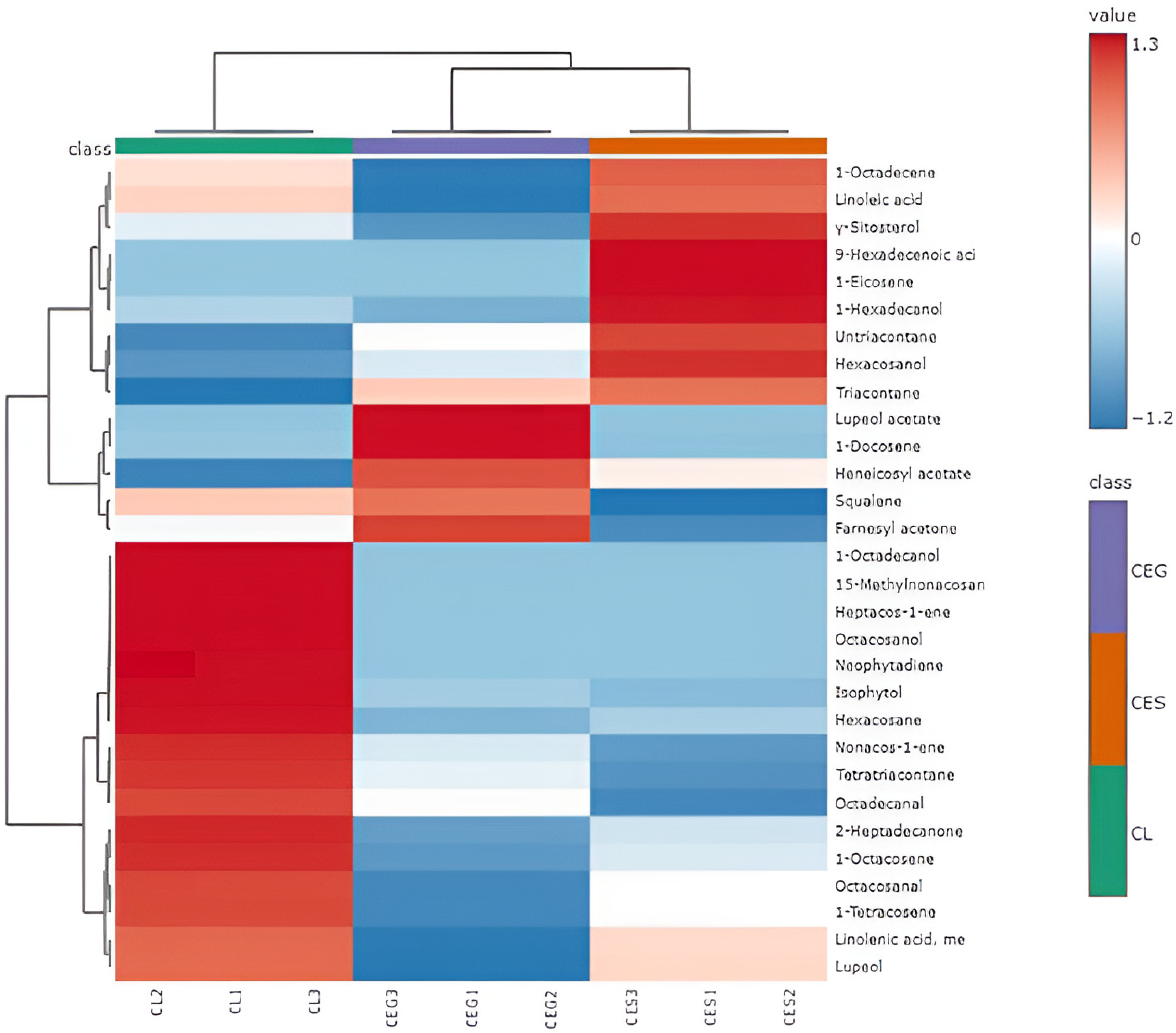
2.2. Chemometric Analysis of the Essential Oils
2.3. Antioxidant Activity
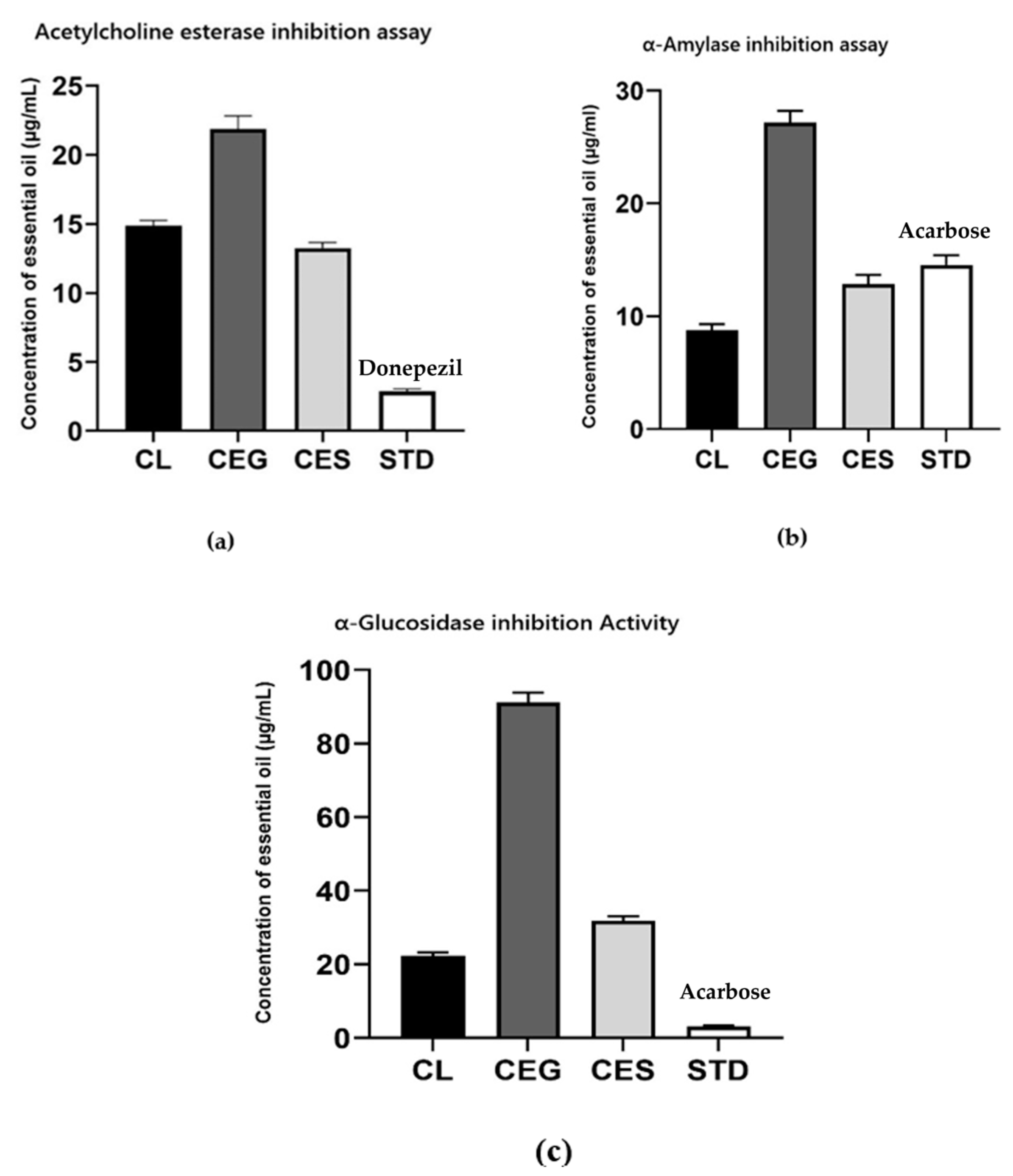
2.4. Enzyme Inhibitory Activities
2.5. Antimicrobial Effect of Essential Oils
3. Discussion
4. Methods
4.1. Plant Collection
4.2. Plant Drying
4.3. Essential Oils Isolation
4.4. GC/MS Analysis of Essential Oils
4.5. Identification of the Oil Components
4.6. Chemometric Analysis
4.7. Antioxidant Assay
4.7.1. DPPH Radical Scavenging Activity
4.7.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.8. Enzyme Inhibition Assays
4.8.1. α-Amylase Inhibition Assay
4.8.2. α-Glucosidase Inhibition Assay
4.8.3. Acetylcholinesterase Inhibition Assay
4.9. Antimicrobial Activity Assay
4.9.1. Microorganisms
4.9.2. Disc-Diffusion Test
4.9.3. Minimum Inhibitory Concentration Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Wang, Q.; Kuang, H.; Su, Y.; Sun, Y.; Feng, J.; Guo, R.; Chan, K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013, 146, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Lisa, S.R.; Islam, M.K.; Qais, N. Plants and plant constituents with analgesic and anti-inflammatory activities: A systematic review. Dhaka Univ. J. Pharm. Sci. 2020, 19, 207–224. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N.S.; El-Labbad, E.M.; Hamoda, A.M.; El-Keblawy, A.A.; El-Shorbagi, A.-N.A.; Mosa, K.A.; Soliman, S.S. The anti-Candida activity of Tephrosia apollinea is more superiorly attributed to a novel steroidal compound with selective targeting. Plants 2022, 11, 2120. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem.-Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucl. 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential—A review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Ismail, L.C.; Hasan, H.; Hashim, M.; Obaid, R.S. A status review on health-promoting properties and global regulation of essential oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
- Afifi, H.S.; Al Marzooqi, H.M.; Tabbaa, M.J.; Arran, A.A. Phytochemicals of Conocarpus spp. as a natural and safe source of phenolic compounds and antioxidants. Molecules 2021, 26, 1069. [Google Scholar] [CrossRef] [PubMed]
- de Morais Lima, G.R.; De Sales, I.R.P.; Caldas Filho, M.R.D.; De Jesus, N.Z.T.; de Sousa Falcão, H.; Barbosa-Filho, J.M.; Cabral, A.G.S.; Souto, A.L.; Tavares, J.F.; Batista, L.M. Bioactivities of the genus Combretum (Combretaceae): A review. Molecules 2012, 17, 9142–9206. [Google Scholar] [CrossRef] [PubMed]
- Bantho, S.; Naidoo, Y.; Dewir, Y.H.; Singh, M. A review on the secretory structures, bioactive compounds and biological activities of selected Combretum species. S. Afr. J. Bot. 2023, 158, 18–30. [Google Scholar] [CrossRef]
- Ramanjaneyulu, A.; Chaitanya, T.; Joseph, B. Conocarpus Tree—A Boon or Bane? Chron. Bioresour. Manag. 2023, 7, 028–034. [Google Scholar]
- Naseer, M.; Hameed, M.; Zahoor, A.; Ahmad, F.; Fatima, S.; Ahmad, M.S.A.; Ahmad, K.S.; Iftikhar, M. Photosynthetic response in buttonwood (Conocarpus erectus L.) to salt stress. Pak. J. Bot 2017, 49, 847–856. [Google Scholar]
- Baroon, Z.; Razzaque, M.A. Nutritional evaluation and palatability trial of ensiled Conocarpus Greenery residues. Exp. Agric. 2012, 48, 138–147. [Google Scholar] [CrossRef]
- Agra, M.d.F.; Freitas, P.F.d.; Barbosa-Filho, J.M. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev. Bras. De Farmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef]
- Altschul, S.v.R. Drugs and Foods from Little-Known Plants: Notes in Harvard University Herbaria; Harvard University Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Von Reis, S.; Lipp, F.J. New Plant Sources for Drugs and Foods From the New York Botanical Garden Herbarium; Harvard University Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Al-Musayeib, N.M.; Mothana, R.A.; Al-Massarani, S.; Matheeussen, A.; Cos, P.; Maes, L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules 2012, 17, 11379–11390. [Google Scholar] [CrossRef]
- Touqeer, S.; Saeed, M.A.; Ansari, F.; Zahra, N.; Masood, Z.; Fareed, M.; Javed, A. Antibacterial and antifungal activity of Conocarpus lancifolius Engl. (Combretaceae). Appl. Pharmacol. 2014, 6, 153–155. [Google Scholar] [CrossRef]
- Rehman, S.; Azam, F.; Rehman, S.; Rehman, T.; Mehmood, A.; Gohar, A.; Samad, A. A review on botanical, phytochemical and pharmacological reports of conocarpus erectus. Pak. J. Agric. Res. 2019, 32, 212–217. [Google Scholar] [CrossRef]
- Khalil, R.; Ali, Q.; Hafeez, M.; Malik, A. Phenolic acid profiling by RP-HPLC: Evaluation of antibacterial and anticancer activities of Conocarpus erectus plant extracts. Biol. Clin. Sci. Res. J. 2020, 2020. [Google Scholar] [CrossRef]
- Raza, S.A.; Chaudhary, A.R.; Mumtaz, M.W.; Adnan, A.; Mukhtar, H.; Akhtar, M.T. Metabolite profiling and antidiabetic attributes of ultrasonicated leaf extracts of Conocarpus lancifolius. Asian Pac. J. Trop. Biomed. 2020, 10, 353–360. [Google Scholar]
- Saadullah, M.; Fakhar-e-Alam, M.; Atif, M.; Asif, M.; Irshad, K.; Ali, Z. Biological and in silico investigation of isolated novel bioactive compound from Conocarpus lancifolius. J. King Saud Univ.-Sci. 2024, 36, 103121. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.-S.S.; Bazaid, S.A.; Shohayeb, M.M. RP-HPLC-UV-ESI-MS phytochemical analysis of fruits of Conocarpus erectus L. Chem. Pap. 2014, 68, 1358–1367. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Perveen, S.; Fawzy, G.A.; Mehmood, R.; Khan, A.; Khan, S.I. New ellagic acid derivative from the fruits of heat-tolerant plant Conocarpus lancifolius Engl. and their anti-inflammatory, cytotoxic, PPAR agonistic activities. Pak. J. Pharm. Sci. 2016, 29, 1833–1837. [Google Scholar] [PubMed]
- Nazir, N.; Zahoor, M.; Uddin, F.; Nisar, M. Chemical composition, in vitro antioxidant, anticholinesterase, and antidiabetic potential of essential oil of Elaeagnus umbellata Thunb. BMC Complement. Med. Ther. 2021, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Tauqeer, H.M.; Hussain, S.; Abbas, F.; Iqbal, M. The potential of an energy crop “Conocarpus erectus” for lead phytoextraction and phytostabilization of chromium, nickel, and cadmium: An excellent option for the management of multi-metal contaminated soils. Ecotoxicol. Environ. Saf. 2019, 173, 273–284. [Google Scholar] [CrossRef]
- Ashraf, F.; Abbas, G.; Murtaza, B.; Amjad, M.; Imran, M.; Naeem, M.A.; Saqib, M.; Khan Niazi, N.; Zakir, A.; Hussain, M. Comparative tolerance and phytostabilization potential of Conocarpus erectus and Eucalyptus camaldulensis grown in cadmium contaminated soil. Pak. J. Agric. Sci. 2018, 55, 521–529. [Google Scholar]
- do Nascimento Santos, D.K.D.; de Oliveira Melo, W.H.; de Oliveira Lima, A.M.N.; da Cruz Filho, I.J.; de Souza Lima, G.M.; da Silva, T.D.; de Moura, M.C.; do Nascimento, M.S.; Maior, A.M.S.; Napoleão, T.H. Conocarpus erectus L., a plant with a high content of structural sugars, ions and phenolic compounds, shows antioxidant and antimicrobial properties promoted by different organic fractions. Asian Pac. J. Trop. Biomed. 2018, 8, 463–470. [Google Scholar]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Kumari, S.; Pundhir, S.; Priya, P.; Jeena, G.; Punetha, A.; Chawla, K.; Firdos Jafaree, Z.; Mondal, S.; Yadav, G. EssOilDB: A database of essential oils reflecting terpene composition and variability in the plant kingdom. Database 2014, 2014, bau120. [Google Scholar] [CrossRef] [PubMed]
- Németh-Zámboriné, E. Natural variability of essential oil components. In Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 87–125. [Google Scholar]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). Nat. Prod. 2013, 10, 973–978. [Google Scholar]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Benomari, F.Z.; Sarazin, M.; Chaib, D.; Pichette, A.; Boumghar, H.; Boumghar, Y.; Djabou, N. Chemical variability and chemotype concept of essential oils from Algerian wild plants. Molecules 2023, 28, 4439. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Yang, S.-b.; Wang, Y.-z. An integrated chemical characterization based on FT-NIR, and GC–MS for the comparative metabolite profiling of 3 species of the genus Amomum. Anal. Chim. Acta 2023, 1280, 341869. [Google Scholar] [CrossRef]
- Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X. Environmental factors on secondary metabolism in medicinal plants: Exploring accelerating factors. Med. Plant Biol. 2024, 3, e016. [Google Scholar] [CrossRef]
- Gad, H.A.; Mukhammadiev, E.A.; Zengen, G.; Musayeib, N.M.A.; Hussain, H.; Bin Ware, I.; Ashour, M.L.; Mamadalieva, N.Z. Chemometric Analysis Based on GC-MS Chemical Profiles of Three Stachys Species from Uzbekistan and Their Biological Activity. Plants 2022, 11, 1215. [Google Scholar] [CrossRef]
- Khalil, R.; Ali, Q.; Hafeez, M.; Malik, A. Phytochemical activities of Conocarpus erectus: An overview. Biol. Clin. Sci. Res. J. 2020, 2020, e008. [Google Scholar] [CrossRef]
- Pan, S.-P.; Pirker, T.; Kunert, O.; Kretschmer, N.; Hummelbrunner, S.; Latkolik, S.L.; Rappai, J.; Dirsch, V.M.; Bochkov, V.; Bauer, R. C13 megastigmane derivatives from Epipremnum pinnatum: β-damascenone inhibits the expression of pro-inflammatory cytokines and leukocyte adhesion molecules as well as NF-κB signaling. Front. Pharmacol. 2019, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Elaguel, A.; Kallel, I.; Gargouri, B.; Ben Amor, I.; Hadrich, B.; Ben Messaoud, E.; Gdoura, R.; Lassoued, S.; Gargouri, A. Lawsonia inermis essential oil: Extraction optimization by RSM, antioxidant activity, lipid peroxydation and antiproliferative effects. Lipids Health Dis. 2019, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N.S.; Nilofar, N.; Zengin, G.; Eldahshan, O.A. Metabolic profiling and enzyme inhibitory activity of the essential oil of citrus aurantium fruit peel. BMC Complement. Med. Ther. 2024, 24, 262. [Google Scholar] [CrossRef] [PubMed]
- Diniz do Nascimento, L.; Barbosa de Moraes, A.A.; Santana da Costa, K.; Pereira Galúcio, J.M.; Taube, P.S.; Leal Costa, C.M.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Guerreiro de Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.K.; Chander, R.; Kumar, R.; Agnihotri, V.K. In-vitro antidiabetic and antimicrobial studies of volatile organic compounds (VOCs) from the roots of Rosa damascena Mill. of North-western Himalaya. S. Afr. J. Bot. 2024, 168, 518–528. [Google Scholar] [CrossRef]
- Fayez, S.; Eldahshan, O.A.; Nilofar, N.; Zengin, G.; Fahmy, N.M. Metabolomics-guided discrimination of constituents in five commercial apple (Malus × domestica Borkh.) cultivars: Assessment of their antioxidant, neuroprotective, and antidiabetic activities for potential use as functional food. Food Biosci. 2024, 61, 104708. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; El Hassab, M.A.; Elhady, S.S.; Fahmy, N.M. Insights on Citrus clementina essential oil as a potential antiaging candidate with a comparative chemometric study on different cultivars. Ind. Crops Prod. 2023, 194, 116349. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Elhady, S.S.; Bannan, D.F.; Malatani, R.T.; Gad, H.A. Citrus reticulata Leaves Essential Oil as an Antiaging Agent: A Comparative Study between Different Cultivars and Correlation with Their Chemical Compositions. Plants 2022, 11, 3335. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.G.; Ashmawy, N.S.; Al-Rawi, A.M.; Abu-Qiyas, A.; Hamoda, A.M.; Hamdy, R.; Dakalbab, S.; Arikat, S.; Salahat, D.; Madkour, M. SARS-CoV-2-free residual proteins mediated phenotypic and metabolic changes in peripheral blood monocytic-derived macrophages in support of viral pathogenesis. PLoS ONE 2023, 18, e0280592. [Google Scholar] [CrossRef] [PubMed]
- Abd Algaffar, S.O.; Satyal, P.; Ashmawy, N.S.; Verbon, A.; van de Sande, W.W.; Khalid, S.A. In Vitro and In Vivo Wide-Spectrum Dual Antimycetomal Activity of Eight Essential Oils Coupled with Chemical Composition and Metabolomic Profiling. Microbiol. Res. 2024, 15, 1280–1297. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; TM, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]

| KI | Compound | Relative Abundance % ± SD | |||||
|---|---|---|---|---|---|---|---|
| No. | Cal. | Rep. | Molecular Formula | CEG | CES | CL | |
| 1. | 1377 | 1374 | Decanoic acid | C12H20O2 | - | 0.14 ± 0.05 | 0.08 ± 0.02 |
| 2. | 1389 | 1388 | β-Damascenone | C13H18O | - | 0.16 ± 0.03 | - |
| 3. | 1407 | 1408 | Hexahydropseudoionone | C13H26O | - | 0.11 ± 0.03 | - |
| 4. | 1433 | 1432 | α-Ionone | C13H20O | 0.19 ± 0.07 | 0.16 ± 0.02 | 0.05 ± 0.08 |
| 5. | 1458 | 1457 | Nerylacetone | C13H22O | 2.83 ± 0.04 | 2.74 ± 0.04 | 2.61 ± 0.14 |
| 6. | 1494 | 1494 | trans-β-Ionone | C13H20O | 1.77 ± 0.09 | 2.88 ± 0.06 | 1.07 ± 0.04 |
| 7. | 1502 | 1500 | PentadecaneI8II | C15H32 | - | 0.15 ± 0.05 | 0.07 ± 0.01 |
| 8. | 1515 | 1518 | Tridecanal | C13H26O | - | 0.16 ± 0.07 | 0.06 ± 0.01 |
| 9. | 1521 | 1519 | 2,4-di-t-Butylphenol | C14H22O | 0.47 ± 0.07 | 1.10 ± 0.02 | 0.17 ± 0.08 |
| 10. | 1534 | 1535 | (E,Z)-Pseudoionone | C13H20O | 0.65 ± 0.04 | 0.79 ± 0.06 | 0.49 ± 0.05 |
| 11. | 1574 | 1574 | Dodecanoic acid | C12H24O2 | 0.46 ± 0.05 | 0.57 ± 0.09 | 0.45 ± 0.04 |
| 12. | 1584 | 1581 | Benzoic acid, hexyl ester | C13H18O2 | - | - | 0.18 ± 0.09 |
| 13. | 1591 | 1590 | (E,E)-Pseudoionone | C13H20O | 0.68 ± 0.02 | 0.63 ± 0.05 | 0.53 ± 0.03 |
| 14. | 1600 | 1588 | 2,2,4-Trimethyl-1,3-pentanediol ester | C16H30O4 | 0.63 ± 0.03 | 3.36 ± 0.07 | 0.42 ± 0.06 |
| 15. | 1610 | 1613 | Tetradecanal | C14H28O | - | 0.29 ± 0.03 | 0.04 ± 0.03 |
| 16. | 1659 | 1656 | Undecylcyclopentane | C16H32 | - | - | 0.05 ± 0.03 |
| 17. | 1665 | 1666 | 2-Methylhexadecane | C17H36 | 0.16 ± 0.02 | 0.09 ± 0.05 | 0.12 ± 0.03 |
| 18. | 1674 | 1680 | 13-Methyltetradecanal | C15H30O | 1.14 ± 0.07 | 0.95 ± 0.05 | 0.81 ± 0.08 |
| 19. | 1698 | 1698 | 2-Pentadecanone | C15H30O | 0.27 ± 0.04 | 0.29 ± 0.01 | 0.15 ± 0.06 |
| 20. | 1702 | 1700 | Heptadecane | C17H36 | 0.28 ± 0.08 | 0.31 ± 0.02 | 0.28 ± 0.01 |
| 21. | 1709 | 1710 | Pentadecanal | C15H30O | 0.22 ± 0.04 | 0.31 ± 0.04 | 0.13 ± 0.04 |
| 22. | 1750 | 1749 | Farnesyl alcohol | C15H26O | 1.00 ± 0.13 | 0.31 ± 0.05 | 0.54 ± 0.15 |
| 23. | 1770 | 1771 | Tetradecanoic acid | C14H28O2 | - | 0.60 ± 0.05 | 0.48 ± 0.01 |
| 24. | 1791 | 1806 | 1-Octadecene | C18H36 | 0.59 ± 0.12 | 1.01 ± 0.05 | 0.86 ± 0.07 |
| 25. | 1800 | 1800 | Octadecane | C18H38 | 0.4 ± 0.08 | 0.31 ± 0.05 | 0.37 ± 0.04 |
| 26. | 1805 | 1811 | Hexadecanal | C16H32O | 0.5 ± 0.11 | 0.43 ± 0.06 | 0.28 ± 0.03 |
| 27. | 1841 | 1837 | Neophytadiene | C20H38 | - | - | 0.26 ± 0.03 |
| 28. | 1849 | 1848 | Hexahydrofarnesyl acetone | C18H36O | 3.28 ± 0.08 | 2.31 ± 0.03 | 3.83 ± 0.03 |
| 29. | 1869 | 1860 | Benzoic acid, 2-phenylethyl ester | C15H14O | 0.27 ± 0.08 | 0.28 ± 0.02 | 0.24 ± 0.03 |
| 30. | 1876 | 1876 | 1-Hexadecanol | C16H34O | 0.67 ± 0.09 | 1.52 ± 0.04 | 0.29 ± 0.08 |
| 31. | 1896 | 1900 | 1-Nonadecene | C19H38 | - | 0.81 ± 0.04 | 0.30 ± 0.06 |
| 32. | 1902 | 1900 | Nonadecane | C19H40 | 0.23 ± 0.05 | 0.15 ± 0.05 | 0.12 ± 0.03 |
| 33. | 1907 | 1906 | 2-Heptadecanone | C17H34O | - | 0.14 ± 0.03 | 0.04 ± 0.03 |
| 35. | 1926 | 1927 | Farnesyl acetone | C18H30O | 6.64 ± 0.13 | 4.55 ± 0.12 | 5.51 ± 0.04 |
| 36. | 1945 | 1942 | Palmitelaidic acid | C16H30O2 | - | 0.10 ± 0.04 | - |
| 37. | 1951 | 1950 | Isophytol | C20H40O | 0.25 ± 0.04 | 0.20 ± 0.08 | 0.82 ± 0.06 |
| 38. | 1971 | 1971 | n-Hexadecanoic acid | C16H32O2 | 2.89 ± 0.11 | 2.96 ± 0.07 | 2.97 ± 0.03 |
| 39. | 1994 | 1994 | 1-Eicosene | C20H40 | 0.4 ± 0.07 | 0.52 ± 0.03 | 0.40 ± 0.02 |
| 40. | 2000 | 2000 | Eicosane | C20H42 | 0.47 ± 0.09 | 0.40 ± 0.06 | 0.38 ± 0.08 |
| 41. | 2005 | 2008 | 1-Hexadecanol acetate | C18H36O2 | 0.15 ± 0.01 | 0.09 ± 0.02 | 0.13 ± 0.03 |
| 42. | 2027 | 2024 | Octadecanal | C18H36O | 0.18 ± 0.06 | 0.16 ± 0.07 | 0.32 ± 0.04 |
| 43. | 2035 | 2034 | Geranyl linalool | C20H34O | 0.15 ± 0.02 | 0.09 ± 0.05 | 0.11 ± 0.08 |
| 44. | 2088 | 2086 | 1-Octadecanol | C18H38O | - | - | 0.12 ± 0.03 |
| 45. | 2095 | 2100 | Henicosane | C21H44 | 0.34 ± 0.06 | 0.86 ± 0.02 | 0.29 ± 0.03 |
| 46. | 2096 | 2100 | 1-Heneicosene | C21H42 | - | 0.3 ± 0.06 | 0.13 ± 0.03 |
| 47. | 2106 | 2107 | Linolenic acid, methyl ester | C19H32O2 | - | 0.13 ± 0.04 | 0.18 ± 0.09 |
| 48. | 2121 | 2122 | Phytol | C20H40O | 13.56 ± 0.05 | 0.85 ± 0.05 | 7.29 ± 0.19 |
| 49. | 2149 | 2145 | Linoleic acid | C18H32O2 | - | 1.08 ± 0.10 | 0.81 ± 0.08 |
| 50. | 2162 | 2164 | Linoleic acid ethyl ester | C20H36O2 | - | 0.14 ± 0.01 | 0.26 ± 0.03 |
| 51. | 2181 | 2185 | Phytanic acid, methyl ester | C21H42O2 | - | - | 0.05 ± 0.02 |
| 52. | 2194 | 2198 | 1-Docosene | C22H44 | 0.92 ± 0.11 | 0.33 ± 0.05 | 0.35 ± 0.08 |
| 53. | 2199 | 2200 | Docosane | C22H46 | 0.10 ± 0.05 | 0.16 ± 0.03 | 0.24 ± 0.05 |
| 54. | 2209 | 2208 | Octadecanol acetate | C20H40O2 | - | - | 0.08 ± 0.02 |
| 55. | 2223 | 2219 | Phytol, acetate | C22H42O2 | 0.12 ± 0.09 | - | 0.27 ± 0.06 |
| 56. | 2301 | 2300 | Tricosane | C23H48 | 0.32 ± 0.04 | 0.62 ± 0.01 | 0.87 ± 0.09 |
| 57. | 2331 | 2333 | Eicosanoic acid, methyl ester | C21H42O2 | - | - | 0.08 ± 0.02 |
| 58. | 2362 | 2365 | 2-Methyl-Tricosane | C24H50 | 0.12 ± 0.06 | 0.12 ± 0.01 | 0.13 ± 0.05 |
| 59. | 2374 | 2380 | Eicosanoic acid | C20H40O2 | 0.11 ± 0.09 | - | 0.08 ± 0.01 |
| 60. | 2391 | 2400 | Tetracosane | C24H50 | 0.65 ± 0.06 | 0.50 ± 0.03 | 1.17 ± 0.12 |
| 61. | 2391 | 2396 | 1-Tetracosene | C24H48 | - | 0.24 ± 0.09 | 0.43 ± 0.06 |
| 62. | 2433 | 2430 | Heneicosanoic acid, methyl ester | C22H44O2 | - | - | 0.06 ± 0.01 |
| 63. | 2501 | 2500 | Pentacosane | C25H52 | 1.32 ± 0.12 | 1.83 ± 0.13 | 5.81 ± 0.07 |
| 64. | 2517 | 2509 | Heneicosyl acetate | C23H46O2 | 0.13 ± 0.02 | 0.08 ± 0.02 | - |
| 65. | 2536 | 2531 | Docosanoic acid, methyl ester | C23H46O2 | - | - | 0.07 ± 0.01 |
| 66. | 2599 | 2600 | Hexacosane | C26H54 | 0.87 ± 0.08 | 1.16 ± 0.07 | 3.06 ± 0.05 |
| 67. | 2637 | 2632 | Tetracosanal | C24H48O | - | 0.11 ± 0.03 | 0.13 ± 0.05 |
| 68. | 2689 | 2684 | Heptacos-1-ene | C27H54 | - | - | 0.06 ± 0.01 |
| 69. | 2704 | 2700 | Heptacosane | C27H56 | 9.41 ± 0.09 | 10.93 ± 0.04 | 23.32 ± 0.03 |
| 70. | 2720 | 2725 | Methyl tetracosanoate | C25H50O2 | 0.12 ± 0.06 | - | - |
| 71. | 2790 | 2800 | Octacosane | C28H58 | 2.88 ± 0.10 | 1.80 ± 0.09 | 2.05 ± 0.08 |
| 72. | 2792 | 2797 | 1-Octacosene | C28H56 | - | 0.84 ± 0.05 | 2.09 ± 0.05 |
| 73. | 2836 | 2835 | Squalene | C30H50 | 1.28 ± 0.02 | 0.52 ± 0.04 | 1.09 ± 0.04 |
| 74. | 2842 | 2848 | Hexacosanol | C26H54O | 0.06 ± 0.01 | 0.15 ± 0.04 | - |
| 75. | 2892 | 2884 | Nonacos-1-ene | C29H58 | 0.22 ± 0.04 | 0.13 ± 0.03 | 0.39 ± 0.07 |
| 76. | 2906 | 2900 | Nonacosane | C29H60 | 23.36 ± 0.03 | 24.54 ± 0.05 | 9.76 ± 0.02 |
| 77. | 2922 | 2929 | 15-Methylnonacosane | C30H62 | - | - | 0.08 ± 0.02 |
| 78. | 2944 | 2940 | Hexacosanoic acid, methyl ester | C27H54O2 | - | - | 0.06 ± 0.01 |
| 79. | 2963 | 2960 | 2-Methylnonacosane | C30H62 | - | - | 0.09 ± 0.01 |
| 80. | 2999 | 3000 | Triacontane | C30H62 | 0.63 ± 0.03 | 0.70 ± 0.11 | 0.44 ± 0.05 |
| 81. | 3046 | 3040 | Octacosanal | C28H56O | - | 0.22 ± 0.04 | 0.39 ± 0.07 |
| 82. | 3101 | 3100 | Untriacontane | C31H64 | 4.11 ± 0.04 | 7.35 ± 0.09 | 1.42 ± 0.06 |
| 83. | 3109 | 3110 | Octacosanol | C28H58O | - | - | 0.05 ± 0.01 |
| 84. | 3199 | 3200 | Dotriacontane | C32H66 | - | 0.08 ± 0.02 | 0.10 ± 0.02 |
| 85. | 3245 | 3251 | Triacontanal | C30H60O | 0.31 ± 0.01 | 0.46 ± 0.15 | 0.32 ± 0.04 |
| 86. | 3292 | 3300 | Tritriacontane | C33H68 | - | 0.11 ± 0.08 | 0.08 ± 0.02 |
| 87. | 3349 | 3351 | γ-Sitosterol | C29H50O | - | 0.16 ± 0.07 | 0.07 ± 0.01 |
| 88. | 3368 | 3327 | β-Amyrone | C30H48O | 0.17 ± 0.01 | 0.14 ± 0.04 | 0.08 ± 0.01 |
| 89. | 3395 | 3400 | Tetratriacontane | C34H70 | 0.67 ± 0.05 | 0.39 ± 0.09 | 1.09 ± 0.04 |
| 90. | 3422 | 3384 | Lupenone | C30H48O | 1.40 ± 0.07 | 1.16 ± 0.04 | 0.61 ± 0.09 |
| 91. | 3451 | 3525 | Lupeol acetate | C32H52O2 | 2.33 ± 0.11 | - | - |
| 92. | 3463 | 3500 | Lupeol | C30H50O | 0.24 ± 0.03 | 2.33 ± 0.01 | 3.29 ± 0.03 |
| Total identified compounds% | 93.57 | 92.65 | 94.9 | ||||
| Plant | DPPH Scavenging Assay | FRAP Scavenging Assay |
|---|---|---|
| C. lancifolius | 470.12 ± 9.84 | 603.74 ± 17.54 |
| C. erectus silver | 349.78 ± 8.26 | 432.48 ± 9.56 |
| C. erectus green | 571.79 ± 16.31 | 796.38 ± 18.91 |
| Microorganism | Conocarpus Species | Controls | |||
|---|---|---|---|---|---|
| CL | CES | CEG | Ketoconazole | Gentamycin | |
| A. niger | 9 | NA | NA | 15 | NT |
| C. albicans | 13 | 15 | 12 | 20 | NT |
| S. aureus | 15 | 10 | 10 | NT | 24 |
| B. subtilis | 14 | 9 | 11 | NT | 26 |
| E. coli | 12 | 14 | 10 | NT | 30 |
| P. vulgaris | 13 | 12 | NA | NT | 25 |
| Microorganisms | Conocarpus Species EOs | Controls | |||
|---|---|---|---|---|---|
| CL | CES | CEG | Ketoconazole | Gentamycin | |
| A. niger | 5000 | NA | NA | 19.54 | NT |
| C. albicans | 1250 | 625 | 1250 | 9.77 | NT |
| S. aureus | 625 | 2500 | 2500 | NT | 4.88 |
| B. subtilis | 1250 | 5000 | 2500 | NT | 4.88 |
| E. coli | 1250 | 625 | 2500 | NT | 9.76 |
| P. vulgaris | 1250 | 1250 | NA | NT | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim, A.; Arasteh, A.A.; Sahrish, R.; Labash, D.; El-Keblawy, A.A.; Gad, H.A.; Ashmawy, N.S. Comparative Metabolic Profiling and Biological Evaluation of Essential Oils from Conocarpus Species: Antidiabetic, Antioxidant, and Antimicrobial Potential. Plants 2025, 14, 464. https://doi.org/10.3390/plants14030464
Salim A, Arasteh AA, Sahrish R, Labash D, El-Keblawy AA, Gad HA, Ashmawy NS. Comparative Metabolic Profiling and Biological Evaluation of Essential Oils from Conocarpus Species: Antidiabetic, Antioxidant, and Antimicrobial Potential. Plants. 2025; 14(3):464. https://doi.org/10.3390/plants14030464
Chicago/Turabian StyleSalim, Aya, Alya Ali Arasteh, Ruqaiya Sahrish, Danya Labash, Ali A. El-Keblawy, Haidy A. Gad, and Naglaa S. Ashmawy. 2025. "Comparative Metabolic Profiling and Biological Evaluation of Essential Oils from Conocarpus Species: Antidiabetic, Antioxidant, and Antimicrobial Potential" Plants 14, no. 3: 464. https://doi.org/10.3390/plants14030464
APA StyleSalim, A., Arasteh, A. A., Sahrish, R., Labash, D., El-Keblawy, A. A., Gad, H. A., & Ashmawy, N. S. (2025). Comparative Metabolic Profiling and Biological Evaluation of Essential Oils from Conocarpus Species: Antidiabetic, Antioxidant, and Antimicrobial Potential. Plants, 14(3), 464. https://doi.org/10.3390/plants14030464








